Abstract
Pathologists have recognized breast cancer heterogeneity for decades, but its causes were unknown. In recent years, basic science and translational studies have demonstrated that cancer stem cells contribute to the heterogeneous histological and functional characteristics of breast cancer. Even more recently, the ability of breast epithelial cells to undergo an epithelial to mesenchymal (EMT) transition has been linked to the acquisition of stem cells properties, and enhanced tumor invasion, metastasis, and resistance to available treatments. The stem cells and cells undergoing EMT are attractive targets for therapy and breast cancer prevention. Despite current challenges, their identification in breast tissue samples would enable pathologists to discover and validate prognostic and predictive markers, as well as identify markers of increased risk for breast cancer.
Keywords: stem cells, epithelial to mesenchymal transition, EMT, metastasis, ALDH1, CD44
Introduction
Pathologists have observed for decades that breast carcinomas are histologically heterogeneous. Not only may the histological features of tumors vary between different patients, but a single tumor may exhibit varying morphologies and growth patterns. However, it was not until recently that the biological basis of histological heterogeneity within breast cancer began to unravel. It is recognized that breast carcinomas harbor oncogenic mutations which through clonal evolution give rise to phenotypic diversity. In more recent years, the discovery of breast cancer stem cells has further enhanced our understanding of tumor heterogeneity. The existence of undifferentiated cells with self-renewal and multilineage differentiating capacity within tumors led to the idea that functional differences of individual tumor cells are the result of differences in their state of differentiation, thus resulting in the varied histological appearance of human breast cancer.
Studies have implicated the transition between epithelial to mesenchymal differentiation states in the generation and maintenance of breast cancer stem cells. The process of epithelial to mesenchymal transition (EMT) involves the dynamic and potentially reversible change from an epithelial morphology to a mesenchymal-like, spindled histological appearance of individual cells. This morphological change is accompanied by an immunophenotypic and molecular transition towards a mesenchymal program. Cancer cells undergoing EMT have enhanced invasive and motile properties, and are thought to trigger metastatic spread.
From a clinical perspective, the importance of identifying and targeting stem cells is based on their reported chemo- and radio-resistance, which may be responsible for the failure of current treatment modalities to cure breast cancer. Breast cancer stem cells, especially those in EMT states, have been highlighted as the drivers of distant metastasis. Furthermore, a number of studies have demonstrated that the presence and abundance of breast cancer stem cells in tissue samples have prognostic significance. Despite all the evidence supporting the importance of EMT and stem cells in breast cancer development and progression, detection of these cells in biopsies and resections has been challenging for pathologists. In this article, we focus on current means of detecting breast cancer stem cells and cells undergoing EMT by immunohistochemistry in breast cancer tissue samples, and discuss their possible utility as tissue-based clinical useful biomarkers.
The discovery of breast cancer stem cells and their isolation in the laboratory
The normal breast consists of a complicated network of ducts culminating in the terminal duct lobular unit (TDLU) which contains different cell types with regenerative properties during puberty and lactation. These cells have self-renewal capacity and are able to differentiate into ductal epithelial, alveolar, and myoepithelial cells. Mammary transplantation models have allowed the identification of stem cells in mouse mammary glands. Further seminal studies have demonstrated the existence of stem cell-like populations in human mammary glands.
The concept that cancers arise from stem cells was first proposed over 150 years ago. Recent advances in stem cell biology have extended and directly tested this hypothesis, providing evidence that breast cancers arise in stem cells through dysregulation of the normally tightly-regulated process of self-renewal. The first demonstration of the existence of breast cancer stem cells was reported in 2003. These investigators showed that human breast cancers contain a cellular population characterized by the expression of cell-surface markers CD44+/CD24low/−/lin− detected using fluorescent-activated cell-sorting (FACS). As few as 200 CD44+/CD24low/−/lin− cells were able to form tumors when implanted in NOD/SCID mice. Furthermore, these tumors recapitulated the cellular heterogenic composition of the primary tumor.
In addition to the detection of the CD44+/CD24low/−/lin− population, breast cancer stem cells can be isolated on the basis of their increased expression of aldehyde dehydrogenase (ALDH) (Aldefluor assay) by flow cytometry, and by their capacity to form mammospheres in vitro. ALDH1 is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes which has been shown to identify and to promote breast cancer stem cell properties in vivo and in vitro. In vitro, breast cancer stem cells are isolated by their ability to grow as spherical colonies or mammospheres under non-adherent substrata in serum free conditions in the presence of growth factors. This assay tests fundamentally the self-renewal capacity characteristic of stem cells.
The process of EMT and the links between EMT and breast stem cells
The epithelial to mesenchymal transition (EMT) is a complex and dynamic biological process characterized by the acquisition of a molecular phenotype marked by dysfunctional cell-cell adhesion, loss of apical-basal polarity, increased resistance to apoptosis, and gain of motility. Both EMT and the reverse process, the mesenchymal to epithelial transition, are fundamental in normal development and formation of the body plan. Indeed, these events are thought to occur as early in development as trophoblast invasion into the endometrial matrix and are involved in gastrulation and subsequent events leading to organ formation (reviewed in).
The study of EMT in development has grown considerably since first officially characterized by Hay more than a decade ago, and a role for EMT has been described in both organ fibrosis and cancer. In contrast to the developmental EMT program, however, pathologic EMT appears to be poorly coordinated, leading to a recent classification system of EMT and stratification into type I (developmental), type II (tissue regeneration and fibrosis), and type III (cancer progression and metastasis). EMT can generally be characterized by the loss of cell-cell adhesion molecules via transcriptional repression and relocalization of cadherins, occludins, claudins, and desmoplakin, with concomitant increases in mesenchymal markers including N-cadherin, vimentin, smooth muscle actin, fibronectin. One of the most important hallmarks of EMT is the switch of the cell-cell adhesion molecule and negative regulator of the canonical Wnt pathway E-cadherin to N-cadherin.
The idea of whether EMT actually occurs in vivo was initially met with some skepticism, in part due to the lack of pathologic evidence at secondary sites and the idea that epithelial cells undergoing EMT may be indistinguishable from fibroblasts; however, generation of several murine systems have strengthened the case for EMT. Indeed, the development of a system to mark and follow the fate of mammary epithelial and stromal cells in a whey acidic protein (WAP-myc) model has provided direct evidence of EMT in vivo. Other animal models of breast cancer including the MMTV-neu and MMTV-polyoma middle T antigen models have recapitulated these findings.
The epithelial to mesenchymal transition is regulated by a group of transcription factors, mostly repressors including Snai1, Snai2 (formerly named Slug), ZEB1 and 2, and Twist1 and 2. These transcriptional repressors directly silence mediators of epithelial adhesion, the most important of which is E-cadherin. These factors bind to E-boxes in the E-cadherin promoter and inhibit E-cadherin transcription. The role of Snail and Twist in EMT is corroborated by findings that their ectopic expression induces EMT in immortalized human mammary epithelial cells. The link between Snail overexpression and metastatic potential is suggested clinically by the fact that in women with breast cancer, high levels of Snail expression are predictive of decreased relapse-free survival.
The transcriptional regulator ZEB1 is also recruited to E-boxes of the E-cadherin promoter, and this recruitment results in transcriptional repression, and loss of cellular polarity, implicating ZEB1 as an EMT-inducer in breast and other solid tumor cells. The overexpression of ZEB1 and subsequent EMT induction has been linked to a number of mechanisms; signaling through TGF- β, TNF- α, IGF1, EGFR, estrogen, progesterone, and COX-2 pathways have been associated with activation of ZEB1 expression. Additionally, mammary epithelial cells made to constitutively express the active subunit of NF-kB have increased expression of ZEB-1 and ZEB-2 and concomitant increases in mesenchymal markers. There is mounting evidence suggesting that ZEB1 represses miRNAs responsible for preventing EMT, migration, and invasion of cancer cells.
In addition to the above described proteins, the cysteine-rich protein CCN6 (or Wnt-1-induced signaling protein 3, WISP3) is an important regulator of EMT and E-cadherin expression in breast epithelial cells. Stable knockdown of CCN6 in breast epithelial cells results in the suppression of epithelial proteins, including E-cadherin, and the upregulation of mesenchymal proteins, consistent with EMT. Suppression of E-cadherin by CCN6 loss occurs via an increase in Snail and ZEB1 mRNA and protein and is associated with axillary lymph node breast cancer metastases (reviewed in).
In recent years, a body of work has provided mechanistic and functional links between EMT and acquisition of both normal and cancer stem cell traits. This connection implicates EMT not only in cancer cell migration and invasion, but also as seeds of metastasis. The molecular connections between EMT and cancer stem cells involve several signaling pathways, including but not limited to Wnt-β-catenin, microRNAs (miR-200b), and epigenetic regulators, principally BMI-1 and Suz12. Understanding of how these processes interact, and how detection of EMT markers in breast tissues samples may allow identification of tumors with higher metastatic potential is of clinical importance.
Tissue-based identification of breast cancer stem cells
Although multiple putative tissue-based breast cancer stem cell markers have been proposed, immunostaining for ALDH1 and dual detection of CD44+/CD24low/− are the most studied and validated breast cancer stem cell marker proteins to date.
Detection of ALDH1 protein
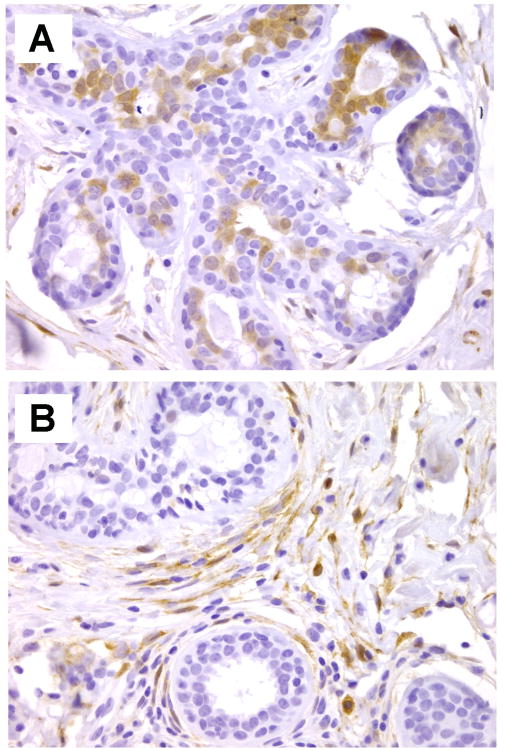
In normal breast tissues, ALDH1 localizes to the cytoplasm of ductal epithelial cells. There are very few studies in the literature on ALDH1 expression in normal breast tissues. The first study describing ALDH1 as a marker of breast stem cells described ALDH1 expression in normal breast epithelial cells as rare, predominantly towards the lumen of the ducts. Later on, ALDH1 positive normal ductal epithelial cells were reported to comprise approximately 1-2% of the epithelial cell population. A subsequent study investigated the expression of ALDH1 in benign breast biopsies found that ALDH1 was expressed in up to 48% of cases. This study noted that ALDH1 was expressed in the intralobular and interlobular stromal cells in approximately 60% of the benign biopsies studied. ALDH1 expression in the ductal epithelial cells as well as in the interlobular stromal cells of benign breast biopsies was associated with a subsequent breast cancer diagnosis. This study provided the first demonstration of the potential utility of ALDH1 detection in breast biopsies and posited ALDH1 as a promising biomarker of breast cancer risk. Figure 1 shows examples of ALDH1 staining in normal breast tissues.
Figure 1.
ALDH1 expression in normal breast. A. ALDH1 positive cells are localized towards the lumen of breast acinar structures. B. Intralobular stromal cells expressing ALDH1 protein are seen surrounding normal acini. Original magnification × 400.
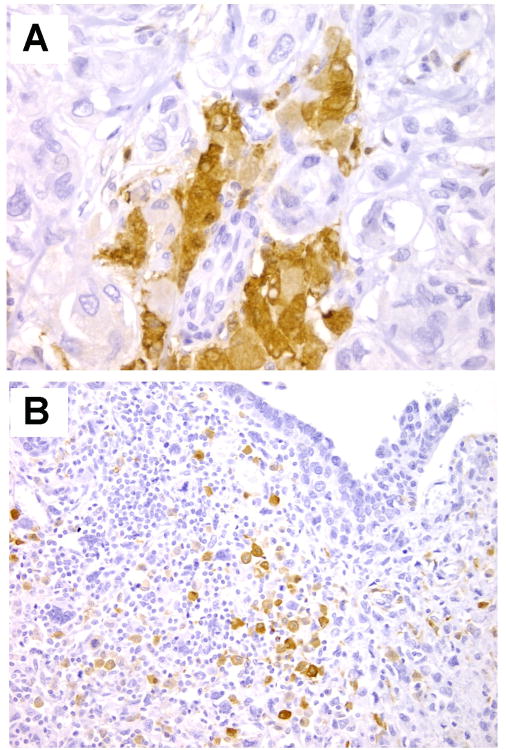
The first study to show the utility of ALDH-1 immunostaining in breast cancer was by Ginestier et al. These investigators evaluated ALDH1 expression in the epithelial component of 481 invasive carcinomas from two independent patient cohorts, arrayed in tissue microarrays. When present, ALDH1 was expressed in an average of 5% of tumor cells. ALDH1 was positive in 19 – 30% of invasive carcinomas. In this study, expression of ALDH1 was associated with high histological grade, HER-2/neu overexpression, negative estrogen and progesterone receptors, and poor overall survival. ADLH1 expression was able to independently predict overall survival. Later on, ALDH1 positivity also identified a subset of patients with inflammatory breast carcinoma with increased risk of recurrence. In these studies, ALDH1 was evaluated exclusively in the cancer cells. More recently, it became apparent that ALDH1 is also expressed by the stromal cells in the tumor microenvironment. A recent report showed that ALDH1 expression in the stromal compartment of breast carcinomas is able to predict disease free survival in triple negative breast cancer. Figure 2 shows representative immunostaining for ALDH1 in breast cancer tissue samples.
Figure 2.
ALDH1 expression in invasive carcinoma. A. Patchy positivity for ALDH1 in the cytoplasm of breast cancer cells. Original magnification × 400. B. ALDH1 is expressed by a group of malignant spindle cells in a case of metaplastic breast carcinoma. Original magnification × 200.
Deng et al. addressed the important and practical question of whether ALDH1 activity detected by the Aldefluor assay correlates with ALDH1 protein expression by immunohistochemistry. Indeed, these investigators found that ALDH1 enzymatic activity was positively correlated with its expression in cancer cells, when determined in breast as well as other solid human tumors. Given these exciting initial results, it is clear that more detailed investigations are warranted on the role of ALDH1 as a marker of breast cancer risk, and as a prognostic and predictive biomarker in breast cancer.
D etection of C D44+/CD24low/− cells
CD24 is a small, two chain glycosylphosphatidyltinositol-anchored protein which is localized to the cell surface and functions an adhesion protein. CD24 is expressed in multiple malignancies including B cell lymphoma, small cell and non-small cell lung carcinoma, hepatocellular carcinoma, and breast. Functionally, CD24 has been identified as a ligand of P-selectin, an adhesion receptor on activated endothelial cells and platelets, which might enhance metastatic potential. In breast cancer, CD24 expression in tissues has been reported as a promising prognostic indicator. Similar to CD24, CD44 is a cell surface glycoprotein, CD44 is also known as homing cell adhesion molecule (HCAM), Phagocytic glycoprotein-1 (PgP-1), ECM-III, HUTCH-1, or Hermes-1. In solid tumors, CD44 has been implicated in cell migration and metastasis.
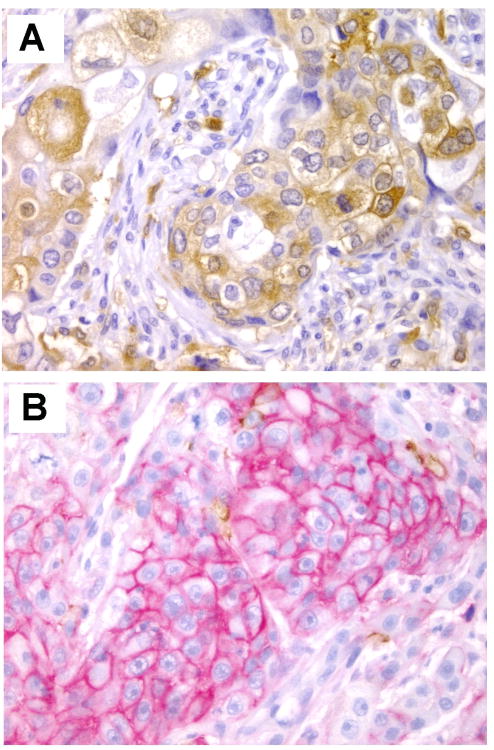
The discovery that CD44+/CD24low/−/lin− marker combination identified a population of breast cancer stem cells in the laboratory provided the basis for studies in tissues. Indeed, there have been multiple studies investigating the expression of CD44 and CD24 in human breast cancer samples. In some studies these markers were detected in separate tissue sections, while in others dual immunostaining has been performed. One of the first studies utilizing double immunostaining for CD44 and CD24 in breast cancer examined 240 invasive carcinomas arrayed in tissue microarrays from women with a median follow up of 5.3 years. They found that CD44+/CD24−/low cells were detected in 31% (75/240) of the tumors. Interestingly, the authors demonstrated that the CD44+/CD24low/− phenotype was associated with basal like breast carcinomas, especially tumors arising in the setting of BRCA1 mutations. Although subsequent studies confirmed the potential utility of CD44 and CD24 proteins as biomarkers in breast cancer, others failed to support these observations. It is clear that further investigations are necessary to draw conclusions about the utility of these markers in breast tissue sections. Figure 3 illustrates dual immunostaining for CD44 and CD24 proteins.
Figure 3.
Dual immunostaining for CD24 (brown) and CD44 (red) in invasive carcinomas of the breast. A. CD24 expressing breast cancer cells. B. Invasive carcinoma cells positive for CD44 and negative for CD24. Notice that there is minimal CD24 (brown) reactivity in this area. The expression of CD24 and CD44 is heterogenous within invasive carcinomas. Original magnification × 400.
It is intriguing indeed that when analysed in the same invasive carcinomas, CD44+/CD24low/− and ALDH1 positive cell populations do not necessarily coincide. It has been suggested that these proteins detect different states (e.g. epithelial vs. mesenchymal) of breast cancer stem cells, which is currently under intensive laboratory-based investigation.
Other promising biomarkers to identify breast cancer stem cells in tissue samples
In addition to ALDH1 and CD44+/CD24low/−, other markers have been studied as cancer stem cell biomarkers, including CD133. CD133, also known as prominin-1, is a pentaspan transmembrane glycoprotein that localizes to membrane protrusions such as microvilli and in the apical surface of some epithelial cells. Expression of CD133 in cancer-initiating cells has been reported in several tumor types including hematopoietic malignancies and solid tumors included breast cancer. Recently, Liu et. al. analyzed the expression of CD133 by immunohistochemistry in triple negative invasive carcinomas of the breast. CD133 could be present in the luminal surface, in the cytoplasm and cytoplasmic membrane, or in predominantly in the cytoplasm of cancer cells, and ranged from 5-24% positive cells. In this study, CD133 positivity was associated with positive lymph nodes and higher histological grade. Further studies are necessary to evaluate the utility of CD133 as a breast cancer stem cell biomarker and to determine its potential clinical utility in breast cancer.
Markers of EMT cells and of the EMT-stem cell connection
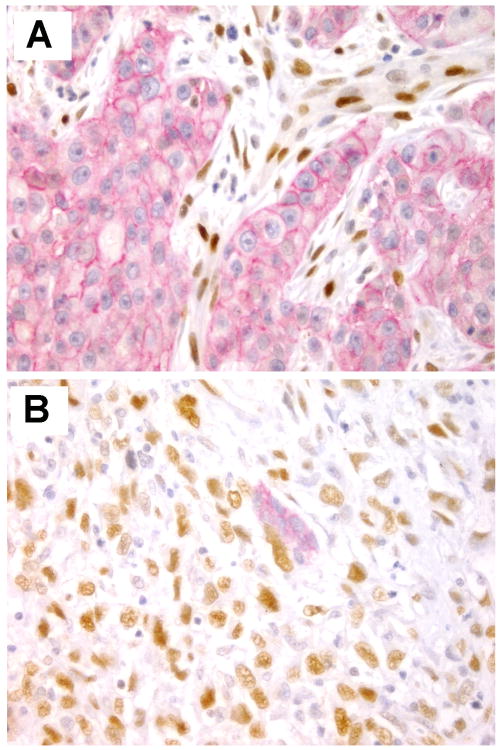
While EMT has been shown in cancer cell lines in culture, whether EMT can be detected in vivo remains incompletely resolved. Demonstration of EMT in tissues is limited by the nature of the EMT process: its transient, dynamic and reversible characteristics. In breast tissue sections, multiple studies have been conducted to test the expression of EMT transcription factors ( including Snai1, Snai2, ZEB1, ZEB2, Twist1, Twist2) as well as E-cadherin. Additionally, studies of invasive breast carcinomas have linked EMT markers with specific cancer phenotypes. For example, several EMT markers (vimentin, N-cadherin, cadherin-11, smooth muscle actin, SPARC, laminin, fascin, and low levels of E-cadherin and cytokeratins) were preferentially found in basal-like breast cancers. Figure 4 illustrates detection of EMT using dual immunohistochemistry for ZEB1 and E-cadherin in breast cancer tissue sections.
Figure 4.
Dual immunostaining to detect E-cadherin (red) and ZEB1 (brown) in invasive carcinomas. A. Invasive ductal carcinoma showing E-cadherin expression at cell membranes. ZEB1 is localized to the nuclei of stromal cells and vessel walls. B. Metaplastic carcinoma of the breast provides a good example of EMT in tissues. The small gland exhibits E-cadherin expression, however, the surrounding invasive metaplastic carcinoma cells have high expression of the EMT transcription factor ZEB1. Original magnification × 400.
The process of EMT is linked to metaplasia, which involves the reversible change of one adult cell type to another and is thought to arise from genetic or epigenetic reprogramming of stem cells. In the breast, metaplastic carcinomas constitute an excellent model to study the interplay between EMT and stem cells, as they are characterized by the presence of a metaplastic, non-glandular component, which most of the times exhibits mesenchymal differentiation including spindle, osseous, or cartilaginous cells. Using metaplastic carcinomas as a model, a recent study provided direct in situ evidence for the reported connection between EMT and stem cells in breast cancer. This study demonstrated that ALDH1 positive and CD44+/CD24−/low stem cells also exhibited markers of EMT markers including decreased E-cadherin and ZEB1 upregulation. Furthermore, the stem cells and cells with EMT features were enriched in the spindle areas and heterologous elements of metaplastic carcinomas.
The link between EMT and cancer stem cells and their relevance as potentially clinically useful biomarkers has been recently highlighted by Mego et al. . In this study, circulating tumor cells and circulating tumor cells with EMT, as measured by detecting EMT transcription factors using quantitative RT-PCR, had prognostic value in patients with metastatic breast carcinoma high-dose chemotherapy (HDCT) and autologous hematopoietic stem cell transplantation (AHSCT).
Conclusion and perspectives
In recent years, basic science studies have provided strong evidence that the EMT and stem cell programs are closely interrelated and are a cause of breast cancer heterogeneity. These processes have been proposed to contribute to breast cancer initiation, invasion, metastasis and resistance to current treatments. Advances in the laboratory have led to the identification of breast cancer stem cells. Although more work is needed, available studies demonstrate our ability to detect and quantify stem cells and EMT cells in normal breast and in breast cancer tissue samples. These studies may lead to the development of predictive tests for treatment response, better prognosticators, and ways to identify women with increased risk for breast cancer.
Acknowledgments
This work was supported by NIH grants R01 CA107469, R01 CA125577 and U01CA154224 (to CGK), the University of Michigan's Cancer Center Support Grant (5 P30 CA46592).
Footnotes
Conflict of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morel AP, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–76. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 4.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):253–60. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 5.Li X, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 6.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 7.Balic M, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12(19):5615–21. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 8.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26(17):2813–20. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginestier C, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356(3):217–26. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 11.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125(10):1921–30. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 12.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 13.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julius Cohnheim (1839-1884) experimental pathologist. JAMA. 1968;206(7):1561–2. [PubMed] [Google Scholar]
- 16.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119(6):1417–9. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 1996;156(3):202–16. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 19.Yamakoshi S, et al. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction. 2012;143(3):377–87. doi: 10.1530/REP-11-0364. [DOI] [PubMed] [Google Scholar]
- 20.Nieto MA. Epithelial-Mesenchymal Transitions in development and disease: old views and new perspectives. Int J Dev Biol. 2009;53(8-10):1541–7. doi: 10.1387/ijdb.072410mn. [DOI] [PubMed] [Google Scholar]
- 21.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154(1):8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugo H, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 24.Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28(1-2):151–66. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 26.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancerarallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–34. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trimboli AJ, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68(3):937–45. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 28.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11(6):213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 30.Burk U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9(6):582–9. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham TR, et al. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68(7):2479–88. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 32.Moody SE, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8(3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Yook JI, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8(12):1398–406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 34.Batlle E, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 35.Cano A, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 36.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66(5):773–87. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grooteclaes ML, Frisch SM. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene. 2000;19(33):3823–8. doi: 10.1038/sj.onc.1203721. [DOI] [PubMed] [Google Scholar]
- 38.Guaita S, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem. 2002;277(42):39209–16. doi: 10.1074/jbc.M206400200. [DOI] [PubMed] [Google Scholar]
- 39.Eger A, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24(14):2375–85. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 40.Spaderna S, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68(2):537–44. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 41.Chua HL, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cellsotential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26(5):711–24. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 42.Bracken CP, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68(19):7846–54. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 43.Korpal M, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–4. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang W, et al. Inhibition of CCN6 (Wnt-1-induced signaling protein 3) down-regulates E-cadherin in the breast epithelium through induction of snail and ZEB1. Am J Pathol. 2008;172(4):893–904. doi: 10.2353/ajpath.2008.070899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Inhibition of CCN6 (WISP3) expression promotes neoplastic progression and enhances the effects of insulin-like growth factor-1 on breast epithelial cells. Breast Cancer Res. 2005;7(6):R1080–9. doi: 10.1186/bcr1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang W, Pal A, Kleer CG. On how CCN6 suppresses breast cancer growth and invasion. J Cell Commun Signal. 2012;6(1):5–10. doi: 10.1007/s12079-011-0148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blick T, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/−) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):235–52. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 48.Taube JH, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107(35):15449–54. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin Cancer Biol. 2012;22(5-6):396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunju LP, et al. EZH2 and ALDH-1 mark breast epithelium at risk for breast cancer development. Mod Pathol. 2011;24(6):786–93. doi: 10.1038/modpathol.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng S, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5(4):e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charafe-Jauffret E, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16(1):45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resetkova E, et al. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123(1):97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 54.Kristiansen G, et al. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9(13):4906–13. [PubMed] [Google Scholar]
- 55.Jackson D, et al. CD24, a signal-transducing molecule expressed on human B cells, is a major surface antigen on small cell lung carcinomas. Cancer Res. 1992;52(19):5264–70. [PubMed] [Google Scholar]
- 56.Aigner S, et al. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89(9):3385–95. [PubMed] [Google Scholar]
- 57.Honeth G, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10(3):R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mylona E, et al. The clinicopathologic and prognostic significance of CD44+/CD24(−/low) and CD44−/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39(7):1096–102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Toy KA, Kleer CG. Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Mod Pathol. 2012;25(2):178–84. doi: 10.1038/modpathol.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S, Clouthier SG, Wicha MS. Role of microRNAs in the regulation of breast cancer stem cells. J Mammary Gland Biol Neoplasia. 2012;17(1):15–21. doi: 10.1007/s10911-012-9242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu TJ, et al. CD133(+) cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2012 doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 62.Wright MH, et al. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10(1):R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu ZQ, et al. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1205822109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oka H, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53(7):1696–701. [PubMed] [Google Scholar]
- 65.Aigner K, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26(49):6979–88. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarrio D, et al. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68(4):989–97. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 67.Kumar V, A A, Fausto N, Aster J. Robbins and Cotran Pathologic Basis of Disease. 8th. Elsevier; 2010. [Google Scholar]
- 68.Mego M, et al. Prognostic Value of EMT-Circulating Tumor Cells in Metastatic Breast Cancer Patients Undergoing High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Transplantation. J Cancer. 2012;3:369–80. doi: 10.7150/jca.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]