Abstract
The possibility of free radical reactions occurring in biological processes led to the development and employment of novel methods and techniques focused on determining their existence and importance in normal and pathological conditions. For this reason the use of Nitrones for spin trapping free radicals came into widespread use in the 1970s and 1980s when surprisingly the first evidence of their potent biological properties was first noted. Since then wide-spread exploration and demonstration of the potent biological properties of phenyl-tert-butylnitrone (PBN) and derivatives were shown in preclinical models of septic shock and then in experimental stroke. The most extensive commercial effort done in order to capitalize on the potent properties of the PBN-Nitrones was for acute ischemic stroke. This occurred during the 1993–2006 time interval where the 2,4-disulfonyl-phenyl PBN derivative, called NXY-059 in the stroke studies, was shown to be safe in humans and was taken all the way through clinical phase 3 trials and was deemed to be ineffective. As summarized in this review because of its excellent human safety profile 2,4-disulfonyl-phenyl PBN, now called OKN-007 in the cancer studies, was tested as an anti-cancer agent in several preclinical glioma models and shown to be very effective. Based on these studies this compound is now scheduled to enter into early clinical trials for astrocytoma/glioblastoma multiform this year. The potential use of OKN-007 in combination with neurotropic compounds such as the lanthionine ketamine esters (LKE) is discussed for glioblastoma multiform as well as for various other indications leading to dementia such as aging, septic shock, and malaria infections. There is much more research and development activity on-going for various indications with the nitrones alone or in combination with other active compounds as briefly noted in this review.
Why should Nitrones be considered Potential Therapeutics?
It is important to ask the question, why are the PBN (α-phenyl-N-tert-butyl-nitrone) Nitrones considered potential therapeutics? The most important reason is that PBN and some of its congeners have been found to have extremely potent biological activity in several experimental biological systems. Historically in the late 1960s and early 1970s the nitrones became important agents to aid in the identification of highly unstable free radical intermediates in chemical reactions because they could react with and stabilize free radicals by what was commonly known as a spin-trapping reaction. To the surprise of some chemists the nitrones PBN and DMPO (5,5-dimethyl-1-pyrroline-N-oxide) also proved to be useful to trap free radicals in biochemical systems. Their use was then extended to biological systems [1]. It was found that extending their use to biological systems became problematic for their intended purpose of identifying the free radical intermediates because the spin-adduct nitroxide was chemically reduced by the reductive biological processes and rendered paramagnetic silent. However it was discovered in several instances that the nitrones had potent biological activity in many biological systems [1, 2] . This report presents a summary of past, present and future prospective of research and development focused on the therapeutic potential of nitrones.
Spin Trapping- History and General Considerations
The spin trapping method, named by Janzen, was developed independently by various researchers in 1968 and 1969 [3–8], to extend the limits of electron spin resonance (ESR) spectroscopy so that lower concentrations of free radicals could be detected indirectly. This method involves the trapping of reactive short-lived free radicals by a diamagnetic spin trap compound via an addition reaction to produce a more stable free radical product or spin adduct. The spin adduct that is formed is paramagnetic and has an ESR spectrum with a hyperfine splitting constant and g-value characteristic of the type of reactive free radical trapped. Thus, the structure of the radical trapped can usually be deduced, although the most difficult aspect of the spin trapping technique is the correct assignment of the nitroxide spectrum to the original radical species.
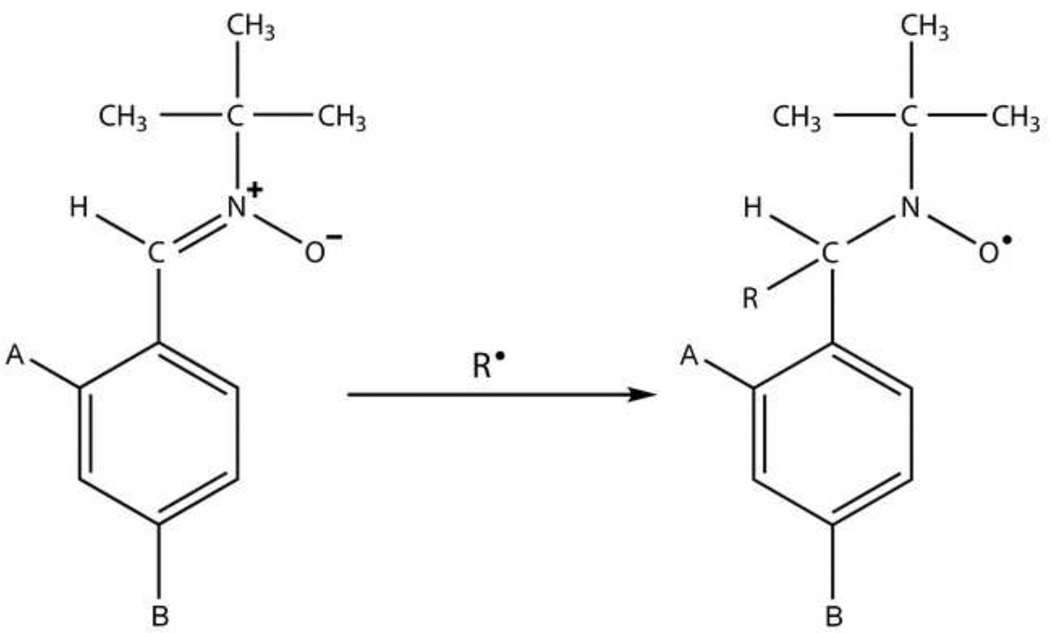
Nitrones are commonly used spin trapping agents in biological systems. The addition of a reactive free radical (R•) with a nitrone spin trap results in the formation of a nitroxide (see Fig. 1). Nitroxides are stable free radicals due to the resonance stabilization of the unpaired electron between the nitrogen and the oxygen of the nitroxyl functional group [9]. The most stable nitroxides are those with inert functional groups attached to the nitrogen atom, such as methylated carbon atoms [10–12]. Examples of commonly used nitrones are PBN [3] and the cyclic nitrone DMPO [8, 13–15]. Interesting derivatives of PBN bound to cyclodextrins, such as permethylated cyclodextrin (Me3CD-PBN) or 2,6-di-O-Me-β-cyclodextrin-grafted PBN (Me2CD-PBN) have been found to trap carbon- and oxygen-centered free radicals with enhanced ESR signal intensities [16]. Recently, DMPO and other cyclic nitrones (e.g. AMPO, EMPO, DEPMPO) have been assessed in their abilities to trap reactive nitrogen species (RNS), such as ●NO2, ONOO−, and ONOOCO2− [17, 18]. Improvements in nitrones for the trapping of superoxide (O2●−) include derivatives of the DEPMPO nitrone (5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide), 4-HMDEPMPO (5-diethoxyphosphoryl-4-hydroxymethyl-5-methyl-1-pyrroline-N-oxide) (160, the β-cyclodextrin (β-CD)-cyclic nitrone conjugate, 5-N-β-cyclodextrin-carboxamide-5-methyl-1-pyrroline N-oxide (CDNMPO) [19] , a cyclic nitrone conjugate of calyx[4]pyrrole (CalixMPO) [20], and a 4-furoxanyl nitrone (FxBN) [21]. For the detection of mitochondrial generated free radicals, a novel cyclic nitrone spin trap containing a phosphonium cation, ([4-(2-methyl-1-oxy-3,4-dihydro-2H-pyrrole-2-carbonyloxy)-butyl]-triphenyl-phosphonium bromide, MitoBMPOBr) trapping superoxide and hydroxyl radicals [22], and a nitrone-containing N-arylpyridinium salts trapping carbon-centered radicals [23], could be useful.
Figure 1.
Spin-Trapping reaction where a free radical has reacted with a general PBN-type nitrone to form a spin-adduct. In the case of PBN A=B=H and with OKN-007 A=B= SO3Na2
The type of spin trap used is an important factor in determining how informative and sensitive the spin trapping technique may be for a given free radical species. It is important to emphasize that no single spin trap is optimal for the trapping of all types of reactive free radicals. The main advantages of using nitrone spin traps are: (i) they are less sensitive to light, oxygen or water vapor, (ii) they are soluble in a large number of solvents at fairly high concentrations (~0.1 M), and (iii) spin adducts are considerably more stable since a carbon atom separates the nitroxide functional group from the trapped radical species [8, 24]. The use of nitrone compounds also has some disadvantages such as: (i) the information regarding the nature and structure of the trapped species is difficult to obtain from the spectrum of the spin adduct, e.g. ESR spectra PBN spin adducts have characteristically small aHβ (β-hyperfine splitting constant) values with little variation in magnitude with different free radical species, although the magnitude of the β-hyperfine splitting is enhanced with the cyclic nitrone (DMPO), and (ii) photolysis of PBN in certain solvents (e.g. tetrahydrofuran) rapidly produces spin adducts derived from solvent radicals [8, 16]. Reviews of interest on the chemistry of nitrone spin traps and their biological importance are found in Towner [25], Floyd et al. [26, 27], Rhodes CJ [28], and Villamena et al. [29].
Recently in the last decade protein radicals generated as a result of oxidative stress processes can be tagged by DMPO to form DMPO-radical adducts, which can be further assessed by immune-spin trapping, a method that utilizes an antibody against DMPO-adducts [30–34]. This application has been used very recently in combination with molecular MRI to visualize membrane-bound radicals in various organs/tissues in a diabetes model [35].
Early demonstrations of the biological effects of nitrones
The very first early reports which indicated that nitrones had biological activity and suggested that they may act as pharmaceutical agents were done by Novelli in 1985 [36] and 1986 [37]. Novelli confined and subjected rats to trauma brought on by placing them in a rotating drum. He demonstrated that pre-administering PBN protected these animals from death. He also demonstrated that pre-administration of PBN protected rats from shock trauma caused by administering lipopolysaccharide (LPS). Soon after Novelli reported, in a book chapter, that PBN protected from LPS induced shock, McKechnie et al. [38] reported results of more extensive studies confirming and expanding upon the primary finding. Hamburger and McCay also reproduced and expanded upon the protective effect of PBN in septic shock as reported in 1989 [39], and then later Pogrebniak et al. [40] explored the role of PBN in mediating blood cytokine down regulation in the rat LPS model when the nitrone was administered prior to shock induction. PBN given after LPS administration was shown not to be protective in this model. As discussed below during this general time-frame we made novel observations which clearly showed PBN had neuroprotective activity in an experimental stroke model.
PBN-related Nitrones for the Treatment of Stroke
Early observations in experimental stroke
In December 1988 we made the novel observation that PBN had neuroprotective activity in experimental ischemic stroke [41–43]. This finding came about serendipitously. We had previously developed the use of salicylate to trap tissue hydroxyl free radicals and demonstrated that they were formed in ischemia/re-perfused brains of gerbils [44]. With this result in mind our initial thought was to use PBN to spin-trap and identify possible secondary free radicals that may be formed by the reaction of hydroxyl free radicals with biomolecules such as membrane lipids in the stroked brain. This experiment was tried and it failed to show secondary PBN-trapped radicals using electron spin resonance to monitor these radicals. The use of PBN to trap secondary free radicals failed because the presumptive PBN-trapped free radical adducts were most likely chemically reduced by the reductive systems of biological tissue and hence became non-paramagnetic, and as such thus were electron spin resonance silent. Even though the desired experimental endpoint was not obtained we did discover that PBN treated animals were protective from stroke even if the PBN was administered up to one hour after the ischemia/re-perfusion insult [41, 43]. In addition it was found that older animals were more susceptible to experimental stroke than younger ones, however if chronic low-levels of PBN were administered to the old animals for 2 weeks they became as resistant to the stroke insult as the younger animals, that this effect was maintained with nearly the same magnitude at 3 days, and was still significant but much less so at 5 days after the last dose of PBN [42, 45, 46]. The half-life of PBN in animals is about 6 hours so the protective effect could not be due to PBN buildup. These were very novel and surprising results and therefore the discoveries were patented [43]. Our results were quickly confirmed and expanded upon by others [47, 48].
Commercial Development of novel nitrone NXY-059 for treatment of Acute Ischemic Stroke
Although we have discussed this topic in detail before [26, 49, 50], it is useful to summarize important aspects of the commercial development of the nitrone NXY-059 for treatment of acute ischemic stroke here. Table 1 summarizes the time-line and important stages of this effort. Even though PBN showed protection from experimental stroke if given after the ischemia/reperfusion insult the window of opportunity was short. Also the compound had been widely used in academic settings and therefore not novel either. We discovered that a novel PBN derivative 2,4-disulfonyl-phenyl-tert-butylnitrone (NXY-059) was much more effective than PBN, and showed a much longer window of opportunity for administration after a stroke [49]. In addition it was shown to be an extremely safe compound when administered i.v. to humans even up to 260µM and therefore it was chosen to enter into phase 3 clinical trials [49] . This decision was made after extensive studies that showed it to be protective in preclinical models of transient and permanent experimental stroke [49].
Table 1.
Time-line and Major Stages of Commercial Development of NXG059 for Stroke
| 1985 – 88 | Extensive studies on brain ischemia/perfusion insult (IRI) in gerbils |
| Demonstrated salicylate trapping of hydroxyl free radicals in brain IRI | |
| Established time dependent IRI-mediated brain damage in hippocampus and forebrain | |
| 1988 | Basic discovery that PBN protected in experimental stroke-Dec 1988 |
| Discovery patented, basic studies continued, made many attempts to license to companies | |
| 1992–1993 | Centaur Pharmaceutical, Inc. founded March 1992 |
| Angel investments made possible, CEO hired | |
| Company hires employees, begins to conduct experiments to add value | |
| Explores extent of intellectual property and revise patents for commercial development | |
| 1995 | Centaur and Astra make co-development relationship – June 1995 |
| Ramp-up studies in stroke, medicinal chemistry for other indications | |
| 1996 | NXY059 chosen as candidate drug for stroke |
| 1996 – 2002 | NXY059 undergoes extensive toxicology and tested in several clinical phase I and 2 trials |
| 2002 lead |
Renovis, Inc buys Centaur assets and moves clinical trials forward with AstraZeneca taking |
| 2003 | NXY059 Phase 3 SAINT-1 efficacy trial began in May 2003 |
| 2005 | SAINT-1 trial shows efficacy in 1700 patients – May 2005 |
| SAINT-2 trial begins for 3200 patients – July 2005 | |
| 2006 | Analysis of combined SAINT-1 and SAINT-2 trials showed NXY059 not effective- Oct 2006 |
In the first phase 3 clinical trial (SAINT-1), which enrolled about 1700 patients and required 2 years to complete, decode and analyze the data, the results showed significant efficacy when utilizing the modified Rankin scale as the endpoint parameter. This result prompted the start of a much larger phase 3 trial (SAINT-2) where about 3200 patients were enrolled. This trial showed no beneficial effect of using NXY-059, and when the data from SAINT-1 and SAINT-2 were pooled and analyzed there was a non-significant effect when the window of administration was 6 hours or less after the stroke (Table 1).
The testing of NXY-059 for acute ischemic stroke was widely followed and engendered much disappointment and much speculation following its failure as we have summarized previously [26]. As we discussed previously [50] there may have been a significant fraction of stroke patients helped if MRI characterization of the patients had been an inclusion criteria for screening the patients to be entered into the trial data base. However the MRI procedure was not in wide use at the time these trials were being designed and carried out.
Anti-Cancer Activity of the Nitrone OKN-007 for Glioblastoma
Glioblastomas (GBM): General Considerations
Forty percent (40%) of all primary central nervous system (CNS) tumors diagnosed are gliomas [51]. Of these, the grade IV glioblastomas (GBM) are the most malignant, with a survival time of about 15 months for most patients [51], despite current standard-of-care therapeutic approaches. High grade gliomas (grades III and IV) are the most common primary brain tumors in adults, and their malignant nature ranks them 4th regarding incidence of cancer death [52]. Grading and identification criteria that can be used to provide information regarding tumor behavior include cell proliferation (cellularity and mitotic activity), nuclear atypia, neovascularization and the presence of necrosis and/or apoptotic regions [53] and which may affect therapeutic outcome. Many of these grading and identification criteria can be measured with the use of magnetic resonance imaging (MRI) and MR spectroscopy (MRS) methods [54, 55] which can be useful information regarding an optimal therapeutic approach.
Current therapies for the treatment of GBM include surgical resection, followed up with radiotherapy and chemotherapy with temozolomide, has only limited effectiveness [56, 57], with an average lifespan of 14.6 months post-diagnosis [58]. Gamma Knife radiosurgery has been used to treat tumor recurrences in select cases and has been successful in prolonging median survival by 8–12 months on average [59]. New targeted therapeutic approaches are classified into functional groups, including inhibitors of growth factors and their receptors, inhibitors of proteins of intracellular signaling pathways, epigenetic gene-expressing mechanisms, inhibitors of tumor angiogenesis, tumor immunotherapy and vaccines [56]. Bevacizumab, a monoclonal antibody against the vascular endothelial growth factor (VEGF), is the only FDA (Food and Drug Administration)-approved molecular drug for GBM [60, 61]. Unfortunately the use of bevacizumab has only improved progression-free survival, but not overall survival [60]. Trials with anti-EGFR (epidermal growth factor receptor) agents have so far failed to show any improved outcomes for GBM [60]. There are other potential therapies that target other signaling pathways, such as heat shock proteins and proteosomes, or multisite kinase inhibitors, that are currently under consideration [60]. Immunotherapies that target key cellular and molecular immune system mediators such as TGF-β, cytotoxic T cells, Tregs, CTLA-4, PD-1 and IDO are also being considered [58] . There are also other approaches being considered that involve the use of microglial cells as therapeutic vectors to transport genes and/or substances to the tumor site, as well as the use of natural toxins conjugated to drugs that bind to over-expressed receptors in cancer cells [57]. Quite recently, a nitrone compound, OKN-007 (previously known as NXY-059), which has known anti-inflammatory characteristics is under investigation as an anti-glioma agent in recurrent glioma patients.
GBM: Diagnosis
MRI plays an important role as an optimal imaging tool in the diagnostic process for human gliomas. It is commonly used to provide information on brain tumor growth, vasculature, biochemical metabolism, and molecular changes in preclinical glioma models. Morphological MRI (T2-weighted or T1-weighted contrast-enhanced imaging) is used to provide information on tumor volumes and growth rates, which can be used to distinguish between tumor grades. Tumor morphology can also provide information on tumor invasiveness and necrotic lesions. Necrotic lesions are depicted as dark void regions in a tumor, of which volumes can be measured from multiple slices [62]. Contrast-enhanced imaging can be used to assesses blood-brain-barrier (BBB) disruption, however this feature can be absent in diffuse infiltrative tumor regions or when assessing therapeutic treatment [63].
GBM: Animal Models
Animal models provide a means to study disease and pathological processes, as well as the efficacy of potential new therapies. The majority of glioma models involve intracerebral implantation of rodent (rat or mouse) or human glioma cells into synergetic rats or mice, or immunocompromised rodents (e.g. nude or athymic rats or mice). Glioma cells are injected into the cerebral cortex of rats or mice (synergetic if cells are transplanted into the same species and strain that they were obtained from, or immune-compromised rats or mice if human cells are used) using a stereotaxic device for precise implantation into a brain region. Although no one model emulates all of the characteristics of GBM, they have provided important insight into specific mechanisms of tumor development [64]. Many of these models have some characteristics associated with human gliomas, such as aggressive tumor growth, angiogenesis, and tumor necrosis. The F98 model is currently used by our group as these gliomas have an infiltrative pattern of growth, have attributes associated with human GBM gliomas, and are classified as anaplastic malignant tumors [65, 66]. C6 and F98 glioma cell lines were obtained from chemical induction as a result of administering ethylnitrosourea (ENU) to pregnant rats, where the progeny developed brain tumors that were isolated, and propagated and cloned in cell culture [66], and both are commonly used as a pre-clinical rodent glioma models. Similar to GBM, U87 tumors in immunocompromised rodents are highly cellular with atypia such as mitotic figures and irregular nucleoli, and profuse neovascularization, however unlike GBM, these tumors have a non-diffuse infiltrative growth pattern and a well-demarcated tumor mass surrounded by reactive astrocytes [67].
Gliomas: Therapeutic effect of OKN-007
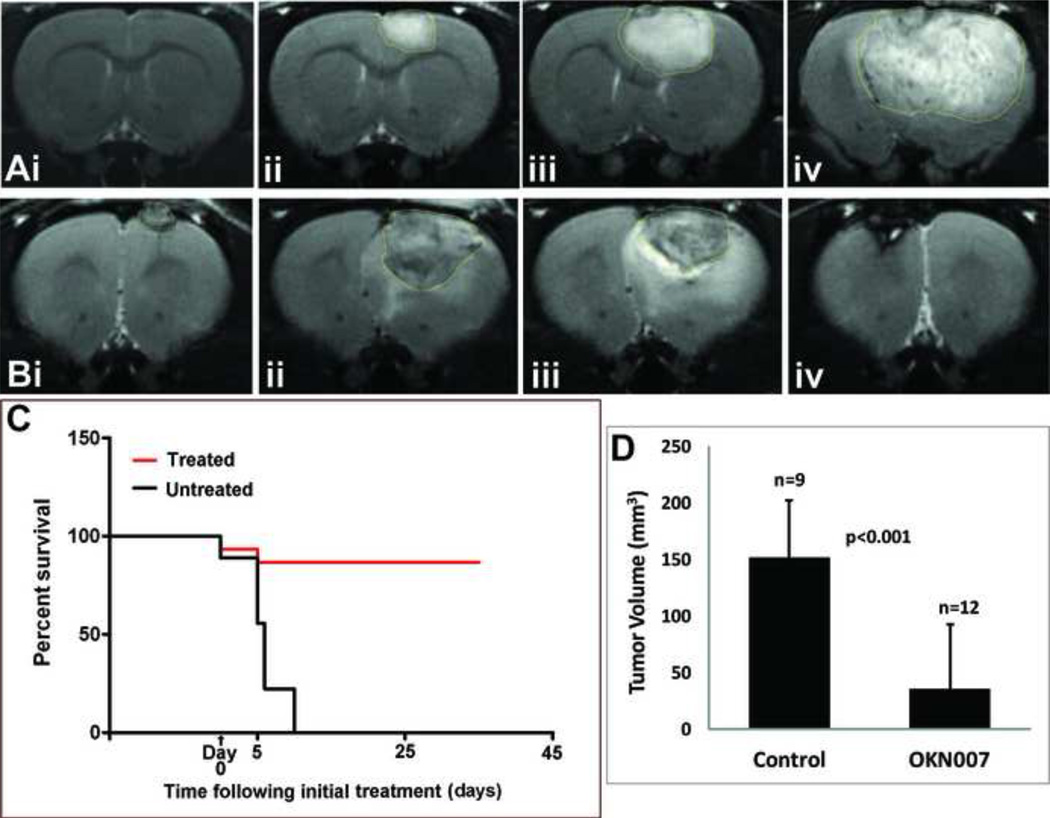
We have previously shown that OKN-007 (previously known as NXY-059) has a dramatic effect on regressing tumor formation in a rat C6 glioma model [68, 69]. Morphological MRI was used to calculate tumor volumes (see Fig. 2Ai-iv and 2D); diffusion-weighted imaging (DWI) was used to measure apparent diffusion coefficients (ADC), which assesses changes in water diffusion due to tissue structural alterations; and perfusion-weighted MRI (pMRI) was used to characterize tissue perfusion rates, which can provide information on alterations in the vascular capillary bed. Rats treated with OKN-007 after tumors were visualized by MRI, were found to have significantly decreased tumor volumes (~3-fold, p<0.05)(Fig. 2Bi-iv and 2D), decreased ADC (~20%, p<0.05), and increased tissue perfusion rates (~60%, p<0.05) in tumors, compared to non-treated rats [68]. Figure 2D presents a very clear example that tumor volumes were significantly decreased by OKN-007 treatment. The tumor volume was about 150 mm3 at days 15–17 in non-treated controls and was about 30 mm3 at days 36–40 in OKN-007 treated rats. It should be noted that the non-treated rats could not be kept alive for 36–40 days because of the huge tumor volume and therefore they had to be killed at about 25 days after tumor cell implantation. OKN-007 was administered in the drinking water at 10 mg/kg/day starting when tumors had reached ~50 mm3 in volume (about day 15 following intracerebral implantation of rat C6 glioma cells), and continued for a total of 10 days [68]. One group of rats was euthanized after the 10 day treatment period, and a second group was monitored for an additional 25 days following the treatment period [68]. In the cohort of animals that were treated for 10 days and then euthanized, percent survival was 92% (p<0.0001)(Fig. 2C), whereas for the rats that were monitored for an additional 25 days the percent survival was greater than 80% (p<0.001) [68]. OKN-007 is currently undergoing phase I/II clinical trials as a therapy against recurrent GBM.
Figure 2.
Treatment of C6 glioma-bearing rats with OKN-007. (A) Representative MR images of a non-treated C6 glioma-bearing rat at 7, 14, 18 and 24 days following intracerebral implantation of rat C6 glioma cells (tumor volumes are 0.0, 23.7, 59.5 and 180.1 mm3, respectively). (B) Representative MR images of an OKN-007 treated C6 treated Glioma at 14, 24, 30 and 40 days following cell implantation (corresponding to 0, 10, 16, and 26 days following initiation of OKN-007 treatment) (tumor volumes are 9.1, 84.5, 71.8 and 2.0 mm3, respectively). (C) Survival curve for untreated (n=9) and OKN-007 treated (n=12) C6 glioma-bearing rats. There is a significant survival for OKN-007 treated animals (p<0.001). (D) C6 tumor volumes as measured from MR images at days 17–21 for untreated rats (n=9; Control) and at days 36–40 for OKN-007 treated rats (n=12). There is a significant decrease in tumor volumes for OKN-007 treated rats compared to untreated controls (p<0.001).
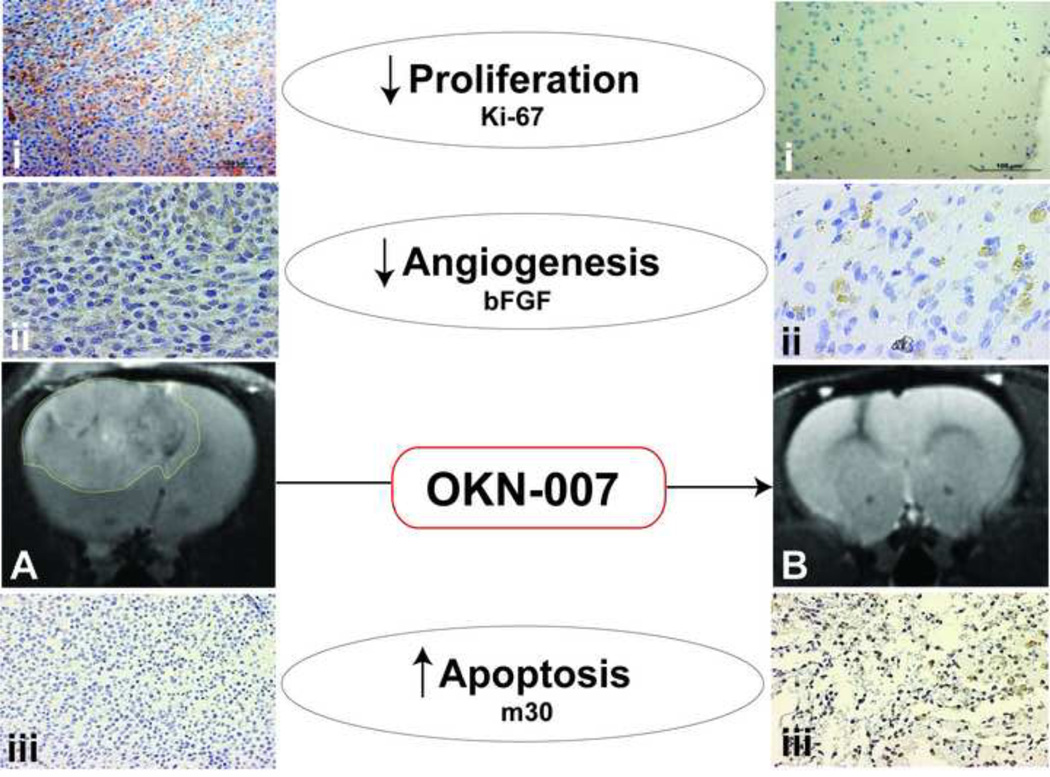
Currently, the known pharmacological effects of the nitrones are primarily anti-inflammatory in nature, in addition in their ability to scavenge free radicals. The parent nitrone compound, PBN (α-phenyl N-tert-bultyl nitrone), is known to inhibit (1) cyclooxygenase-2 (COX-2), (2) inducible nitric oxide synthase (iNOS), and (3) nuclear factor kappaB (NF-κB)[26]. OKN-007 has been found to be neuroprotective in a rat transient middle cerebral artery (MCA) occlusion model for neurological ischemia-reperfusion injury [49]. The neuroprotective effect of OKN-007 has been suggested to be attributed in part to its ability to restore functionality of the brain endothelium [70]. The anti-cancer activity of PBN-nitrones has been possibly attributed to the suppression of nitric oxide production, suppression of iNOS expression, suppression of S-nitrosylation of critical proteins (caspases, Bcl-2, OGG1 DNA repair enzyme, and PTEN tumor suppressor protein, which are respectively involved in shutting down apoptosis, enhanced mutation events and enhanced Akt-mediated signaling in oncogenesis), and inhibition of NF-κB activation [27]. We determined previously from immunohistochemistry (IHC) evaluation that OKN-007 in C6 gliomas decreases cell proliferation (Ki-67) and the basic fibroblast growth factor (bFGF) which is associated with angiogenesis, as well as increases apoptosis (m30 antigen) as shown in Fig. 3 [69]. Current investigations are ongoing using microarray and RT-PCR to establish a common target(s) that are responsible for genetic alterations associated with the multiple anti-cancer effect of OKN-007. It is becoming more widely acceptable that single-target agents are not as effective as a combination therapeutic approach. Perhaps either nitrones as single agents that can affect multiple targets may be more effective as anti-cancer agents or when nitrones are used in combination with other single-target agents.
Figure 3.
(A) MRI of an untreated C6 glioma with an outline of the tumor. (B) MRI of an OKN-007 treated C6 glioma depicting a totally regressed tumor. (i) Immunohistochemistry (IHC) of cell proliferation marker, Ki-67, in untreated (left) and OKN-007 treated (right) C6 gliomas. Note decreased cell proliferation following OKN-007 treatment. (ii) IHC of the angiogenesis marker, bFGF (basic fibroblast growth factor), in untreated (left) and OKN-007 treated (right) gliomas. Note decreased levels of bFGF in OKN-007 treated IHC slide. (iii) IHC of the apoptosis marker, M30, in untreated (left) and OKN-007 treated (right) gliomas. Note increased apoptosis following OKN-007 treatment. Images shown are modified from He et al.[69].
Lanthionines- A novel class of neurotropic agents with therapeutic potential to synergize with nitrone neuroprotectants
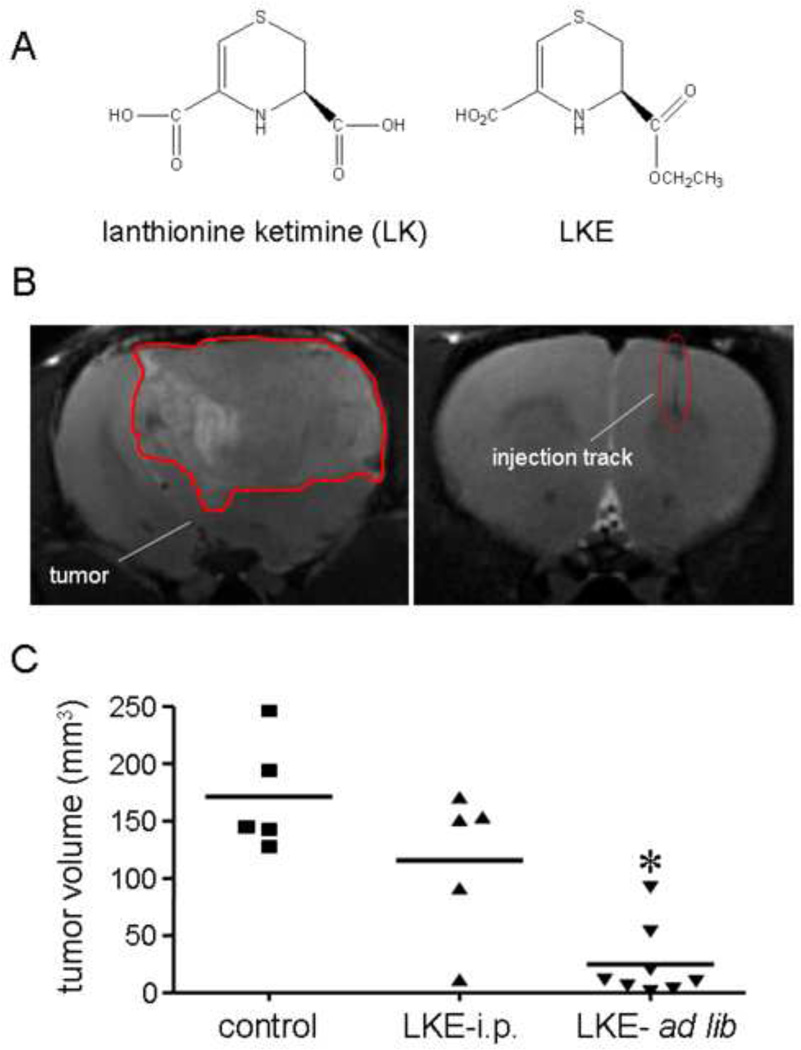
Lanthionine [L-cysteine-S-(β-L-alanine] is a non-proteogenic, natural amino acid found in the mammalian brain and central nervous system. Currently, lanthionine is thought to arise from promiscuous reactivity of transsulfuration enzymes [71] though we have speculated that the glutathione-binding brain protein LanCL1 (lanthionine synthetase-like protein-1) may catalyze lanthionine formation from glutathione (GSH) [71–73]. Whereas lanthionine itself has no known bioactivities, its natural metabolites may be important lead compounds for neurodegeneration and other conditions. In particular lanthionine is converted to an unusual cyclic thioether, lanthionine ketimine (KH Fig 4), through reactions catalyzed by brain kynurenine pathway enzymes [71]. Lanthionionine ketimine (LK) and its cell-penetrating ethyl ester, LKE (Fig. 4), possess several intriguing biological activities including the ability to promote growth factor-dependent neurite extension at low nanomolar concentrations [71, 73, 74]. Additionally LK and LKE suppress neurotoxic microglial activation [71, 73]. Proteomics studies suggest that LK/LKE act, in part through the protein CRMP2 (collapsin mediator protein-2) in order to bring about these biological effects [74]. Additionally, LK/LKE are potent antioxidants and protect cultured neurons against oxidative stress [73]. LKE is orally bioavailable, brain penetrating, and can be safely administered for weeks-months to laboratory animals [71] and (KH, personal observations).
Figure 4.
A: Structure of natural lanthionine ketimine (LK) and its synthetic ethyl ester, LKE. Both structures naturally exist in tautomeric equilibria, but only the ene-amine form is depicted here. B: MRI image of a growing glioma (post implantation day 18) seeded into a rat brain as described in the text, and an analogous image from a sham-injected animal (right-hand image of B) showing the injection track. The boundaries of the tumor in this coronal section are highlighted (image in this figure and the graphical abstract is a modified version published in Floyd et al.[26]). C: Oral LKE (3000 ppm / 300 mg/kg/d) significantly slowed the growth of implanted C6 gliomas in the rat model. Data were collected at 21 d post-implantation (28 d cumulative dosage with LKE); p<0.05 by Mann-Whitney test.
Lanthionine ketimine derivatives would be logical to consider alone or in combination with nitrones as treatments for both brain tumors and neurodegeneration, because one would anticipate a synergistic benefit from combining the neurotrophic effects of LKE with the neuroprotective effects of PBN. Lanthionines may be especially interesting to consider in the context of brain tumor therapy, where radiotherapy can cause undesirable collateral oxidative stress to healthy neurons. Keeping in mind this idea, pilot studies were performed to test LKE effects in a rat glioma implant model. LKE was administered either intraperitoneally (100 mg/kg/d in saline vehicle) or orally (3 ppt / 300 mg/kg/d ad libitum in AIN93G defined rodent chow; Dyets, Inc. Bethlehem PA) to male Sprague-Dawley rats for 10 days prior to an intracerebral injection of 100,000 C6 glioma cells into the neocortex. Control rats in the intraperitoneal study received vehicle (saline) injections. In the ad libitum study, control rats received AIN93G diet without drug. In both cases, the treatments continued post-implantation. At twenty-one days following C6 implantation, brain tumor volumes were assessed by magnetic resonance imaging (MRI) according to published methods [69]. Both intraperitoneal injections of LKE and ad libitum LKE treatments tended to slow the glioma growth, with statistically significant effects observed in the ad libitum group (Fig.4). Given that PBN can slow glioma growth in the same model [69], there is compelling rationale to further explore lanthionines as brain tumor treatment agents either alone or in combination with nitrone-based therapeutics.
Potential of Nitrone Therapy in Cognitive Decline and Dementia in Aging and after Critical Illness
Cognitive decline and dementia in aging
Cognitive impairment and dementia at older ages commonly results from accumulating vascular and neurodegenerative pathology in the brain and are experienced by more than half of adults aged 85 or over [75] . Major risk factors for cognitive impairments in later life include the APOE e4 haplotype, female sex, and head injury [76], but the underlying biological mechanisms are still unclear. Alzheimer’s disease—a progressive and irreversible neurodegenerative disorder—is the most common form of dementia among older adults, affecting more than 4 million people in the USA and almost 30 million worldwide [77]. Furthermore, a growing consensus is emerging that neurocognitive declines are common, long lasting, and likely permanent for patients who have been critically ill with sepsis, acute respiratory distress syndrome (ARDS), and multiple organ failure [78].
Although these are seemingly distinct scenarios and groups of patients, one must ask whether common features exist among these and potentially other populations of patients experiencing cognitive decline. Aside from generally affecting older people, these conditions of decreased cognition also share a paucity of effective therapeutic or prophylactic measures as a troublesome characteristic.
No theory has been widely accepted to explain the aging process. However, the oxidative stress hypothesis proposed by D. Harman in 1956—the Free Radical Theory of Aging—offers an appealing mechanistic explanation of aging and age related diseases [79, 80]. Harman’s theory posits a single process modified by genetic and environmental factors that is responsible for aging and related diseases. The central idea is that aging is primarily the result of cumulative damage to macromolecules resulting from free radical attacks - oxidative stress - originated mainly by mitochondrial processes [79–81]. Although fast replicating cells with low levels of oxygen consumption are less susceptible to free radical damage, highly differentiated cells with high levels of oxygen consumption are more vulnerable to oxidative stress [80].
Brain function is almost totally dependent on a continuous supply of glucose and oxygen from the arterial circulation. Although the brain represents only 2% of the body weight, it receives 15% of the cardiac output, consumes 20% of total body oxygen and 25% of total body glucose. Neurotransmission and ionic homeostasis account for most of brain energy expenditure. Glutamate re-uptake by astrocytes is a key event required to maintain brain homeostasis. The entry of glutamate into astrocytes is coupled to the activation of the Na+/ K+ ATPase pump to normalize ionic gradients across plasma membranes in an ATP-dependent manner [82]. Thus, glutamatergic neurotransmission accounts for a considerable part of energy consumption in the brain. Among the brain cell types, neurons are the most oxygen-dependent cells and numerous studies demonstrate a strong metabolic cooperation between astrocytes and neurons in terms of neurotransmitter reuptake, oxidative stress defense, and energy substrate delivery. Furthermore, all of these processes are critically depend on energy metabolism [83]. Consequently, neurons, as highly specialized and oxygen dependent cells, are especially vulnerable to mitochondrial damage by free radicals [84]. Thus, the detrimental effects of the aging process are pronounced in the brain, where irreversibly damaged cells cannot be replaced.
Sepsis induced Dementia
Experimental and clinical evidence indicate that multiple organ dysfunction in sepsis is associated with increased production of reactive oxidant species and depletion of antioxidants, leading to oxidative stress. Reactive oxygen species (ROS) are frequently associated to endothelial cell injury, blood brain barrier disruption, recruitment of neutrophils, lipid peroxidation, and oxidative modifications of target proteins in sepsis [85]. Ascorbate levels were significantly lower in cerebrospinal fluid in patients with septic encephalopathy, being correlated with the severity of neurologic symptoms [86]. Oxidative stress is also implicated in pathophysiological mechanisms of cognitive impairment that is detected in over 60% of the patients that survive critical illness such as ARDS and sepsis. These are usually patients over 60 years old, and they frequently have long-lasting, and perhaps permanent, cognitive decline after being released from the intensive care unit. Importantly, cognitive sequelae are predicted by the duration of delirium, as a manifestation of sepsis-associated encephalopathy, during the acute phase of the disease [87]. Although the clinical evidence is limited because of ethical dilemmas, experimental data using the cecum ligation and puncture (CLP) rodent model of sepsis show that there is an increase in superoxide, nitrites, and lipid peroxide content in the brain capillaries as long as 48 hours after induction of sepsis, and that glutathione concentration is significantly decreased even at longer times [88]. Importantly, it seems that the hippocampus is especially sensitive to oxidative stress since evidence for oxidative damage can be detected even at 10 days after the induction of sepsis in this, but not in other, brain regions [89]. Importantly, anti-oxidant treatment appears to protect mice from oxidative damage and death [90]. The most robust evidence linking oxidative damage and long-term cognitive impairment comes from animal studies. Barichello and co-workers demonstrated that the combined administration of the anti-oxidants N-acetylcysteine and deferoxamine during the acute phase of sepsis induced by the CLP model prevented oxidative damage and long-term cognitive decline [91]. Similarly, antioxidants including vitamins E and C can enhance learning and memory and prevent memory deficits in other experimental conditions including Alzheimer’s disease, aging, and menopause [92].
Inflammation is another key player in the cognitive decline of neurodegenerative diseases and after critical illness. In fact, chronic low-level inflammation has been proposed as a major mechanism underlying cognitive decline and dementia and has been implicated in the neuropathological cascade leading to the late onset of Alzheimer disease [93, 94]. Proinflammatory cytokines can modulate energy metabolism and oxidative stress defense by astrocytes, which may contribute to increased neuronal vulnerability and neurodegeneration (see above). In pathological conditions associated with neuroinflammation, such as in sepsis-associated encephalopathy, excitatory glutamatergic neurotransmission may be increased and this can be the cause of a bioenergetic crisis involving mitochondrial activity and can directly affect brain function [95]. Neuroinflammation is also linked to cell death that may occur by apoptosis or by necrosis due to hypoxia, oxidative stress, and other cytotoxic factors [95]. Brain imaging studies of septic patients with sepsis-associated encephalopathy detected sites of tissue ischemia and lesions in the white matter, suggesting blood-brain barrier dysfunction [96–98]. MRI studies in experimental sepsis also demonstrated cytotoxic and vasogenic edema as well as neuronal damage during the early phase of sepsis [99].
Malaria induced Dementia
Neuroinflammation and oxidative stress are not only connected to cognitive decline in aged patients with neurodegenerative diseases or after severe systemic infectious/inflammatory syndromes. These two injurious mechanisms have also major roles in patients, particularly children, who develop long term, and potentially permanent, cognitive impairment after cerebral malaria. Cerebral malaria affects African children with high incidence and high mortality [100, 101] and there is substantial evidence that patients who are successfully treated and survive cerebral malaria develop long term cognitive decline in as much as 26% of the cases [102–105]. Precise identification of molecular mechanisms that underlie sustained cognitive dysfunction after cerebral malaria is yet to be achieved, which makes the development of efficient therapy more challenging. Recent studies provided evidence that neuroinflammation and oxidative stress play fundamental roles in cerebral malaria [106]. In fact, intravascular accumulation of monocytes in the brain has long been observed both in mice [109–111] with cerebral malaria. Furthermore, sequestered monocytes and macrophages are more abundant in pediatric patients with cerebral malaria than in those with non-cerebral malaria or non-malarial encephalopathy [112]. The accumulation of leukocytes in the brains of patients and mice with cerebral malaria is evidence of activation of cytokine/chemokine cascade, which has been shown in experimental and human studies [106, 113]. The expression of monocyte-secreted cytokines and chemokines, such as TNF, CXC-10, CCL2 and RANTES, varies with mouse genotype and correlates with resistance versus susceptibility to disease [113]. Moreover the deletion of adhesion molecules, such as αmβ2 or αdβ2, was shown to have protective effects in experimental cerebral malaria [114, 115], (Vieira-de-Abreu et al., manuscript in preparation). In a recent study, Reis et al. [116] obtained clear evidence of oxidative damage of the brain during experimental cerebral malaria and demonstrated that animals that survived experimental malaria after rescue with anti-malarial drugs developed a long term cognitive impairment similar to what is observed in the clinical setting and in experimental or clinical sepsis (see above). Furthermore, administration of two antioxidants in combination, N-acetylcysteine and desferroxamine, as adjunctive treatment together with rescue antimalarial therapy prevented late cognitive impairment, and reduced microvascular sequestration in several areas of the brain [116]. Again these results are similar to studies with models of polymicrobial sepsis [91].
In light of the results discussed so far, one is compelled to speculate that neuroinflammation and oxidative stress are major events underlying cognitive decline observed in neurodegenerative diseases as well as in patients who survived severe systemic infectious/inflammatory syndromes with cerebral involvement. Although neuroinflammation and oxidative stress may not be the whole story, these events are certainly consequent and common to a variety of clinical conditions associated to cognitive decline, and therefore, suitable targets for new therapeutic measures. Therefore, the unique antioxidant and anti-inflammaory actions of PBN-nitrones discussed before in this review implicate them as a suitable pharmacological strategy to prevent and/or imporove the outcome of dementia from different etiologies. Importantly, neuroinflammation and oxidative stress are highly interconnected phenomena where the cause-consequence relation is difficult to identify. For instance, pathological activation of astrocytes and microglia, a hallmark of neuroinflammation, leads to enhanced production of reactive oxygen and nitrogen species, including H2O2 and NOO−, largely through cytokine mediated activation of NADPH oxidase system and induction of iNOS expression [117, 118]. On the other hand, highly pro-oxidative stress such as ischemia-reperfusion injury induces activation of microglia, neuroinflammation and cognitive decline [119]. This situation suggests that efficacious therapeutic measures would only likely be achieved with drugs that interfere with these two central events that operate in neurodegenerative diseases and in patients with severe systemic infectious/inflammatory syndromes.
Potential of PBN-Nitrones to treat Dementia
The PBN-nitrones provide an excellent example of an agent considered antioxidant, but with independent and potent activity in suppressing neuroinflammation as demonstrated in animal models using the rat kainic acid-induced epileptic-type seizures [120] . PBN-nitrones are also potential modulators of neuroinflammation induced by intracisternal injection of LPS in rats [121] . In these models PBN-nitrones prevented microglia activation, loss of NMDA receptors and cellular killing in the brain [50].
Testing candidate drugs for prevention of cognitive decline in clinical trials has the potential to yield both mechanistic and therapeutic information, but clinical trials are costly and time consuming, and are frequently unpowered to detect less than dramatic benefits. On the other hand, pre-clinical models of disease are easy to control, provide useful mechanistic information, and are much less expensive and time consuming, although appropriate care must be taken in extending results in rodents and other models to humans. Therefore, candidate drugs for clinical trials should often be first selected on the basis their results in pre-clinical models that mimic the cognitive impairment associated with aging or severe systemic inflammatory/infectious syndromes, such as sepsis and cerebral malaria.
Several pre-clinical models exist for studying dementia and Alzheimer disease with similar features to the clinical syndromes. They all have advantages and disadvantages that vary with the experimental protocol to be employed [122–124]. Recently, models suited to the study of the cognitive decline associated with severe systemic inflammatory/infectious syndromes in sepsis and malaria have been reported [91, 116]. Characteristically, these models employ animals that developed the acute phase of the disease with documented neuroinflammation and/or cerebral oxidative stress and were rescued by conventional treatment with antibiotics, fluid reposition or anti-malarial drugs. Typically, surviving animals showed no evidence of the acute disease at late time points and were then followed for late cognitive sequela using specific task or behavioral tests that provided information about learning abilities and different types of memory [91, 116]. These models have been used both for mechanistic studies [89, 91, 116, 125–128] and for testing of candidate therapies, particularly antioxidant drugs [91, 116, 125–127]. These models resemble clinical situations in which patients develop the acute critical illness before starting conventional treatment. In the experimental models, adjuvant candidate drugs may be used in addition to the conventional treatment and cognitive endpoint investigated in survivors at later time points. One positive and informative aspect of this kind of model is that the animals are no longer under the effect of the candidate drugs when they are tested for cognitive performance, providing direct links, that are mechanistically relevant, between events that took place during the acute phase of the disease and the late cognitive sequel [91, 116, 129].
PBN-nitrones make ideal candidates to be tested using such experimental models because of their spin trapping and anti-inflammatory activities, and because they have low toxicity, and are also suited for multiple dose regimens [50]. Furthermore, Novelli and colleagues showed that PBN-nitrones were active in protecting rats from traumatic shock or LPS injection [36]. Also, PBN-nitrones were reported to protect the brain from damage caused by stroke even if the PBN was administered after the brain was reperfused [41, 42]. Furthermore administration of PBN-nitrones protected rats from axonal and neuronal injury induced by intracerebral IL-1β as indicated by decreased expression of β-amyloid precursor protein. Protection of PBN was linked to attenuated oxidative stress as indicated by decreased elevation of 8-isoprostane content and by the reduced number of 4-hydroxynonenal or malondialdehyde or nitrotyrosine positive cells following IL-1β exposure. PBN also attenuated IL-1β-stimulated inflammatory as indicated by the reduced activation of microglia [130]. Together, these observations indicate that PBN-nitrones may play a critical role in modulating brain injury associated with oxidative stress and neuroinflammation and therefore can be considered as a potential therapeutic strategy for dementia that follows sever systemic infectious/inflammatory syndromes. In addition Garcia-Alloza and colleagues [131] using a mouse model of Alzheimer’s Disease (APPswe/PS1dE9 mice) and multiphoton microscopy to asses in vivo the characteristics of senile plaques and neurites in APP mice provided compelling evidence suggesting that PBN-nitrone therapy can alleviate the neuronal abnormalities of APP mice showing a straightening effect on curved neurites as was detected as soon as 4 days after commencing the treatment and persisted 15 days after stopping the PBN-nitrone administration. Senile plaques have been associated with abnormal curvature of nearby neurites [132] [133], synaptic loss [134] [135] and neuritic dystrophies [136] and these alteration have been shown to be at least partially responsible for the disruption of cortical synaptic integration [137]. These data indicate that amelioration of the neuritic distortions associated with senile plaques by PBN-nitrones is both rapid and long lasting and may have beneficial effects on the progression of Alzheimer’s Disease.
These observations warrant new investigations in this area and open up considerable interest in the potential of PBN-nitrones in neurodegenerative diseases and other age-related diseases and as adjuvant drugs for prevention of cognitive decline in syndromes of cerebral inflammation or infection. In addition, PBN-nitrones should also be considered in combination with N-acetyl cysteine in models of cognitive impairment after sepsis or malaria, since this combination yielded promising outcomes in experimental noise-related hearing loss [138].
Summary and Prospective Future Directions
This report presents an overview of some of the active research and development efforts that is ongoing using a specific nitrone (OKN007) for a specific indication (GBM). In addition another potential areas where therapeutics are needed includes dementia which may be an excellent target indication for specific nitrones and/or for a specific nitrone combined with another compound such as lanthionine which can act as a neurotropic agent. Although not presented here, current R&D efforts at various stages of development are now ongoing where specific therapeutic nitrones are being targeted for: A) treatment of noise-induced hearing loss [138–141], B) treatment for macular dementia [142], and C) treatment for brain damage and hearing loss caused by explosive blasts [143]. With so much R&D focused on nitrones as therapeutics it provides optimism that treatment for one or more of the indications discussed above may arise from these efforts.
Bullet Points.
Specific nitrones have been shown to have potent therapeutic potential
Nitrone OKN-007 has potent anti-cancer activity in glioblastoma models
Lanthionine ethyl ester (LKE) had anti-cancer activity in a glioblastoma model
Combining a nitrone and lanthionine has enhanced therapeutic potential
Nitrones show potential for treating dementia in aging, sepsis, and malaria infection
Acknowledgements
This work was supported in part by grants from the National Institutes of Health 5R37HL044525 to GAZ and to KH NIH grants R01-AG031553 and R21-NS066279 as well as the Judith and Jean Pape Adams Charitable Foundation. Support from the Oklahoma Center for the Advancement of Sciences and Technology (OCAST) grant no. AR092-049 (RAT) and the Oklahoma Medical Research Foundation (RAT and RAF) is also acknowledged. HCCFN is 1A Researcher from CNPq and is supported by grants from: PRONEX, Rede Malaria, CNPq and FAPERJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. Some of the authors (RAF,RAT and KH) are inventors on issued or pending US patents and RAF is a co-founder of Otologic Pharmaceutics, INC which has in commercial development a treatment consisting of a nitrone combined with an antioxidant for noise-induced hearing loss.
Reference List
- 1.Floyd RA. Protective action of nitrone-based free radical traps against oxidative damage to the central nervous system. Adv Pharmacol. 1997;38:361–378. doi: 10.1016/s1054-3589(08)60991-6. [DOI] [PubMed] [Google Scholar]
- 2.Floyd RA. Nitrones as therapeutics in age-related diseases. Aging Cell. 2006;5:51–57. doi: 10.1111/j.1474-9726.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 3.Janzen EG, Blackburn BJ. Detection and identification of short-lived free radicals by an electron spin resonance trapping technique. J Am Chem Soc. 1968;90:5909–5910. [Google Scholar]
- 4.Janzen EG, Blackburn BJ. Detection and identification of short-lived free radicals by electron spin resonance trapping techniques (spin trapping). Photolysis of organolead, -tin, and -mercury compounds. J Am Chem Soc. 1969;91:4481–4490. [Google Scholar]
- 5.Forshult S, Lagercrantz C, Torssell K. Use of nitroso compounds as scavengers for the study of short-lived free radicals in organic reactions. Acta Chem Scand [B] 1969;23:522–530. [Google Scholar]
- 6.Chalfont GR, Perkins MJ. A probe for homolytic reactions in Solstion. II. The polymerization of styrene. J Am Chem Soc. 1968;90:7141–7142. [Google Scholar]
- 7.Leaver IH, Ramsay GC. Trapping of Radical Intermediates in the Photoreduction of Benzophenone. Tetrahedron Lett. 1969;25:5669–5675. [Google Scholar]
- 8.Terabe S, Konaka R. Electron spin resonance studies on oxidation with nickel peroxide. Spin trapping of free-radical intermediates. Journal of the American Chemical Society. 1969;91:5655–5657. [Google Scholar]
- 9.Janzen EG. A critical review of spin trapping in biological systems. In: Pryor WA, editor. Free radicals in biology. Academic Press, Inc.; 1980. pp. 115–154. [Google Scholar]
- 10.Thornalley PJ. Theory and bilogical application of the electron spin resonance technique of spin trapping. Life Chemistry Reports. 2010:1–56. [Google Scholar]
- 11.Janzen EG, Stronks HJ, DuBose CM, Poyer JL, McCay PB. Chemistry and biology of spin-trapping radicals associated with halocarbon metabolism in vitro and in vivo. Environ Health Perspect. 1985;64:151–170. doi: 10.1289/ehp.8564151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins MJ. Spin Trapping. Adv Phys Org Chem. 1980;17:1–64. [Google Scholar]
- 13.Iwamura M, Inamoto N. Novel formation of nitroxide radicals by radical addition to nitrones. Bull Chem Soc Jpn. 1967;40:703. [Google Scholar]
- 14.Janzen EG, Liu JIP. Radical addition reactions of 5,5-dimethyl-1-pyrroline-1-oxide. ESR spin trapping with a cyclic nitrone. J Magn Reson. 1973;9:510–512. [Google Scholar]
- 15.Iwamura M, Inamoto N. Reactions of nitrones with free radicals. I. Radical 1,3-addition to nitrones. Bull Chem Soc Jpn. 1970;43:856–860. [Google Scholar]
- 16.Bardelang D, Charles L, Finet JP, Jicsinszky L, Karoui H, Marque SR, et al. Alpha-phenyl-N-tert-butylnitrone-type derivatives bound to beta-cyclodextrins: syntheses, thermokinetics of self-inclusion and application to superoxide spin-trapping. Chemistry. 2007;13:9344–9354. doi: 10.1002/chem.200700369. [DOI] [PubMed] [Google Scholar]
- 17.Nash KM, Rockenbauer A, Villamena FA. Reactive Nitrogen Species Reactivities with Nitrones: Theoretical and Experimental Studies. Chem Res Toxicol. 2012 doi: 10.1021/tx200526y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalier F, Hardy M, Ouari O, Rockenbauer A, Tordo P. Design of new derivatives of nitrone DEPMPO functionalized at C-4 for further specific applications in superoxide radical detection. J Org Chem. 2007;72:7886–7892. doi: 10.1021/jo071070s. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Tuccio B, Lauricella R, Villamena FA. Improved spin trapping properties by beta-cyclodextrin-cyclic nitrone conjugate. J Org Chem. 2008;73:7108–7117. doi: 10.1021/jo8007176. [DOI] [PubMed] [Google Scholar]
- 20.Kim SU, Liu Y, Nash KM, Zweier JL, Rockenbauer A, Villamena FA. Fast Reactivity of a Cyclic Nitrone-Calix[4]pyrrole Conjugate with Superoxide Radical Anion: Theoretical and Experimental Studies (dagger) J Am Chem Soc. 2010 doi: 10.1021/ja105198c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barriga G, Olea-Azar C, Norambuena E, Castro A, Porcal W, Gerpe A, et al. New heteroaryl nitrones with spin trap properties: Identification of a 4-furoxanyl derivative with excellent properties to be used in biological systems. Bioorg Med Chem. 2010;18:795–802. doi: 10.1016/j.bmc.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Kalyanaraman B. Synthesis and ESR studies of a novel cyclic nitrone spin trap attached to a phosphonium group-a suitable trap for mitochondria-generated ROS? Free Radic Res. 2007;41:1–7. doi: 10.1080/10715760600911147. [DOI] [PubMed] [Google Scholar]
- 23.Robertson L, Hartley RC. Synthesis of N-arylpyridinium salts bearing a nitrone spin trap as potential mitochondria-targeted antioxidants. Tetrahedron. 2009;65:5284–5292. doi: 10.1016/j.tet.2009.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janzen EG. Spin trapping. Ace Chem Res. 1971;4:31–40. [Google Scholar]
- 25.Towner RA. In: Chemistry of spin-trapping in toxicology of the human environment: The Critical Role of Free Radicals. Rhodes CJ, editor. New York: Taylor & Francis; 2000. pp. 7–24. [Google Scholar]
- 26.Floyd RA, Kopke RD, Choi CH, Foster SB, Doblas S, Towner RA. Nitrones as therapeutics. Free Radic Biol Med. 2008;45:1361–1374. doi: 10.1016/j.freeradbiomed.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Floyd RA, Chandru HK, He T, Towner R. Anti-cancer activity of nitrones and observations on mechanism of action. Anticancer Agents Med Chem. 2011;11:373–379. doi: 10.2174/187152011795677517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes CJ. Current commentary: Can free radicals be good for you? Sci Prog. 2011;94:451–462. doi: 10.3184/003685011X13215432034594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villamena FA, Das A, Nash KM. Potential implication of the chemical properties and bioactivity of nitrone spin traps for therapeutics. Future Med Chem. 2012;4:1171–1207. doi: 10.4155/fmc.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med. 2004;36:1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez DC, Gomez Mejiba SE, Mason RP. Mechanism of hydrogen peroxide-induced Cu,Zn-superoxide dismutase-centered radical formation as explored by immuno-spin trapping: the role of copper- and carbonate radical anion-mediated oxidations. Free Radic Biol Med. 2005;38:201–214. doi: 10.1016/j.freeradbiomed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez DC, Gomez-Mejiba SE, Corbett JT, Deterding LJ, Tomer KB, Mason RP. Cu,Zn-superoxide dismutase-driven free radical modifications: copper- and carbonate radical anion-initiated protein radical chemistry. Biochem J. 2009;417:341–353. doi: 10.1042/BJ20070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Detweiler CD, Deterding LJ, Tomer KB, Chignell CF, Germolec D, Mason RP. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic Biol Med. 2002;33:364–369. doi: 10.1016/s0891-5849(02)00895-x. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Mejiba SE, Zhai Z, Akram H, Deterding LJ, Hensley K, Smith N, et al. Immuno-spin trapping of protein and DNA radicals: "tagging" free radicals to locate and understand the redox process. Free Radic Biol Med. 2009;46:853–865. doi: 10.1016/j.freeradbiomed.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towner RA, Smith N, Saunders D, Henderson M, Downum K, Lupu F, et al. In Vivo Imaging of Immunospin-Trapped Radicals With Molecular Magnetic Resonance Imaging in a Mouse Diabetes Model. Diabetes. 2012 doi: 10.2337/db11-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novelli GP, Angiolini P, Tani R, Consales G, Bordi L. Phenyl-T-butyl-nitrone is active against traumatic shock in rats. Free Radic Res Commun. 1985;1:321–327. doi: 10.3109/10715768609080971. [DOI] [PubMed] [Google Scholar]
- 37.Novelli G, Angiolini P, Cansales G, Lippi R, Tani R. Anti-shock action of phenyl-t-butyl-nitrone, a spin trapper. In: Novelli GP, Ursini F, editors. Oxygen Free Radicals in Shock. Florence: Karger, Basel; 1986. pp. 119–124. [Google Scholar]
- 38.McKechnie K, Furman BL, Parratt JR. Modification by oxygen free radical scavengers of the metabolic and cardiovascular effects of endotoxin infusion in conscious rats. Circ Shock. 1986;19:429–439. [PubMed] [Google Scholar]
- 39.Hamburger SA, McCay PB. Endotoxin-induced mortality in rats is reduced by nitrones. Circ Shock. 1989;29:329–334. [PubMed] [Google Scholar]
- 40.Pogrebniak HW, Merino MJ, Hahn SM, Mitchell JB, Pass HI. Spin trap salvage from endotoxemia: The role of cytokine down-regulation. Surgery. 1992;112:130–139. [PubMed] [Google Scholar]
- 41.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–2597. [PubMed] [Google Scholar]
- 42.Carney JM, Starke-Reed PE, Oliver CN, Landrum RW, Chen MS, Wu JF, et al. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spacial memory by chronic administration of the spin-trapping compound N-tert-butyl-α-phenylnitrone. Proc Natl Acad Sci USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carney JM, Floyd RA. Phenyl butyl nitrone compositions and methods for treatment of oxidative tissue damage. #5,025,032. U.S. Patent. 1991 Jun 18;:1–14. Ref Type: Patent.
- 44.Cao W, Carney JM, Duchon A, Floyd RA, Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett. 1988;88:233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- 45.Carney JM, Floyd RA. Protection against oxidative damage to CNS by α-phenyl-tert-butyl nitrone (PBN) and other spin-trapping agents: A novel series of nonlipid free radical scavengers. J Mol Neurosci. 1991;3:47–57. doi: 10.1007/BF02896848. [DOI] [PubMed] [Google Scholar]
- 46.Floyd RA, Carney JM. Free radical damage to protein and DNA: Mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32:S22–S27. doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- 47.Phillis JW, Clough-Helfman C. Protection from cerebral ischemic injury in gerbils with the spin trap agent N-tert-butyl-α-phenylnitrone (PBN) Neurosci Lett. 1990;116:315–319. doi: 10.1016/0304-3940(90)90093-o. [DOI] [PubMed] [Google Scholar]
- 48.Cao X, Phillis JW. α-Phenyl-tert-butyl-nitrone reduces cortical infarct and edema in rats subjected to focal ischemia. Brain Res. 1994;644:267–272. doi: 10.1016/0006-8993(94)91689-6. [DOI] [PubMed] [Google Scholar]
- 49.Maples KR, Green AR, Floyd RA. Nitrone-related therapeutics: potential of NXY-059 for the treatment of acute ischaemic stroke. CNS Drugs. 2004;18:1071–1084. doi: 10.2165/00023210-200418150-00003. [DOI] [PubMed] [Google Scholar]
- 50.Floyd RA, Towner RA, He T, Hensley K, Maples KR. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med. 2011;51:931–941. doi: 10.1016/j.freeradbiomed.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Central Brain Tumor Registry of the United States (CBTRUS) 2011 CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. Central Brain Tumor Registry of the United States (CBTRUS) 2011:54–57. [Google Scholar]
- 52.Niclou SP, Fack F, Rajcevic U. Glioma proteomics: status and perspectives. J Proteomics. 2010;73:1823–1838. doi: 10.1016/j.jprot.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Gudinaviciene I, Pranys D, Juozaityte E. Impact of morphology and biology on the prognosis of patients with gliomas. Medicina (Kaunas) 2004;40:112–120. [PubMed] [Google Scholar]
- 54.Waldman AD, Jackson A, Price SJ, Clark CA, Booth TC, Auer DP, et al. Quantitative imaging biomarkers in neuro-oncology. Nat Rev Clin Oncol. 2009;6:445–454. doi: 10.1038/nrclinonc.2009.92. [DOI] [PubMed] [Google Scholar]
- 55.Young GS. Advanced MRI of adult brain tumors. Neurol Clin. 2007;25:947–973. viii. doi: 10.1016/j.ncl.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Polivka J, Jr, Polivka J, Rohan V, Topolcan O, Ferda J. New molecularly targeted therapies for glioblastoma multiforme. Anticancer Res. 2012;32:2935–2946. [PubMed] [Google Scholar]
- 57.Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC, et al. Glioblastoma: Therapeutic challenges, what lies ahead. Biochim Biophys Acta. 2012;1826:338–349. doi: 10.1016/j.bbcan.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Wainwright DA, Nigam P, Thaci B, Dey M, Lesniak MS. Recent developments on immunotherapy for brain cancer. Expert Opin Emerg Drugs. 2012;17:181–202. doi: 10.1517/14728214.2012.679929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thumma SR, Elaimy AL, Daines N, Mackay AR, Lamoreaux WT, Fairbanks RK, et al. Long-term survival after gamma knife radiosurgery in a case of recurrent glioblastoma multiforme: a case report and review of the literature. Case Report Med. 2012;2012:545492. doi: 10.1155/2012/545492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel M, Vogelbaum MA, Barnett GH, Jalali R, Ahluwalia MS. Molecular targeted therapy in recurrent glioblastoma: current challenges and future directions. Expert Opin Investig Drugs. 2012 doi: 10.1517/13543784.2012.703177. [DOI] [PubMed] [Google Scholar]
- 61.Braghiroli MI, Sabbaga J, Hoff PM. Bevacizumab: overview of the literature. Expert Rev Anticancer Ther. 2012;12:567–580. doi: 10.1586/era.12.13. [DOI] [PubMed] [Google Scholar]
- 62.Towner RA, Smith N, Doblas S, Garteiser P, Watanabe Y, He T, et al. In vivo detection of inducible nitric oxide synthase in rodent gliomas. Free Radio Biol Med. 2010;48:691–703. doi: 10.1016/j.freeradbiomed.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Waerzeggers Y, Monfared P, Viel T, Winkeler A, Jacobs AH. Mouse models in neurological disorders: applications of non-invasive imaging. Biochim Biophys Acta. 2010;1802:819–839. doi: 10.1016/j.bbadis.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Huszthy PC, Daphu I, Niclou SP, Stieber D, Nigro JM, Sakariassen PO, et al. In vivo models of primary brain tumors: pitfalls and perspectives. Neuro Oncol. 2012 doi: 10.1093/neuonc/nos135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 66.Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94:299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobs VL, Valdes PA, Hickey WF, De Leo JA. Current review of in vivo GBM rodent models: emphasis on the CNS-1 tumour model. ASN Neuro. 2011;3:e00063. doi: 10.1042/AN20110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garteiser P, Doblas S, Watanabe Y, Saunders D, Hoyle J, Lerner M, et al. Multiparametric assessment of the anti-glioma properties of OKN007 by magnetic resonance imaging. J Magn Reson Imaging. 2010;31:796–806. doi: 10.1002/jmri.22106. [DOI] [PubMed] [Google Scholar]
- 69.He T, Doblas S, Saunders D, Casteel R, Lerner M, Ritchey JW, et al. Effects of PBN and OKN007 in rodent glioma models assessed by (1)H MR spectroscopy. Free Radic Biol Med. 2011;51:490–502. doi: 10.1016/j.freeradbiomed.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 70.Culot M, Mysiorek C, Renftel M, Roussel BD, Hommet Y, Vivien D, et al. Cerebrovascular protection as a possible mechanism for the protective effects of NXY-059 in preclinical models: an in vitro study. Brain Res. 2009;1294:144–152. doi: 10.1016/j.brainres.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 71.Hensley K, Venkova K, Christov A. Emerging biological importance of central nervous system lanthionines. Molecules. 2010;15:5581–5594. doi: 10.3390/molecules15085581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung CH, Kurien BT, Mehta P, Mhatre M, Mou S, Pye QN, et al. Identification of lanthionine synthase C-like protein-1 as a prominent glutathione binding protein expressed in the mammalian central nervous system. Biochem. 2007;46:3262–3269. doi: 10.1021/bi061888s. [DOI] [PubMed] [Google Scholar]
- 73.Hensley K, Christov A, Kamat S, Zhang XC, Jackson KW, Snow S, et al. Proteomic Identification of Binding Partners for the Brain Metabolite Lanthionine Ketimine (LK) and Documentation of LK Effects on Microglia and Motoneuron Cell Cultures. J Neurosci. 2010;30:2979–2988. doi: 10.1523/JNEUROSCI.5247-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hensley KVKCAGWPJ. Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Mol Neurobiol. 2011;43(3):180–191. doi: 10.1007/s12035-011-8166-4. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- 75.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hughes T, Ganguli M. Modifiable midlife risk factors for late-life dementia. Rev Neurol. 2010;51:259–262. [PubMed] [Google Scholar]
- 77.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement Geriatr Cogn Disord. 2006;21:175–181. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- 78.Hopkins RO, Brett S. Chronic neurocognitive effects of critical illness. Curr Opin Crit Care. 2005;11:369–375. doi: 10.1097/01.ccx.0000166399.88635.a5. [DOI] [PubMed] [Google Scholar]
- 79.Gil D. V. Oxidative stress in aging: Theoretical outcomes and clinical evidences in humans. Biomed Pharmacother. 2010 doi: 10.1016/j.biopha.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Ashok BT, Ali R. The aging paradox: free radical theory of aging. Exp Gerontol. 1999;34:293–303. doi: 10.1016/s0531-5565(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 81.Biesalski HK. Free radical theory of aging. Curr Opin Clin Nutr Metab Care. 2002;5:5–10. doi: 10.1097/00075197-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 82.Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- 83.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 85.Zimmerman JJ. Defining the role of oxyradicals in the pathogenesis of sepsis. Crit Care Med. 1995;23:616–617. doi: 10.1097/00003246-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Voigt K, Kontush A, Stuerenburg HJ, Muench-Harrach D, Hansen HC, Kunze K. Decreased plasma and cerebrospinal fluid ascorbate levels in patients with septic encephalopathy. Free Radio Res. 2002;36:735–739. doi: 10.1080/10715760290032557. [DOI] [PubMed] [Google Scholar]
- 87.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ninkovic M, Malicevic I, Jelenkovic A, Jovanovic DM, Dukic M, Vasiljevic I. Oxidative stress in the rats brain capillaries in sepsis-the influence of 7-nitroindazole. Acta Physiol Hung. 2006;93:315–323. doi: 10.1556/APhysiol.93.2006.4.7. [DOI] [PubMed] [Google Scholar]
- 89.Comim CM, Cassol OJ, Jr, Constantino LS, Felisberto F, Petronilho F, Rezin GT, et al. Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochem Res. 2011;36:304–311. doi: 10.1007/s11064-010-0320-2. [DOI] [PubMed] [Google Scholar]
- 90.Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med. 2004;32:342–349. doi: 10.1097/01.CCM.0000109454.13145.CA. [DOI] [PubMed] [Google Scholar]
- 91.Barichello T, Machado RA, Constantino L, Valvassori SS, Reus GZ, Martins MR, et al. Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Crit Care Med. 2007;35:2186–2190. doi: 10.1097/01.ccm.0000281452.60683.96. [DOI] [PubMed] [Google Scholar]
- 92.Yamada K, Tanaka T, Han D, Senzaki K, Kameyama T, Nabeshima T. Protective effects of idebenone and alpha-tocopherol on beta-amyloid-(1–42)-induced learning and memory deficits in rats: implication of oxidative stress in beta-amyloid-induced neurotoxicity in vivo. EurJ Neurosci. 1999;11:83–90. doi: 10.1046/j.1460-9568.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 93.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N YAcad Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- 94.Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol Neurobiol. 2010;41:115–128. doi: 10.1007/s12035-010-8106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siami S, Annane D, Sharshar T. The encephalopathy in sepsis. Crit Care Clin. 2008;24:67–82. viii. doi: 10.1016/j.ccc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-ldrissi S, et al. Elevated serum levels of S-100 beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34:1967–1974. doi: 10.1097/01.CCM.0000217218.51381.49. [DOI] [PubMed] [Google Scholar]
- 97.Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- 98.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40:S20–S23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bozza FA, Garteiser P, Oliveira MF, Doblas S, Cranford R, Saunders D, et al. Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab. 2010;30:440–448. doi: 10.1038/jcbfm.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Higgins SJ, Kain KC, Liles WC. Immunopathogenesis of falciparum malaria: implications for adjunctive therapy in the management of severe and cerebral malaria. Expert Rev Anti Infect Ther. 2011;9:803–819. doi: 10.1586/eri.11.96. [DOI] [PubMed] [Google Scholar]
- 101.de Souza JB, Hafalla JC, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137:755–772. doi: 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 102.Carter JA, Mung'ala-Odera V, Neville BG, Murira G, Mturi N, Musumba C, et al. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476–481. doi: 10.1136/jnnp.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carter JA, Ross AJ, Neville BG, Obiero E, Katana K, Mung'ala-Odera V, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 104.Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e360–e366. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–e99. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 107.Rest JR. Pathogenesis of cerebral malaria in golden hamsters and inbred mice. Contrib Microbiol Immunol. 1983;7:139–146. [PubMed] [Google Scholar]
- 108.Curfs JH, Hermsen CC, Kremsner P, Neifer S, Meuwissen JH, Van RN, et al. Tumour necrosis factor-alpha and macrophages in Plasmodium berghei-induced cerebral malaria. Parasitology. 1993;107(Pt 2):125–134. doi: 10.1017/s0031182000067226. [DOI] [PubMed] [Google Scholar]
- 109.Elamin AM. Cerebral malaria in adult Zambian Africans. East Afr Med J. 1981;58:124–129. [PubMed] [Google Scholar]
- 110.Pongponratn E, Riganti M, Harinasuta T, Bunnag D. Electron microscopy of the human brain in cerebral malaria. Southeast Asian J Trop Med Public Health. 1985;16:219–227. [PubMed] [Google Scholar]
- 111.Porta J, Carota A, Pizzolato GP, Wildi E, Widmer MC, Margairaz C, et al. Immunopathological changes in human cerebral malaria. Clin Neuropathol. 1993;12:142–146. [PubMed] [Google Scholar]
- 112.Coltel N, Combes V, Hunt NH, Grau GE. Cerebral malaria -- a neurovascular pathology with many riddles still to be solved. Curr Neurovasc Res. 2004;1:91–110. doi: 10.2174/1567202043480116. [DOI] [PubMed] [Google Scholar]
- 113.Hanum PS, Hayano M, Kojima S. Cytokine and chemokine responses in a cerebral malaria-susceptible or - resistant strain of mice to Plasmodium berghei ANKA infection: early chemokine expression in the brain. Int Immunol. 2003;15:633–640. doi: 10.1093/intimm/dxg065. [DOI] [PubMed] [Google Scholar]
- 114.Favre N, Ryffel B, Rudin W. The development of murine cerebral malaria does not require nitric oxide production. Parasitology. 1999;118(Pt 2):135–138. doi: 10.1017/s0031182098003606. [DOI] [PubMed] [Google Scholar]
- 115.Miyazaki Y, Bunting M, Stafforini DM, Harris ES, McIntyre TM, Prescott SM, et al. Integrin alphaDbeta2 is dynamically expressed by inflamed macrophages and alters the natural history of lethal systemic infections. J Immunol. 2008;180:590–600. doi: 10.4049/jimmunol.180.1.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reis PA, Comim CM, Hermani F, Silva B, Barichello T, Portella AC, et al. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog. 2010;6:el000963. doi: 10.1371/journal.ppat.1000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hensley K, bdel-Moaty H, Hunter J, Mhatre M, Mou S, Nguyen K, et al. Primary glia expressing the G93A-SOD1 mutation present a neuroinflammatory phenotype and provide a cellular system for studies of glial inflammation. J Neuroinflammation. 2006;3:2. doi: 10.1186/1742-2094-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hensley K, Mhatre M, Mou S, Pye QN, Stewart C, West M, et al. On the relation of oxidative stress to neuroinflammation: lessons learned from the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Antioxid Redox Signal. 2006;8:2075–2087. doi: 10.1089/ars.2006.8.2075. [DOI] [PubMed] [Google Scholar]
- 119.Zhou J, Huang WQ, Li C, Wu GY, Li YS, Wen SH, et al. Intestinal ischemia/reperfusion enhances microglial activation and induces cerebral injury and memory dysfunction in rats*. Crit Care Med. 2012;40:2438–2448. doi: 10.1097/CCM.0b013e3182546855. [DOI] [PubMed] [Google Scholar]
- 120.Floyd RA, Hensley K, Bing G. Evidence for enhanced neuro-inflammatory processes in neurodegenerative diseases and the action of nitrones as potential therapeutics. J Neural Trans. 2000;60:337–364. doi: 10.1007/978-3-7091-6301-6_28. [DOI] [PubMed] [Google Scholar]
- 121.Biegon A, Alvarado M, Budinger TF, Grossman R, Hensley K, West MS, et al. Region-selective effects of neuroinflammation and antioxidant treatment on peripheral benzodiazepine receptors and NMDA receptors in the rat brain. J Neurochem. 2002;82:924–934. doi: 10.1046/j.1471-4159.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 122.Hainsworth AH, Brittain JF, Khatun H. Pre-clinical models of human cerebral small vessel disease: Utility for clinical application. J Neurol Sci. 2012 doi: 10.1016/j.jns.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 123.Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang QL, Jia L, Jiao X, Guo WL, Ji JW, Yang HL, et al. APP/PS1 transgenic mice treated with aluminum: an update of Alzheimer's disease model. Int J Immunopathol Pharmacol. 2012;25:49–58. doi: 10.1177/039463201202500107. [DOI] [PubMed] [Google Scholar]
- 125.Barichello T, Santos AL, Savi GD, Generoso JS, Otaran P, Michelon CM, et al. Antioxidant treatment prevents cognitive impairment and oxidative damage in pneumococcal meningitis survivor rats. Metab Brain Dis. 2012 doi: 10.1007/s11011-012-9315-9. [DOI] [PubMed] [Google Scholar]
- 126.Petronilho F, Perico SR, Vuolo F, Mina F, Constantino L, Comim CM, et al. Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun. 2012;26:904–910. doi: 10.1016/j.bbi.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 127.Comim CM, Cassol OJ, Jr, Abreu I, Moraz T, Constantino LS, Vuolo F, et al. Erythropoietin reverts cognitive impairment and alters the oxidative parameters and energetic metabolism in sepsis animal model. J Neural Transm. 2012 doi: 10.1007/s00702-012-0774-2. [DOI] [PubMed] [Google Scholar]
- 128.Barichello T, Lemos JC, Generoso JS, Carradore MM, Moreira AP, Collodel A, et al. Evaluation of the brain-derived neurotrophic factor, nerve growth factor and memory in adult rats survivors of the neonatal meningitis by Streptococcus agalactiae. Brain Res Bull. 2012 doi: 10.1016/j.brainresbull.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 129.Zimmerman GA, Castro-Faria-Neto H. Persistent cognitive impairment after cerebral malaria: models, mechanisms and adjunctive therapies. Expert Rev Anti Infect Ther. 2010;8:1209–1212. doi: 10.1586/eri.10.117. [DOI] [PubMed] [Google Scholar]
- 130.Fan L, Mitchell H, Tien L, Rhodes P, Cai Z. Interleukin-1beta-induced brain injury in the neonatal rat can be ameliorated by alpha-phenyl-n-tert-butyl-nitrone. Exp Neurol. 2009;220:143–153. doi: 10.1016/j.expneurol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garcia-Alloza M, Borrelli L, Hyman B, Bacskai B. Antioxidants have a rapid and long-lasting effect on neuritic abnormalities in APP:PS1 mice. Neurobiol Aging. 2010;31:2068. doi: 10.1016/j.neurobiolaging.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.D'Amore J, Kajdasz S, McLellan M, Bacskai B, Stern E, Hyman B. In vivo multiphoton imaging of a transgenic mouse model of Alzheimer disease reveals marked thioflavin-S-associated alterations in neurite trajectories. J Neuropathol Exp Neurol. 2003;62:137–145. doi: 10.1093/jnen/62.2.137. [DOI] [PubMed] [Google Scholar]
- 133.Spires T, Meyer-Luehmann M, Stern E, McLean P, Skoch J, Nguyen P, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 1990;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Masliah E, Terry R, DeTeresa R, Hansen L. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989;103:234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- 135.Scheff S, DeKosky S, Price D. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 136.Lombardo J, Stern E, McLellan M, Kajdasz S, Hickey G, Bacskai B, et al. Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neutitic alterations. J Neurosci. 2003;23:10879–10883. doi: 10.1523/JNEUROSCI.23-34-10879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stern E, Bacskai B, Hickey G, Attenello F, Lombardo J, Hyman B. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi CH, Chen K, Vasquez-Weldon A, Jackson RL, Floyd RA, Kopke RD. Effectiveness of 4-hydroxy phenyl N-tert-butylnitrone (4-OHPBN) alone and in combination with other antioxidant drugs in the treatment of acute acoustic trauma in chinchilla. Free Radic Biol Med. 2008;44:1772–1784. doi: 10.1016/j.freeradbiomed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 139.Choi CH, Chen K, Du X, Floyd RA, Kopke RD. Effects of delayed and extended antioxidant treatment on acute acoustic trauma. Free Radic Res. 2011;45:1162–1172. doi: 10.3109/10715762.2011.605360. [DOI] [PubMed] [Google Scholar]
- 140.Du X, Choi CH, Chen K, Cheng W, Floyd RA, Kopke RD. Reduced formation of oxidative stress biomarkers and migration of mononuclear phagocytes in the cochleae of chinchilla after antioxidant treatment in acute acoustic trauma. Int J Otolaryngol. 2011;2011:612690. doi: 10.1155/2011/612690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Du X, Chen K, Choi CH, Li W, Cheng W, Stewart C, et al. Selective degeneration of synapses in the dorsal cochlear nucleus of chinchilla following acoustic trauma and effects of antioxidant treatment. Hear Res. 2011;283:1–13. doi: 10.1016/j.heares.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 142.Mandal MN, Moiseyev GP, Elliott MH, Kasus-Jacobi A, Li X, Chen H, et al. Alpha-phenyl-N-tert-butylnitrone (PBN) prevents light-induced degeneration of the retina by inhibiting RPE65 protein isomerohydrolase activity. J Biol Chem. 2011;286:32491–32501. doi: 10.1074/jbc.M111.255877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ewert DL, Lu J, Li W, Du X, Floyd R, Kopke R. Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hear Res. 2012;285:29–39. doi: 10.1016/j.heares.2012.01.013. [DOI] [PubMed] [Google Scholar]