Abstract
Although microglial activation is associated with all CNS disorders, many of which are sexually dimorphic or age-dependent, little is known about whether microglial basal gene expression is altered with age in the healthy CNS or if it is sex-dependent. Analysis of microglia from the brains of 3 day (P3) - to 12 month-old male and female C57Bl/6 mice revealed distinct gene expression profiles during postnatal development that differ significantly from adulthood. Microglia at P3 are characterized by relatively high iNOS, TNFα and arginase-1 mRNA levels, whereas P21 microglia have increased expression of CD11b, TLR4 and FcRγI. Adult microglia (2-4 months) are characterized by low pro-inflammatory cytokine expression that increases by 12 months of age. Age-dependent differences in microglial gene expression suggest that microglia likely undergo phenotypic changes during ontogenesis, although in the healthy brain they did not exclusively express either M1 or M2 phenotypic markers at any time. Interestingly, microglia were sexually dimorphic only at P3 when females had higher expression of inflammatory cytokines than males, although there were no sex differences in estrogen receptor expression at this or any other time evaluated here. Compared to microglia in vivo, primary microglia prepared from P3 mice had considerably altered gene expression with higher levels of TNFα, CD11b, arginase-1 and VEGF suggesting that culturing may significantly alter microglial properties. In conclusion, age- and sex-specific variances in basal gene expression may enable differential microglial responses to the same stimulus at different ages, perhaps contributing to altered CNS vulnerabilities and/or disease courses.
Keywords: microglia, development, aging, sexual dimorphism, M1/M2 phenotype
INTRODUCTION
Microglia, the resident innate immune cells in the central nervous system (CNS), are associated with the pathogenesis of virtually all CNS disorders or injuries. One important characteristic of these cells is high morphologic and functional plasticity. They acquire an activated, amoeboid morphology in response to invading pathogens and/or CNS damage. At the same time, they increase their production of a wide array of chemokines, cytokines, nitric oxide and reactive oxygen species that mediate neuroinflammation (Graeber et al. 2011; Hoek et al. 2000; Streit et al. 2005). In contrast, microglia in the healthy adult CNS are characterized by a quiescent morphology with numerous thin ramified processes. Although commonly considered “resting”, emerging evidence suggests that quiescent microglia are highly motile and are actively involved in many physiological processes that include making dynamic contacts with neurons (Graeber 2010; Kettenmann et al. 2011; Paolicelli et al. 2011; Parkhurst and Gan 2010; Tremblay and Majewska 2011; Tremblay et al. 2011; Wake et al. 2009). However, the gene expression profiles of “resting” microglia in the healthy CNS are not well characterized, and even less is known about whether microglia undergo changes in gene expression that accompany their functional alterations from postnatal development to aging. In the postnatal brain, microglia are important for synaptic pruning (Paolicelli et al. 2011) and they have an activated, amoeboid morphology with high phagocytic activity (Schwarz et al. 2012). Microglial changes towards an amoeboid morphology are also associated with aging (Conde and Streit 2006; von Bernhardi et al. 2010), suggesting that their gene expression profiles may also be altered at these times. Therefore, in the present study, we hypothesized that in the normal CNS, microglia undergo age-dependent gene expression changes that reflect the morphologic and functional plasticity they exhibit during development and aging. We focused on key genes associated with the classical pro-inflammatory (M1) and alternative anti-inflammatory (M2) phenotypes, hypothesizing that microglia will express more M1 markers in the postnatal and aging CNS when they display an activated morphology, whereas in the young adult CNS, microglia will be polarized towards the M2 phenotype.
Another aspect of microglial biology that is rarely studied is whether microglial gene expression is sex-dependent (Sierra et al. 2007). Many neurodegenerative diseases characterized by neuroinflammation are sexually dimorphic. For example, women are at higher risk for developing Alzheimer disease and multiple sclerosis, whereas men are more likely to develop amyotrophic lateral sclerosis and Parkinson disease (Logroscino et al. 2010; Payami et al. 1996; Voskuhl and Gold 2012; Wirdefeldt et al. 2011). Although the causes of these sex differences remain poorly understood, potential sexual dimorphisms in microglia may play a role. Estrogen receptors (ER) in the CNS mediate the effects of estrogens in females as well as the effects of testosterone in males that is converted in the brain to estrogen by aromatase (Balthazart and Ball 1998). ERs underlie sex-dependent differences in neurons (Bloch et al. 1992; Flores et al. 2003; Shughrue et al. 2002; Simerly et al. 1997), and suppress inflammatory responses of microglia and macrophages (Arevalo et al. 2012; Smith et al. 2011; Vegeto et al. 2006). Therefore, we hypothesized that microglial inflammatory gene expression would be sex-dependent, and that alterations in estrogen receptor expression would accompany these changes. A major goal of this study was to determine at what age potential sexual dimorphisms in microglial gene expression would be evident.
To address our hypotheses, we evaluated estrogen receptor, and M1 and M2 marker gene expression in microglia from healthy C57Bl/6 male and female mice ranging in age from 3 days to 12 months. Lastly, primary microglial cultures derived from neonatal animals are an invaluable tool to study many aspects of microglial activities. Therefore, we also compared their gene expression profiles to those of microglia in vivo from neonates of the same age, to determine if and how in vitro culturing alters their properties.
MATERIALS AND METHODS
Animals
C57Bl/6 mice were purchased from Charles River. All animals were maintained in the AALAC-accredited animal facility with a 12 hour light/dark cycle regime and access to food and water ad libitum. The 7-8 week old and 4 month-old females were virgins. The 12 month-old mice were retired breeders, with females not having born a litter for at least two months prior to their use to minimize the possibility that hormones associated with pregnancy/lactation would interfere with microglial activities. All experiments were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee.
We examined microglial gene expression at different ages, selected based on important developmental milestones. Postnatal day 3 is a time following the testosterone surge in males (that occurs on the day of birth) that is responsible for masculinization of the still developing CNS. In addition, primary microglial cultures are usually prepared from mice of this age. Postnatal day 21 is a time proximal to weaning and represents an important transition before the onset of puberty that begins during the fourth week of age in this mouse strain (Witham et al. 2012). 7-8 week old mice are young adults that have acquired full reproductive capacity, and 4 month-old mice represent sexually mature young adults. These adult ages are also the most commonly used ages in most studies. Finally, 12 month-old mice represent older animals at a time when both male and female reproductive potential and gonadal hormone levels are beginning to decline. C57Bl/6 mice usually do not produce litters after 1 year of age (Liu et al. 2013).
CD11b+ cell isolation
CD11b+ cells were isolated as previously described (Crain et al. 2009; Nikodemova and Watters 2012). Briefly, mice were euthanized and perfused with cold phosphate buffered saline (PBS). Whole brains (including cerebellum and brainstem) were dissected and dissociated into a single cell suspension using the Neural Tissue Dissociation Kit containing papain (Miltenyi Biotec, Germany). Myelin was removed by centrifugation in 0.9 M sucrose in Hank's Buffered Salt Solution. Cells were stained with PE-conjugated anti-CD11b antibodies followed by magnetic bead-conjugated secondary antibodies against PE. Magnetically-tagged CD11b+ cells were then separated using MS columns according to the manufacturer's protocol (Miltenyi Biotec, Germany). Reagents were used at 4°C and the cells were kept on ice during the isolation process. The average purity of isolated cells having the characteristics of microglia was >97% as determined by flow cytometry for CD11b/CD45 staining (Crain et al. 2009; Nikodemova and Watters 2012). We recently showed that the isolation efficiency of microglia expressing low and high CD11b levels were equal; therefore microglial isolation is not expected to be affected by variations in CD11b expression at different time points (Nikodemova and Watters 2012).
Genotyping
The sex of the 3 day (3d)-old mice was verified by genotyping for the sex-determining region Y (SRY) gene, which is located on the Y chromosome, as previously described (Crain et al. 2009). Genomic DNA was isolated by digestion of a small section of tail and then used in PCR for SRY.
Primary microglial cultures
Primary neonatal microglial cultures were prepared as previously described, from approximately 50% female and 50% male litters (Nikodemova et al. 2007). Briefly, 3 day-old C57Bl/6 mice were euthanized, brains were dissected and cleaned of meninges and visible blood vessels, and then dissociated by incubation in 0.25% trypsin and DNase I followed by trituration with a Pasteur pipette. Cells were plated in T75 flasks containing Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. Microglia were harvested by shaking 10-14 days later and cultured for two days in the medium described above. The purity of microglial cultures was >96% as assessed by CD11b+ staining, as described previously (Nikodemova et al. 2007).
RNA extraction and Quantitative PCR
RNA was extracted from either primary microglial cultures or freshly-isolated microglia using TriReagent (Sigma-Aldrich, St. Louis, MO). cDNA was synthesized from 1μg of total RNA and MMLV reverse transcriptase (Invitrogen,) as previously described (Crain et al. 2009). Quantitative PCR was performed on an ABI 7300 system using Power SYBR Green (Applied Biosystems, Carlsbad, CA). The primer sequences are given in Table 1 and were designed to span introns whenever possible. Primer efficiency was tested by serial dilution. ERβ expression was tested using three independent primer sets whose efficiency was verified using cDNA from ovary as a positive control. The Ct values for ERβ were ~20 in the ovaries (highly expressed), 26 in whole brain tissue homogenates, and > 33 in isolated microglia (defined as undetectable).
CT values from duplicate measurements of each sample were averaged and relative expression levels were determined by the ΔΔCT method. The expression of each gene was normalized to the levels of 18s and/or β-actin within each sample as previously described (Crain et al. 2009).
Statistical analysis
Data are expressed as the mean ± SEM of n = 8-14 mice in each group. Results for primary microglial cultures are from three independent culture preparations. Statistical analyses were performed using Sigma Stat 3.1 software. One-way ANOVA was used to determine statistically significant differences in gene expression over time in the same sex, and Two-way ANOVA followed by the Holm-Sidak test was used to determine differences in age-dependent gene expression between females and males. Statistical significance was set at p < 0.05. Levels of gene expression are displayed relative to 3 day-old males which enabled comparison over time and between sexes. In some cases, a Student's t-test was used to determine differences between primary cultures and postnatal day 3 (P3) microglia, or differences in expression between males and females at the same time point, as indicated in the Results. Gene expression in microglial cultures was compared to both P3 male and female microglia.
RESULTS
Age- and sex-dependent M1 gene expression
We examined basal expression levels of key pro-inflammatory genes typically associated with the M1 phenotype: iNOS, TNFα, IL-1β and IL-6 in naïve mice at P3, P21, 7-8 weeks, 4 months and 12 months of age. Notably, the expression of each gene displayed a unique time course suggesting their independent regulation with age. The levels of mRNA for each gene are expressed relative to 3 day-old males.
iNOS mRNA levels were highest at P3 followed by a significant 70% down-regulation by P21 (Fig 1A). In adult mice, iNOS expression was only 10-20% of that seen at P3 (two-way ANOVA p<0.001). We did not detect any sex differences in the expression of iNOS at any age.
Fig. 1. Basal expression of pro-inflammatory genes in microglia.
The expression of iNOS (A) and TNFα (B), but not IL-1β (C), was significantly higher in microglia isolated from whole brains of 3-day old mice. On the contrary, IL-6 (D) expression was lowest at P3 compared to other ages. Females had higher expression of TNFα, IL-1β and IL-6 than males at P3. Gene expression in primary microglia was significantly affected by culturing in vitro. Gene expression data are expressed as fold change relative to 3 day-old males. + depicts significant age-dependent differences vs. 3 day-old males. * depicts significant age-dependent differences vs. 3 day-old females. # depicts significant differences between males and females of the same age. Symbol “a” depicts significant difference in gene expression in primary microglia vs. 3 day-old males. The number of symbols corresponds to the following p-values: *p<0.05; **p<0.01; ***p<0.001. N.D., not determined.
TNFα was highly expressed at P3, but it was significantly lower between P21 and 4 months of age (Fig 1B). A second peak of TNFα mRNA levels occurred at 12 months in both sexes. A two-way ANOVA revealed significant age-dependent changes in TNFα expression (p<0.001) without significant interaction with sex (p<0.2). Although a two-way ANOVA analysis did not reveal significant sex-dependent TNFα expression, females had 60% higher TNFα mRNA levels at P3 than males, a difference that was statistically significant by Student t-test (p<0.007).
In males, there were no significant age-dependent changes in the expression of IL-1β (Fig 1C) and in females, IL-1β decreased by 50% at 7 weeks of age compared to P3. By 12 months, IL1-β appeared to be upregulated in both sexes, but this increase did not reach statistical significance as determined by the one-way or two-way ANOVA. A sex difference was observed in IL-1β mRNA levels at P3 where expression was significantly higher in females than males (t-test p<0.001).
Contrary to other pro-inflammatory genes that have high expression at P3, IL-6 expression was lowest at P3 compared to adult animals which had 3-4 times greater IL-6 mRNA levels (two-way ANOVA p<0.002). Interestingly, whereas females had higher IL-6 mRNA levels at P3 (t-test p<0.001), this sexual dimorphism did not persist in adulthood (Fig 1D).
Age- and sex-dependent M2 gene expression
We investigated the expression of genes often used to indicate the M2 phenotype: the anti-inflammatory cytokine IL-10, arginase-I, and the growth factor VEGF. We detected no significant age-dependent changes in the expression of IL-10 in males (one-way ANOVA, p=0.12; Fig 2A); however, females showed decreased expression at 7 weeks of age (one-way ANOVA, p< 0.04). Females also had almost 2-fold higher levels of IL-10 mRNA at P3 than males (two-way ANOVA, p<0.03).
Fig 2. Basal expression of anti-inflammatory and trophic factor genes in microglia.
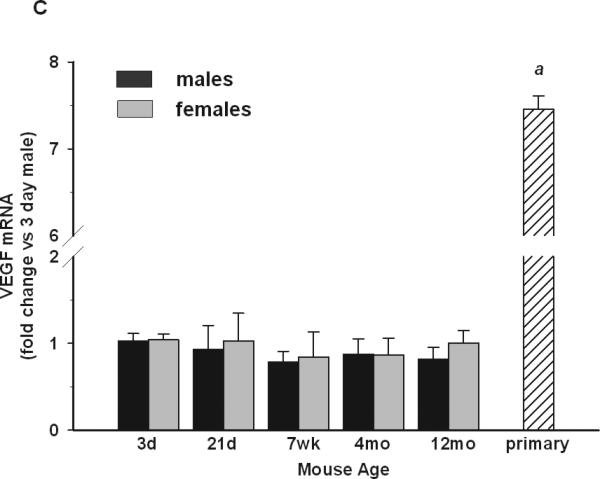
P3 females had higher microglial expression of IL-10 (A) than males. Arginase-I (B) expression was highest at P3 both in males and females compared to other ages. There were no sex- or age-dependent changes in VEGF (C) expression. Primary microglia cultures had significantly lower expression of IL-10 compared to males or females in vivo, whereas VEGF was significantly upregulated compared to any age or sex in vivo. Gene expression data are expressed as fold change relative to 3 day-old males. + depicts significant age-dependent differences vs. 3 day-old males. * depicts significant age-dependent differences vs. 3 day-old females. # depicts significant differences between males and females of the same age. Symbol “a” depicts significant difference in gene expression in primary microglia vs. 3 day-old males. The number of symbols corresponds to the following p-values: *p<0.05; **p<0.01; ***p<0.001
The time course of arginase-I was very similar to that of iNOS. The highest expression was observed at P3 followed by down-regulation by P21 to 30% of P3 levels (Fig 2B). In adulthood, the levels of arginase-I mRNA were <10% of P3 expression (two-way ANOVA, p<0.001). No differences were observed between males and females.
The expression of VEGF, a growth factor that supports neuronal survival, was unchanged at all time-points evaluated (Fig 2C), and no differences between males and females were observed.
Age- and sex-dependent expression of membrane proteins
Toll-like receptors (TLRs) play an important role in the activation of innate immune cells, including microglia. We analyzed the expression of TLR4 and TLR2 because they are associated with CNS disorders such as ischemia, infections, multiple sclerosis and others, and males and females are differentially predisposed to these disorders. The highest expression of TLR4 was observed at P21 in both sexes (Fig 3A, two-way ANOVA, p<0.02). TLR2 expression also exhibited age-dependent changes (two-way ANOVA, p<0.016) with the lowest mRNA levels being observed at 7 weeks of age (Fig 3B). There were no statistically significant sex-dependent differences in the expression of TLR2 or TLR4.
Fig 3. Basal expression of membrane proteins in microglia.
P21 microglia were characterized by elevated expression of TLR4 (A), FcRγI (C) and CD11b (D), but not TLR2 (B) compared to other ages. Interestingly, primary microglial cells had elevated CD11b levels compared to male or female P3 pups in vivo. Gene expression data are expressed as fold change relative to 3 dayold males. + depicts significant age-dependent differences vs. 3 day-old males. * depicts significant age-dependent differences vs. 3 day-old females. # depicts significant differences between males and females of the same age. Symbol “a” depicts significant difference in gene expression in primary microglia vs. 3 day-old males. The number of symbols corresponds to the following p-values: *p<0.05; **p<0.01; ***p<0.001.
Fc receptors mediate antibody-dependent phagocytosis, and morphological studies indicate differences in the prevalence of amoeboid microglia in postnatal males and females (Schwarz et al. 2012). We evaluated the expression of FcRγI that binds IgG, the most common class of antibodies (Fig 3C). FcRγI mRNA levels were significantly up-regulated at P21 compared to all other ages (two-way ANOVA, p<0.002), but there were no differences between males and females.
Lastly, we examined the expression of CD11b, an integrin involved in cell adhesion, phagocytosis, chemotaxis and inflammation. CD11b is often up-regulated upon microglial activation. CD11b mRNA levels were lowest at P3 followed by highest expression at P21 (one-way ANOVA p<0.001 for males, p<0.01 for females; Fig 3D). Although CD11b expression was down-regulated after P21, its expression still remained higher than at P3. We found no significant differences in CD11b expression between males and females at any age.
Age- and sex-dependent expression of estrogen receptors
We also evaluated ERα and ERβ expression in microglia given the sexual dimorphisms in several neurologic disorders. ERα mRNA expression was very low at P3 but increased at P21. Its expression further increased by 7-8 weeks of age after which time its levels remained constant until 12 months of age in both sexes (Fig. 4). Compared to P3, ERα mRNA levels were approximately 4-fold higher at 21days, and 6-7 fold higher at the other time points. Importantly, there were no differences observed in microglial ERα expression between males and females at any age. ERβ mRNA expression was not detectable at any age evaluated, in either male or female mice, suggesting that this gene is not expressed in microglia from healthy animals.
Fig 4. Basal expression of estrogen receptors in microglia.
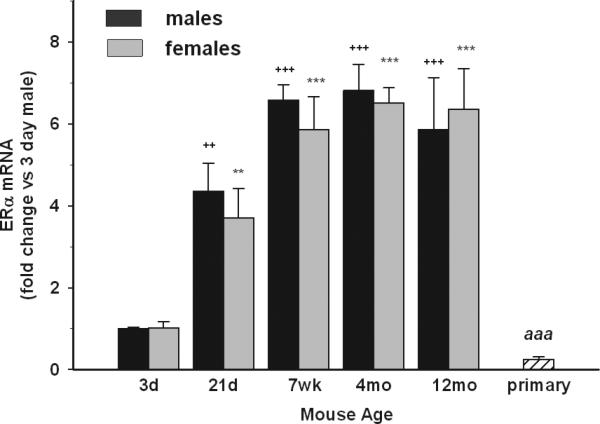
The expression levels of ERα and ERβ were evaluated by qRT-PCR and are expressed as fold change relative to 3 day-old males. P3 male and female microglia had the lowest expression of ERα compared to other ages. No sex-dependent differences were detected in ERα expression at any age. Primary microglia had downregulated ERα levels compared to P3 male and female microglia in vivo. ERβ was undetectable in all ages. + depicts significant age-dependent differences vs. 3 day-old males. * depicts significant age-dependent differences vs. 3 day-old females. # depicts significant differences between males and females of the same age. Symbol “a” depicts significant difference in gene expression in primary microglia vs. 3 day-old males. The number of symbols corresponds to the following p-values: *p<0.05; **p<0.01; ***p<0.001.
Basal gene expression in primary microglia
Because mixed-sex primary microglial cultures are commonly used to study microglia in vitro, we compared gene expression in cultured cells to that of microglia freshly-isolated from animals of the same age (P3) from which the primary cultures were prepared. We found that gene expression in primary microglia was significantly different from that of male and female P3 microglia in vivo. Moreover, primary microglial gene profiles did not match the profile of microglia at any age evaluated here.
TNFα mRNA levels were highly up-regulated in neonatal microglia cultures compared to male but not female P3 microglia (Fig 1B), whereas iNOS expression was significantly down-regulated, with levels comparable to those observed in male and female adult microglia (Fig 1A). IL-10 mRNA levels were also significantly lower in primary cultures compared to male and female microglia of any age (Fig 2A). Arginase-I mRNA was comparable to levels in male and female P3 microglia (Fig 2B). Interestingly, the expression of VEGF was increased by 7-fold in primary cultures compared to freshly-isolated male and female microglia from mice of any age. CD11b mRNA levels in primary cultures were 16-18 times higher than at P3 in males and females (Fig 3), whereas the expression of TLR2, TLR4 and FcRγI was comparable to P3 mice. Lastly, ERα mRNA levels in primary microglial cells were significantly down-regulated compared to male and female microglia in vivo from any age, whereas ERβ mRNA levels remained undetectable (Fig 4).
DISCUSSION
Microglia possess great morphological and functional plasticity that enable their rapid response to specific physiological or pathological signals. However, it is not known whether microglial properties differ in the healthy CNS of postnatal, adult and aged mice, as no studies have systematically evaluated microglia over time. Our data demonstrate that basal microglial gene expression significantly varies in the postnatal and adult brain, perhaps enabling microglial acquisition of specific age-dependent phenotypes. Interestingly, however, microglia in the healthy CNS are not fully committed to either an inflammatory or anti-inflammatory phenotype at any age, but rather display some M1 and M2 markers with variable age-dependent expression levels.
At postnatal day 3 microglia were characterized by high expression of iNOS, TNFα and arginase-I mRNA levels relative to other ages. Thus, during the early postnatal period, microglia express concomitant M1 (iNOS, TNFα) and M2 (arginase-I) markers suggesting that they either acquire a unique phenotype related to specific developmental needs at this age, or that there are several microglial subpopulations that may be region-specific. The latter is supported by the presence of at least three different microglial morphologies found in many CNS regions at this age, with the amoeboid morphology being the most prevalent (Schwarz et al. 2012). Both TNFα and nitric oxide (produced by iNOS) exert pleiotropic effects. Besides their well-known role in inflammation, both are involved in neuronal apoptosis, and synaptic plasticity and pruning, frequent processes during early CNS development in which microglia are actively involved (McCoy and Tansey 2008; Zhou and Zhu 2009). The significance of arginase-I expression at this age is not yet clear. Both iNOS and arginase-I use arginine as a substrate for their enzymatic activities thus competing for arginine availability. Some studies suggest that arginase-I may function as a modulator of iNOS activity to prevent overproduction of nitric oxide in immune cells (Chang et al. 1998; Mori 2007). On the other hand, in macrophages, arginase-I activity is important for extracellular matrix production, thus facilitating wound healing (Bansal and Ochoa 2003). Since at P3 the brain is still developing and growing, it is possible that microglia participate in extracellular matrix building through arginase-I activities.
At P21, iNOS, TNFα and arginase-I are down-regulated whereas IL-6, CD11b, TLR4 and FcRγI mRNAs are significantly increased suggesting that microglia at P21 are phenotypically and functionally distinct from both P3 and adult microglia. The functional significance of elevated CD11b and TLR4 expression at P21 is not yet clear and warrants further study. Fc receptors mediate antibody dependent phagocytosis and are important modulators of microglial activities. Although increased IgG and Fc receptor levels are evident in the aged CNS and during neurodegenerative disease in animal models and humans (Cribbs et al. 2012; Lira et al. 2011; Lunnon et al. 2011), the role of microglial Fc receptors during CNS development is unknown. Our data suggest that they may play a role in the postnatal period when increased phagocytosis may be necessary for clearing debris from neuronal remodeling processes.
Between two and four months of age, microglia express low levels of the M1 markers iNOS and TNFα mRNA. IL-10 and arginase-I expression, markers of two different M2 subtypes (Edwards, 2006) are also low, suggesting that microglia in the healthy adult brain are not polarized to either the M1 or M2 phenotype. However, by 12 months of age, microglial TNFα mRNA had increased to the levels found during early postnatal development. IL-1β mRNA was also increased at 12 months, although not significantly. Similar results have been reported in other studies (Godbout et al. 2005; Sierra et al. 2007), and suggest that at this older age, microglia may be polarizing towards the M1 phenotype.
Interestingly, the only significant sexual dimorphisms we observed in microglial gene expression were in the early postnatal period (P3). Microglia from female mice had higher mRNA levels for TNFα, IL-1β, IL-6 and IL-10 than males. The testosterone surge occurring in male mice shortly after birth may underlie this sex-related differences as androgens reportedly reduce the expression of pro-inflammatory cytokines in macrophages (Brown et al. 2007; Vignozzi et al. 2012) and they are converted to estrogens in the CNS which also exert anti-inflammatory effects. ERα levels were lowest at P3 relative to other ages and no differences between males and females were observed at this age or at any other tested. In addition, we did not detect ERβ mRNA in microglia at all, consistent with a report by Sierra et al.(Sierra et al. 2008). Previous studies have shown effects of ERβ in activated microglial cell lines and primary cultures, and in ischemically injured non-human primates (Baker et al. 2004; Lewis et al. 2008; Mor et al. 1999; Takahashi et al. 2004), but there are no reports to our knowledge demonstrating effects of ERβ activation on inflammatory gene expression in quiescent microglia. Together, these data suggest that ERβ is not expressed in microglia in the healthy CNS, and that neither ERα nor ERβ underlie the sexual dimorphisms observed in early postnatal microglial gene expression. However, a recent study by Sato et al. showed that some effects of male sex hormones in the CNS are mediated via androgen receptors (Sato et al. 2004) so they may be responsible for some sex-dependent differences in microglial gene expression, although androgen receptors have not been detected in microglia (Sierra et al. 2008). Regardless, the absence of sex-dependent differences in inflammatory gene expression in adult microglia is surprising given the considerable sexual disparities in many CNS disorders in adulthood. Perhaps this reflects effects of sex hormones on other CNS cell types instead of microglia, as shown in EAE where the beneficial effects of estrogens are mediated through astrocytes (Spence et al. 2011), or alterations in estrogen receptor signaling independent of the expression of M1/M2 genes in quiescent microglia. In addition, despite a lack of obvious differences in microglial basal gene expression, microglial responses may be sex-dependent. However, we examined only a limited number of genes, and we did not evaluate microglia in animals beyond 12 months of age, so we cannot exclude the possibility that sex-dependent differences in microglial properties emerge at later time points.
The alterations in basal microglial gene expression with age likely reflect the changing environment in the CNS. Microglia express receptors for many neurotransmitters, neurohormones, neuromodulators and ion channels that enable them to communicate with other CNS cells and sense abnormal neuronal activities or disturbances in homeostasis (Kettenmann et al. 2011). We have previously shown age- and sex-dependent alterations in microglial expression of purinergic receptors that are important sensors of extracellular ATP and its metabolites (Crain et al. 2009). However, our understanding of the regulation of microglial activities in the healthy CNS has only begun to evolve, and more studies are needed to fully understand microglial interactions with other cell types in the CNS.
Lastly, we show here that in vitro culturing has a profound effect on basal gene expression in microglia. The expression profile of genes assessed here did not correspond to any single age evaluated in vivo. At least two factors may contribute to the altered microglial phenotype in vitro. First, microglia and mixed primary cultures from which microglia are derived are cultured in medium supplemented with serum, whereas in the healthy CNS, microglia and other cell types are not exposed to serum directly. A recent study by Foo et al. showed that the exposure of astrocytes to serum in vitro induced more than 365 genes that are not normally expressed in these cells in vivo (Foo et al. 2011); thus serum may have similar effects on microglia. In addition, since microglia are cultured for 10-14 days before harvesting in mixed cultures with astrocytes in media containing serum, phenotypically altered astrocytes may affect microglial gene expression. Second, in primary cultures, neurons are absent. Thus, the loss of neuronal signals that are important modulators of microglial activities may also contribute to alterations in microglial properties in vitro. Overall, our study suggests that many factors should be considered when interpreting in vitro data that may be complex and quite distinct from the in vivo condition.
Conclusion
Microglia display specific gene expression profiles in the early postnatal (P3) and late postnatal (P21) periods that are distinct from adult microglia, likely reflecting their specific roles during development. At 12 months of age, microglia appear to begin polarization towards a pro-inflammatory (M1) phenotype. Sex- and age-dependent microglial gene expression patterns such as those shown in this study may induce different microglial responses to the same physiological or pathological stimulus, which in turn could significantly affect CNS vulnerability and/or the outcome of many CNS disorders at specific ages.
ACKNOWLEDGEMENTS
We would like to thank Mr. Alex Wessel and Ms. Alissa Small for their excellent technical assistance and laboratory support. This work was supported by the NIH/NINDS R01NS049033 and NIH Training Grant R25GM083252 (JMC).
REFERENCES
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. Journal of neuroendocrinology. 2012;24(1):183–190. doi: 10.1111/j.1365-2826.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145(11):5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase). Trends Neurosci. 1998;21(6):243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Current opinion in clinical nutrition and metabolic care. 2003;6(2):223–228. doi: 10.1097/00075197-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Kurth SM, Akesson TR, Micevych PE. Estrogen-concentrating cells within cell groups of the medial preoptic area: sex differences and co-localization with galanin-immunoreactive cells. Brain Res. 1992;595(2):301–308. doi: 10.1016/0006-8993(92)91064-l. [DOI] [PubMed] [Google Scholar]
- Brown CM, Xu Q, Okhubo N, Vitek MP, Colton CA. Androgen-mediated immune function is altered by the apolipoprotein E gene. Endocrinology. 2007;148(7):3383–3390. doi: 10.1210/en.2006-1200. [DOI] [PubMed] [Google Scholar]
- Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. The American journal of physiology. 1998;274(1 Pt 2):H342–348. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65(3):199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J Neuroinflammation. 2009;6:24. doi: 10.1186/1742-2094-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9(1):179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CA, Shughrue P, Petersen SL, Mokha SS. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience. 2003;118(3):769–778. doi: 10.1016/s0306-4522(02)01000-x. [DOI] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, Barres BA. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71(5):799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19(10):1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS letters. 2011;585(23):3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol. 2008;195(1-2):47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira A, Kulczycki J, Slack R, Anisman H, Park DS. Involvement of the Fc gamma receptor in a chronic N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of dopaminergic loss. J Biol Chem. 2011;286(33):28783–28793. doi: 10.1074/jbc.M111.244830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Human reproduction. Oxford; England: 2013. Resveratrol protects against age-associated infertility in mice. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Traynor BJ, Hardiman O, Chio A, Mitchell D, Swingler RJ, Millul A, Benn E, Beghi E. Incidence of amyotrophic lateral sclerosis in Europe. Journal of neurology, neurosurgery, and psychiatry. 2010;81(4):385–390. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunnon K, Teeling JL, Tutt AL, Cragg MS, Glennie MJ, Perry VH. Systemic inflammation modulates Fc receptor expression on microglia during chronic neurodegeneration. J Immunol. 2011;186(12):7215–7224. doi: 10.4049/jimmunol.0903833. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, Naftolin F. Estrogen and microglia: A regulatory system that affects the brain. Journal of neurobiology. 1999;40(4):484–496. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. The Journal of nutrition. 2007;137(6 Suppl 2):1616S–1620S. doi: 10.1093/jn/137.6.1616S. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J Neuroinflammation. 2012;9(1):147. doi: 10.1186/1742-2094-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282(20):15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Gan WB. Microglia dynamics and function in the CNS. Current opinion in neurobiology. 2010;20(5):595–600. doi: 10.1016/j.conb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. American journal of human genetics. 1996;58(4):803–811. [PMC free article] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101(6):1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120(6):948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Progress in brain research. 2002;139:15–29. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94(25):14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Das A, Butler JT, Ray SK, Banik NL. Estrogen or estrogen receptor agonist inhibits lipopolysaccharide induced microglial activation and death. Neurochemical research. 2011;36(9):1587–1593. doi: 10.1007/s11064-010-0336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108(21):8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system's immune response. Neurol Res. 2005;27(7):685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Tonchev AB, Koike K, Murakami K, Yamada K, Yamashima T, Inoue M. Expression of estrogen receptor-beta in the postischemic monkey hippocampus. Neurosci Lett. 2004;369(1):9–13. doi: 10.1016/j.neulet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Communicative & integrative biology. 2011;4(2):220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147(5):2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, Cellai I, Maneschi E, Serni S, Gacci M, Carini M, Piccinni MP, Saad F, Adorini L, Vannelli GB, Maggi M. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. The Journal of endocrinology. 2012;212(1):71–84. doi: 10.1530/JOE-11-0289. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Tichauer JE, Eugenin J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112(5):1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nature reviews Neurology. 2012;8(5):255–263. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. European journal of epidemiology. 2011;26(Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Witham EA, Meadows JD, Shojaei S, Kauffman AS, Mellon PL. Prenatal Exposure to Low Levels of Androgen Accelerates Female Puberty Onset and Reproductive Senescence in Mice. Endocrinology. 2012 doi: 10.1210/en.2012-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2009;20(4):223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]







