Abstract
Oxytocin (OT) promotes social and reproductive behaviors in mammals, and OT deficits may be linked to disordered social behaviors like autism and severe anxiety. Male rat sexual behavior is an excellent model for OT regulation of behavior, as its pattern and neural substrates are well characterized. We previously reported that OT microinjected into the medial preoptic area (MPOA), a major integrative site for male sexual behavior, facilitates copulation in sexually experienced male rats, whereas intra-MPOA injection of an OT antagonist (OTA) inhibits copulation. In the present studies, copulation on the day of sacrifice stimulated OTR mRNA expression in the MPOA, irrespective of previous sexual experience, with the highest levels observed in first-time copulators. In addition, sexually experienced males had higher levels of OTR protein in the MPOA than sexually naïve males and first-time copulators. Finally, intra-MPOA injection of OT facilitated mating in sexually naive males. Others have reported a positive correlation between OT mRNA levels and male sexual behavior. Our studies show that OT in the MPOA facilitates mating in both sexually naive and experienced males, some of the behavioral effects of OT are mediated by the OTR, and sexual experience is associated with increased OTR expression in the MPOA. Taken together, these data suggest a reciprocal interaction between central OT and behavior, in which OT facilitates copulation and copulation stimulates the OT/OTR system in the brain.
Keywords: Oxytocin, oxytocin receptor, sexual behavior, sexual experience, medial preoptic area, hypothalamus, rats
1.0 Introduction
The nonapeptide oxytocin (OT) plays a major role in the control of a number of behaviors that help ensure reproductive success and survival and is primarily known for its facilitative effects on social and reproductive behaviors in mammals. It inhibits anxiety (Neumann et al., 2000) and facilitates social preference (Lukas et al., 2011), social recognition (Ferguson et al., 2001), sexual behavior (Arletti et al., 1985; Arletti et al., 1992; Caldwell et al., 1986; Melis and Argiolas, 2011), maternal behavior (Caughey et al., 2011; Pedersen et al., 1982), and pair bond formation (Williams et al., 1994). There is growing evidence that OT influences human social and emotional behaviors as well. In human subjects, OT inhibits the stress response and decreases anxiety (Heinrichs et al., 2003), decreases the fear response (Kirsch et al., 2005), is linked to feeling trusted and reciprocating those feelings to others (Zak et al., 2005), is associated with the sexual response in men and women (Carmichael et al., 1987), and facilitates social cognition (Guastella et al., 2008; Hollander et al., 2007). OT also influences motivated behaviors. For example, intranasal OT improves libido in men (MacDonald and Feifel, 2012), and there is evidence that OT may block withdrawal symptoms in alcohol-dependent patients (Pedersen et al., 2012). Thus, OT appears to play similar roles in humans and in rodents, which strongly supports the use of animal models in the study of central OT regulation of behavior.
One of the best examples of a successful animal model for central OT regulation of behavior is maternal behavior. OT facilitates maternal behavior in virgin female rats (Pedersen et al., 1982), and differences in maternal behavior are associated with differences in central OT activity. Specifically, in female rats, levels of OT receptor (OTR) binding are higher in high licking and grooming (LG) mothers and females that are more maternally responsive, compared to low LG mothers and females that are less responsive to pups, respectively (Champagne et al., 2001; Francis et al., 2000). Male rat sexual behavior is another excellent model for the study of central OT regulation of behavior, because its pattern and neural substrates have been well characterized. OT neurons in the brain are activated following copulation (Witt and Insel, 1994) or ex copula non-contact erections (Baskerville et al., 2009). Microinjection of OT into the brain facilitates copulation (Arletti et al., 1985) and induces ex copula erections (Argiolas et al., 1985), whereas an OT antagonist (OTA) injected into the brain blocks the behavioral effects of OT (Arletti et al., 1992; Melis et al., 1986; Melis et al., 1999) and inhibits copulation (Argiolas et al., 1988). OT can also restore copulation in males whose copulatory behavior had been impaired by chronic fluoxetine (Cantor et al., 1999), and sexual impotence and inefficiency have been linked to reduced central OT mRNA expression in the brain (Arletti et al., 1997).
The paraventricular nucleus (PVN), hippocampus, ventral tegmental area (VTA), and amygdala have all been identified as sites of action of OT (Melis et al., 2007; Melis et al., 2009; Melis and Argiolas, 2011; Pfaus et al., 2012; Succu et al., 2007; Succu et al., 2008; Succu et al., 2011), and we have recently reported that microinjection of OT into the medial preoptic area (MPOA) facilitates copulation in sexually experienced male rats, whereas injection of an OTA into the same site inhibits certain aspects of copulation (Gil et al., 2011). These pharmacological data suggest that OT and its receptor in the MPOA are associated with improved sexual efficiency. To further explore the link between MPOA OT/OTR and sexual efficiency, we studied the role of OTR activity in the MPOA in the phenomenon of experience-induced facilitation of copulation. Sexually experienced males are on average more efficient copulators compared to sexually naïve males. They have shorter latencies to the first mount and intromission, have more intromissions relative to unsuccessful mount attempts, achieve their first ejaculation in a shorter period of time, and have more ejaculations in a 30 min copulation test. Thus, we tested whether sexual experience increases OTR mRNA expression and protein in the MPOA. To determine whether the facilitative effects of intra-MPOA injection of OT on copulation are dependent on sexual experience, we also tested whether OT microinjected into the MPOA facilitates copulation in sexually naïve males.
2.0 Methods
All procedures were in accordance with the National Institutes of Health Guidelines for the Use of Animals and were approved by the University’s Institutional Animal Care and Use Committee.
2.1 Animals
Adult male Long-Evans/Blue-Spruce rats (Harlan, Indianapolis, IN) were housed singly in large plastic cages in a climate-controlled room, with food and water available ad lib. The light:dark cycle was 14:10, with lights off at 11:00 a.m. and on at 9:00 p.m.. Stimulus females of the same strain were housed singly in a different room. Females were ovariectomized, using bilateral flank incisions, under ketamine hydrochloride (75 mg/kg) and xylazine hydrochloride (10 mg/kg) anesthesia and allowed 2 weeks to recover. They were injected with 10 μg estradiol benzoate 48 h, and 500 μg progesterone 4 h, before a copulation test. Receptivity of the female was confirmed by allowing three intromissions by a stud male; only females that showed lordosis in response to the stud male were used for sexual experience sessions and behavioral testing.
2.2 Sexual experience sessions and tissue punches
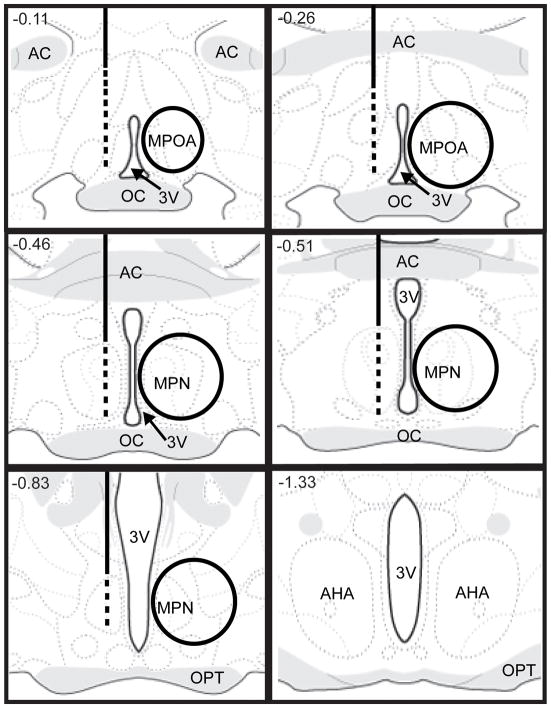
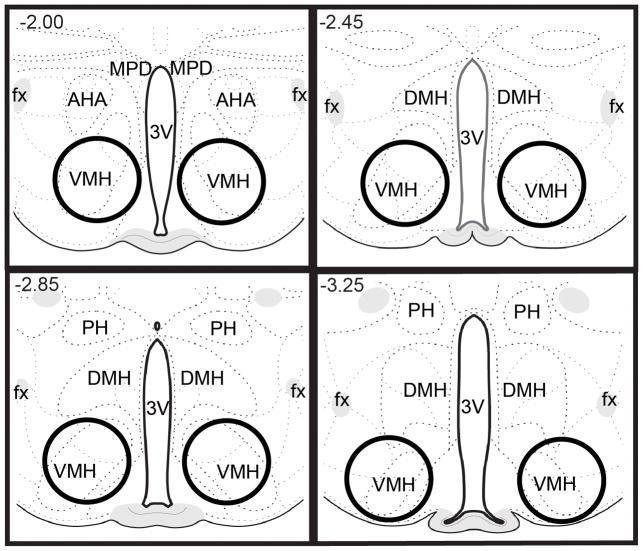
Male rats were randomly divided into four groups: Naïve No Sex, NNS; Naïve Sex, NS; Experienced No Sex, ENS; and Experienced Sex, ES. Males in the ENS and ES groups were paired with receptive females for 30 min on five different occasions over a two week period. Sexual experience sessions began at 1:00 p.m.. In order to test the acute effects of copulation in first time copulators and sexually experienced animals, males in the NS and ES groups were transferred to a testing room, paired with receptive females, and allowed to copulate to one ejaculation. After achieving an ejaculation, the female was removed from the male’s home cage. One hour after ejaculation, males were deeply anesthetized with sodium pentobarbital, decapitated, and their brains were removed. Males in the NNS and ENS groups were also transferred to a testing room, but they were not paired with a receptive female; an hour later they were anesthetized with sodium pentobarbital, decapitated, and their brains were removed. Fresh brains were immediately flash frozen with 2-methyl butane, then stored in aluminum foil in a −80° C freezer. Using a cryostat, brains were sliced, and bilateral tissue punches were taken from the MPOA and ventromedial hypothalamus (VMH) from approximately 4 1-mm thick sections using a Harris Unicore sampling tool. Fig. 1 shows approximate location of tissue punches taken from the MPOA; Fig. 2 shows approximate location of tissue punches taken from the VMH. Tissue from two subjects that received the same treatment were pooled. For the RT-PCR experiment, 24 males were randomly divided into the 4 above mentioned groups. For the western immunoblotting experiment, 32 males were also randomly divided into those 4 groups.
Figure 1.
Left side of 3rd ventricle: approximate placements of guide cannulae for intra-MPOA injections of oxytocin; the solid lines represent guide cannulae ending just above the MPOA, while the dotted lines indicate the range of placements for the bottom tip of injector cannulae within the MPOA. Right side of 3rd ventricle: approximate size of tissue punches from MPOA for analysis of OTR mRNA and protein. AC, anterior commissure; AHA, anterior hypothalamic area; MPN, medial preoptic nucleus; MPOA, medial preoptic area; OC, optic chiasm; OPT, optic tract; 3V, 3rd ventricle. Images adapted and modified from Swanson (2004), with permission from Elsevier.
Figure 2.
Approximate size of tissue punches from VMH for analysis of OTR protein. AHA, anterior hypothalamic area; DMH, dorsomedial hypothalamic nucleus; fx, fornix; PH, posterior hypothalamic nucleus; 3V, 3rd ventricle. Images adapted and modified from Swanson (2004), with permission from Elsevier.
2.3 RT-PCR with TaqMan Probe
Tissue was homogenized in lysis buffer, and total RNA was extracted using a High Pure RNA tissue kit (Roche). Complimentary DNA synthesis was performed on total RNA using a transcriptor first strand cDNA Synthesis kit (Roche). RNA used for RT-PCR was taken from samples that were used to generate microarray probes. Oligo (dT) primer (500 ng) was added to RNA (1 μg ); this template primer mixture was denatured by heating the tube for 10 min at 65°C and then chilled on ice for 2 min. Transcriptor Reverse Transcriptase reaction buffer (5x), Protector RNase Inhibitor (20 units) dNTPs mix 10mM each, and Transcriptor Reverse Transcriptase (10 units) were then added. The 20 μl reaction was incubated for 30 min at 55°C followed by a final incubation at 85°C for 5 min for termination. The resulting cDNA product was quantified, and 25 ng of product was used in each subsequent RT-PCR reaction. Quantitative PCR was carried out on a real-time detection instrument (ABI 7500) in 96-well optical plates using Fast Start TaqMan probe Master (Rox). Results were normalized against an endogenous control, or housekeeping gene. Delta Ct was calculated by subtracting the Ct of the housekeeping gene (beta actin; forward primer: 5′-CCCGCGAGTACAACCTTCT, reverse primer: 5′-CGTCATCCATGGCGAACT) from the Ct of the gene of interest (OTR; forward primer: 5′-AGCGTTTGGGACGTCAAT, reverse primer: 5′-GTTGAGGCTGGCCAAGAG). Fold changes in different groups were calculated by using the ΔΔCT values in a formula 2−ΔΔCT.
2.4 Western Immunoblotting
Tissue was homogenized in RIPA Buffer [50 mM Tris (pH=7.4),100 mM NaCl, 2.5 mM EDTA, 1% Triton x-100, 0.5% NP-40, 2.5 mM Na3VO4, 1 mM phenylmethylsulfonyl (PMSF), 25 ug/ml aprotinin and leupeptine], incubated on ice for 30 min, and then centrifuged for 10 min at 1,200 g. The supernatant was removed and stored in the −80°C freezer. After determining protein concentrations for all samples, using a spectrophotometer, equal amounts of protein from each group were loaded onto a 10% SDS-polyacrylamide gel. Gel electrophoresis was performed at 100 V for approximately 90 min. The fractionated protein was transferred to a polyvinylidene difluoride (PVDF) membrane, which was then rinsed in Tris buffered saline + 0.1% Tween-20 (TBST) for 10 min, followed by 60 min in super block T20 (TBS). The membrane was incubated overnight in 4°C with the anti-oxytocin receptor antibody (C-20: Santa Cruz Biotechnology; 1:1000). The next morning, the membrane was incubated with the secondary antibody (donkey anti-goat horseradish peroxidase-linked; Pierce Biotechnology; 1:2000) for 1 h at room temperature. This was followed by 3 rinses in super block for 10 min each. The membrane was stripped and then probed with beta actin antibody as an internal control. Membrane signal was enhanced with a chemiluminescence kit (ECL; Amersham Biosciences, Piscataway, NJ) for 2 min, and developed on Kodak BioMax film. Films were exposed for an appropriate amount of time, digitally scanned and optical densities measured using NIH Image-J software.
2.5 Stereotaxic surgeries
Twenty-five sexually naive male rats were anesthetized with ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (4 mg/kg), and then implanted with a 23g stainless steel guide cannula ending 1 mm above the MPOA. Stereotaxic coordinates (from bregma, AP +2.1; ML +.4; DV -.65 from dura) used for the anterior MPOA were adapted from Pellegrino et al., (1979). The guide cannula was secured in place by stainless steel screws and dental acrylic, and a stainless steel stylet was inserted into the guide cannula to maintain patency.
2.6 Oxytocin microinjections and copulation tests
Behavioral tests started approximately one week after surgery and occurred between 12.00 and 17.00 h. Males were randomly divided into three groups that received saline, 8 ng OT (Bachem Americas, Inc.), and 12.5 ng OT. Each male was taken from the animal colony to another room. The stylet was removed and replaced with an injection cannula, which was 1 mm longer than the guide cannula. Each animal received 0.5 μl of solution (drug or saline) into the MPOA, administered at a rate of 0.5 μl/min using a Harvard infusion pump. The injection cannula was left in place for 1 min to allow for adequate diffusion of solution and was then replaced with the stylet. The animal was then returned to its home cage and taken to a testing room. A receptive stimulus female was introduced into the male’s home cage approximately 5 min after the microinjection.
Each test lasted for 30 min after the male’s first intromission, or for 30 min after introduction of the female if no intromission occurred. The following measures were recorded: frequency of anogenital investigation (AGI) during the first 5 min of the 30-min copulation test, latency to first mount (ML), latency to first intromission (IL), latency from first intromission to first ejaculation (EL), postejaculatory interval (PEI, period of quiescence between first ejaculation and subsequent intromission), mount frequency preceding first ejaculation (MF1), mount frequency preceding second ejaculation (MF2), total mount frequency for 30-min test (MFT), intromission frequency preceding first ejaculation (IF1), intromission frequency preceding second ejaculation (IF2), total intromission frequency for 30-min test (IFT), intromission ratio for first copulatory series [IR1, IR1 = IF1/(IF1 + MF1)], intromission ratio for second copulatory series [IR2, IR2 = IF2/(IF2 + MF2)], intromission ratio for 30 min test [IRT, IRT = IFT/(IFT + MFT)], inter-intromission interval for the first copulatory series (III, III = EL/IF1), and total ejaculation frequency for the 30-min test (EF).
2.7 Statistics
A two-way ANOVA, with previous sexual experience and copulation on day of sacrifice as factors, was used to identify significant main effects and/or interactions. A one-way between-subjects ANOVA followed by a post hoc Tukey’s test was used to compare behavioral effects of OT in naïve animals. A chi-square analysis was also used to compare differences among groups in the incidence of mounting, intromitting, and ejaculating during a 30 min copulation test. Statistical significance was defined as p ≤ 0.05.
3.0 Results
3.1 Association between sexual experience and OTR mRNA expression in the MPOA
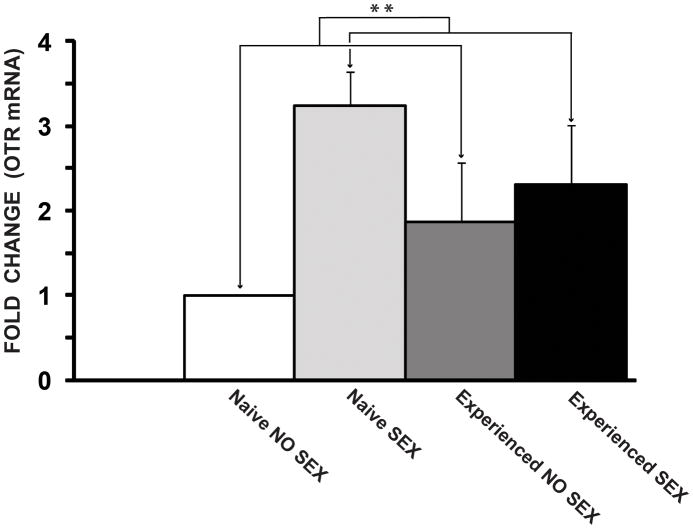
There was a significant main effect of copulation on the day of sacrifice on relative expression of the OTR gene (F(1,8) = 10.13, p < 0.01; Fig. 3). Copulation increased OTR mRNA levels in both first time copulators (NS) and experienced males (ES), compared to sexually naïve males (NNS) and experienced males that did not copulate (ENS). The highest OTR mRNA levels were observed in first time copulators, although this comparison was not statistically significant. There was no effect of previous sexual experience, and there was no interaction between previous experience and copulation on the day of sacrifice.
Figure 3.
Copulation (i.e., acute effects of sexual activity) increases OTR mRNA levels in the MPOA. Values represent expression levels of the OTR gene normalized to beta actin and expressed relative to the control group (NNS), which was set to 1. Values are given as mean (±SEM). NNS= Naïve, no sex; ENS= Experienced, no sex; NS= Naïve, sex; ES= Experienced, sex. p< 0.01**.
3.2 Association between sexual experience and OTR protein levels in the MPOA and VMH
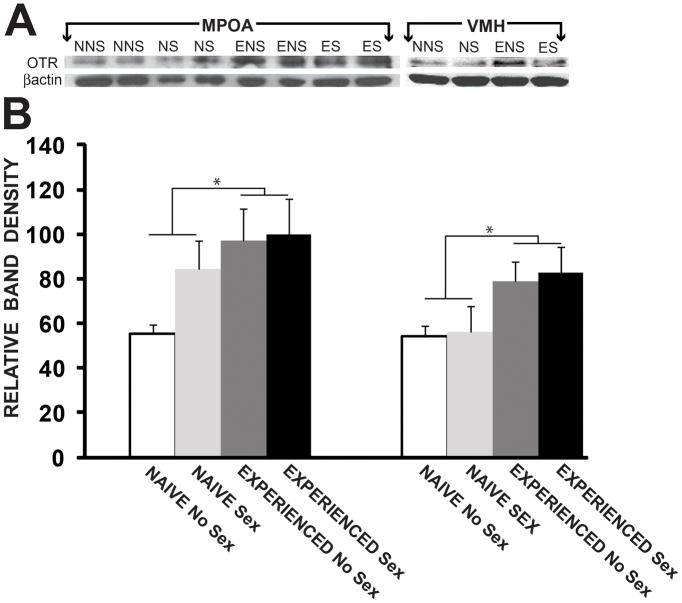
There were significant main effects of previous sexual experience on levels of OTR protein in both the MPOA (F(1,12) = 4.96, p < 0.05; Fig. 4) and the VMH (F(1,8) = 6.62, p< 0.05). Previous sexual experience increased OTR protein levels in the MPOA and VMH of experienced males (ENS and ES), compared to sexually naïve males and first time copulators (NNS and NS). There were no effects of copulation on the day of sacrifice, and there were no interactions between previous experience and copulation on the day of sacrifice.
Figure 4.
Previous sexual experience increases OTR protein levels in the MPOA. Values represent relative band density and are given as mean (±SEM). NNS, naïve, no sex; ENS, experience, no sex; NS, naïve, sex; ES, experienced, sex. MPOA, medial preoptic area; VMH, ventromedial hypothalamus. p< 0.05*.
3.3 Effects of intra-MPOA OT on copulation in sexually naive male rats
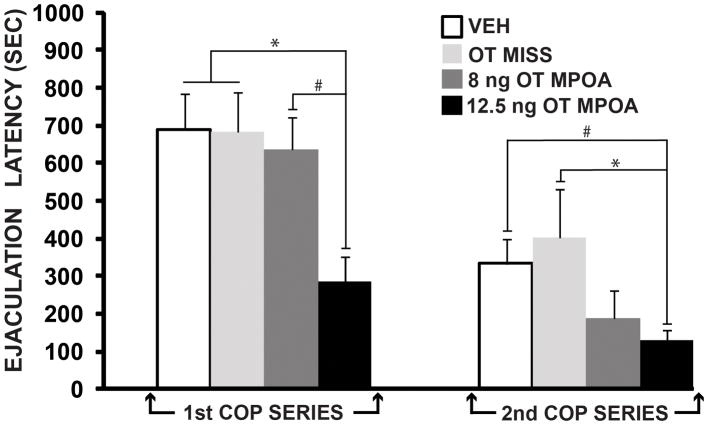
Of the initial 25 cannulated naive males, 4 saline-treated animals, 8 of the 12.5 ng OT-treated animals, and 5 of the 8 ng OT-treated animals had correct cannula placements in the MPOA. Figure 1 shows approximate placement of guide cannulae for intra-MPOA injections of OT. Three saline-treated animals, 3 of the 8 ng OT-treated males, and 2 of the 12.5 ng OT-treated animals had misplaced cannulae. Data from all saline-treated animals (hits and misses) were combined into one group (VEH, n = 7). As an additional control, data from animals that received 8 or 12.5 ng OT outside of the MPOA were combined into a second group (OT MISS, n = 5). Fourteen of the 25 males copulated to at least one ejaculation. There was a significant difference among groups for the 1st ejaculation latency (F(3, 15) = 6.00, p < 0.01; Fig. 5). Tukey post hoc comparisons revealed a significant decrease in ejaculation latency in males that received 12.5 ng of OT (n = 7), compared to those that received saline (n = 6; p < 0.01) or OT outside of the MPOA (n = 3; p < 0.05), and there was a trend for a decrease compared to the 8 ng OT group (n = 4; p = 0.08). There was also a significant effect of OT on the 2nd ejaculation latency (F(3,13) = 4.29, p < 0.05; Fig. 5). Tukey post hoc comparisons revealed significantly shorter latencies in males that received 12.5 ng of OT (n = 7), compared to those that received OT outside of the MPOA (n = 3; p< 0.05), and a trend for a decrease compared to the saline group (n = 4; p = 0.10). The chi-square analysis revealed that the incidence of males achieving 3 or more ejaculations was significantly higher for males that received 12.5 ng of OT, compared to males that received saline, 8 ng of OT, or OT outside of the MPOA (X2(3, N = 25) = 13.28, p < 0.01). In fact, 5 of the 8 males that received 12.5 ng of OT achieved 3 or more ejaculations during the 30 min copulation test; none of the males in the other 3 groups achieved that many ejaculations.
Figure 5.
Oxytocin microinjected into the MPOA shortens the 1st and 2nd ejaculation latencies in sexually naïve male rats. Values are given as mean (±SEM). Bars with different symbols indicate a significant difference between groups. VEH, vehicle; MPOA, medial preoptic area; OT, oxytocin; COP, copulatory; SEC, seconds. p< 0.01*, p<0.10#.
4.0 Discussion
4.1. Sexual experience increases OTR mRNA expression and protein in the MPOA
The hypothesis that sexual experience is associated with increased OTR activity in the MPOA is supported by the results of the first two experiments. Copulation (NS, ES) stimulated OTR gene expression in the MPOA in male rats. The acute effects of copulation were somewhat more pronounced in first time copulators (NS). However, given the relatively low sample sizes and that the stress associated with handling and transporting animals from one room to another (as well as the novelty of sexual activity for first-time couplators) may have influenced OTR expression, our results showing a trend toward higher levels of OTR mRNA in first-time copulators is quite remarkable. Animals with previous sexual experience (ENS and ES) had higher OTR protein levels in the MPOA and VMH, compared to sexually naive males (NS and NNS). These data coincide well with previous reports that female reproductive behaviors are associated with OTR activity in the MPOA. Taken together, the results of the present study and previous findings in females suggest that the expression of reproductive behaviors in both sexes may be dependent, in part, on enhanced OTR gene expression and protein in the MPOA.
It should be noted, however, that these data are correlational and should be interpreted with caution. Although sexual experience improves copulatory efficiency and increases OTR gene expression and protein in the MPOA, this increased OTR expression may not play a role in experience-induced facilitation of copulation. This, however, seems unlikely when one considers the pharmacology data. In the present study, OT in the MPOA facilitated copulation in sexually naive males. Therefore, collectively, our data suggest a reciprocal interaction, in which OT in the MPOA facilitates copulation, and sexual experience enhances OTR expression in the MPOA.
4.2. MPOA OT facilitates the onset and rate of copulation, improves sexual efficiency, and facilitates ejaculation
A large body of evidence indicates that the MPOA is a major site of integration for male sexual behavior in all vertebrate species studied thus far (reviewed in Hull and Rodriguez-Manzo, 2009). The MPOA receives chemosensory information from the medial amygdala and bed nucleus of the stria terminalis, and genital somatosensory information via the subparafascicular nucleus of the thalamus; it is reciprocally connected with all major sensory modalities, which enables the MPOA not only to receive sensory information but also to influence the processing of that information (Simerly and Swanson, 1986; Simerly and Swanson, 1988). Thus, one major function of the MPOA may be to receive and organize sexually relevant sensory information and facilitate appropriate behavioral output.
We previously reported that intra-MPOA OTA decreases anogenital investigation (AGI) and delays the initiation of copulation in sexually experienced male rats (Gil et al., 2011). Intra-MPOA OTA administration also reduces sexual efficiency following the first ejaculation. Interestingly, in female rats, OT in the MPOA stimulates the onset of maternal behavior and is associated with LG behavior toward pups (Numan and Stolzenberg, 2009; Pedersen et al., 1994). These results suggest that OTR activation in the MPOA facilitates the initiation of reproductive behavior in both males and females.
We also reported that intra-MPOA injection of OT improves sexual efficiency by increasing the intromission ratio (IF/MF+IF) in experienced animals; conversely, intra-MPOA injection of an OTA decreases the intromission ratio following the first ejaculation (Gil et al., 2011). These data suggest that OT in the MPOA increases sexual efficiency. Intra-MPOA injection of OT also facilitates ejaculation and decreases the postejaculatory interval in sexually experienced males (Gil et al., 2011). In the present study, we found that intra-MPOA injection of OT decreased the latencies to the first and second ejaculations in sexually naive males. In summary, our data suggest that MPOA OT promotes the initiation of copulation, facilitates the rate of copulation, improves sexual efficiency, and facilitates ejaculations. And the effects of MPOA OT on AGI, initiation of copulation, sexual efficiency following the first ejaculation, and facilitation of ejaculations are mediated, at least in part, by the OTR.
4.3. OT may interact with other neurotransmitter systems to facilitate natural motivated behaviors
OT interacts with other neurotransmitters in several brain areas to facilitate behavior. OT in the ventral tegmental area (VTA) and paraventricular nucleus (PVN) stimulates the mesolimbic DA system and genital function, and DA stimulates OT neurons in the PVN (Argiolas and Melis, 2004; Melis et al., 2007; Melis and Argiolas, 2011; Succu et al., 2008). It seems reasonable that DA and OT may interact in the MPOA to facilitate male reproductive behavior. DA in the MPOA is a major contributor to male sexual behavior in rats (reviewed in Hull and Rodriguez-Manzo, 2009). Therefore, OT may facilitate copulation in both sexually naïve and experienced male rats, in part, by stimulating DA release and/or facilitating DA receptor activity in the MPOA. OT can also interact with nitric oxide (NO)-dependent mechanisms. OT administered into the PVN elicits penile erections, mediated in part by stimulation of NO synthase (NOS); NO apparently acts as an intracellular messenger, stimulating OT neurons that project to other brain areas (Argiolas and Melis, 2004). NO in the MPOA also promotes mating in male rats, at least in part by stimulating DA release (Sato and Hull, 2006). Furthermore, sexual experience increases NOS in the MPOA (Dominguez et al., 2006), and a NOS inhibitor, microinjected into the MPOA, inhibits copulation in both sexually naive and experienced male rats (Lagoda et al., 2004)
In addition to facilitating the consummatory phase of natural motivated behaviors like copulation, OT may interact with central dopamine to facilitate motivation and/or incentive learning. For example, partner preference formation in female prairie voles induced by a D2-like receptor agonist is blocked by an OTA in the nucleus accumbens (NAc), and OT-induced partner preference formation is blocked by a D2-like receptor antagonist (Liu and Wang, 2003). Behavioral sensitization to d-amphetamine facilitates both the appetitive and consummatory aspects of male sexual behavior in sexually naïve rats (Fiorino and Phillips, 1999), and a similar facilitation of maternal behavior and maternal aggression occurs following cocaine pretreatment in female rats (Nephew and Febo, 2010). Interestingly, blocking D1 receptors in the MPOA inhibits stimulus sensitization, the enhancement of mating ability after repeated noncopulatory exposures to a receptive female in male rats (McHenry et al., 2012). Thus, in addition to the mesolimbic reward system, the hypothalamus also appears to play an important role in dopamine-mediated facilitation of natural motivated behaviors, and our results raise the possibility that the experience-induced increase in OTR expression in the MPOA may contribute to stimulus sensitization and/or the experience-induced enhancement of sexual reward.
4.4. Individual differences and OTR levels
The relationship between central OT and maternal behavior in female rats has been well documented. OTRs increase in the MPOA during lactation, and an OTA microinjected into the MPOA decreases arched-back nursing (reviewed in Bosch and Neumann, 2012). Lactation-induced OTR levels are higher in high LG mothers than in low LG mothers (Francis et al., 2000; Shahrokh et al., 2010). In addition, females that are more maternally responsive to pups have higher OTR levels in the amygdala, BNST, and MPOA compared to mothers that are less responsive (Champagne et al., 2001). The high vs. low LG behavioral phenotype can be transmitted from mother to offspring, at least partially by the mother’s behavior toward her offspring. In addition, postweaning environmental enrichments can increase OTR binding in the MPOA of pups raised through weaning by low LG dams, as well as increasing the daughters’ LG and exploratory behaviors (Champagne and Meaney, 2007). Postweaning isolation had the opposite effects on daughters of high LG females. Thus, OTR binding in the MPOA reflects both the maternal and exploratory behaviors of the offspring of high and low LG dams.
In a previous study we explored the relationship between male sexual behavior and OTR binding in the MPOA. We found that, in sexually experienced animals, sexually efficient male rats have lower levels of OTR binding in the rostral MPOA compared to inefficient animals (Gil et al., 2011). This finding seems inconsistent with the results of the present study and seems to undermine our hypothesis that OT and OTR stimulation in the MPOA facilitate male sexual behavior. However, as we previously suggested, the inverse relationship between sexual efficiency and OTR binding may be due to internalization or transcriptional suppression of OTRs in the MPOA of efficient copulators, a possible response to higher levels of OT in the rostral MPOA, compared to inefficient males. There is also evidence that high levels of OT in lactating female rats significantly reduce OTR binding in the PVN (Freund-Mercier et al., 1994). It should be noted, however, that in our previous study the inverse relationship between sexual efficiency in experienced males and OTR binding was detected only in the rostral MPOA. More research is needed to determine whether correlations exist between sexual efficiency and OTR binding in other areas of the MPOA.
Though increased central OT neurotransmission in the MPOA is associated with higher levels of pup-directed behaviors in females and copulation in males, this positive relationship may not exist between other types of reproductive behaviors and the neural substrates that influence and/or regulate them. For example, multiparous female rats are more aggressive than less-experienced, primiparous females, and this enhanced aggression by multiparous females is associated with decreased OT and OTR gene expression in the PVN, compared to less-experienced females (Nephew et al., 2009). Clearly, when investigating the role of central OT in the regulation of behavior, it is important to keep in mind that OT may influence different aspects of reproductive behavior in different ways. Therefore, more research is needed to identify the specific role of the OT/OTR system within various nodes of highly inter-connected neural circuits (e.g., social behavior and reward systems) that regulate reproductive behavior.
4.5. Summary and implications
In summary, copulation increased OTR mRNA in the MPOA of both first-time copulators and experienced males; there was no effect of previous experience, though the relatively small sample sizes and the stress associated with handling and transport may have influenced the effects of experience on OTR mRNA expression. Previous experience did increase OTR protein in both the MPOA and VMH, with no effect of copulation on the day of sacrifice. Thus, the increased mRNA with copulation is effective in increasing protein synthesis. In addition, OT microinjected into the MPOA of naïve males decreased the latencies of both the first and second ejaculations and increased the number of males exhibiting three or more ejaculations. As noted above, OT is important for numerous social functions, including pair bonding in prairie voles, maternal behavior in rats, social trust in humans, and stress buffering and anxiolysis, as well as sexual behaviors, in both humans and nonhuman mammals.
The use of OT in conjunction with behavioral-based therapies is starting to yield promising results in the treatment of mental disorders that are characterized by social dysfunction (Meyer-Lindenberg et al., 2011). However, more research is needed to identify the neural substrates and mechanisms of action for the potential therapeutic effects of OT on behavior, as well as the effects of behavior on central OTR expression. Our present and previous findings, in addition to reports by other research groups, suggest that sexual inefficiency is linked to central OT dysfunction, and this type of sexual inefficiency in rodents may serve as a useful model for sexual dysfunction in humans and cases of autism that are also characterized by central OT dysfunction. Our data also suggest that in males with reduced levels of central OT production, sexual experience may compensate for this reduction by increasing the number of OTRs in the MPOA and other brain areas, which may improve sexual efficiency. Thus, male sexual behavior is an ideal model for investigating the therapeutic effects of OTR and behavioral-based treatments that may be mediated by changes in OTR activity, as opposed to vasopressin receptors that also bind OT. Moreover, the hypothalamus should figure prominently in future investigations of OT regulation of behavior, as it is rich in OT neurons and is a major site of action for the facilitative effects of OT on behavior.
Acknowledgments
This work was supported by NIH grant MH040826 to EMH and was part of the PhD dissertation of MG.
Footnotes
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Contributors
Dr. Mario Gil designed and participated in all experiments, and also wrote the first draft of this manuscript. Ms. Katie Picotte participated in behavioral experiments. Dr. Renu Bhatt wrote the protocols for western immunoblotting and real-time PCR. Dr. Elaine M. Hull supervised all experiments, and also contributed significantly to the preparation and editing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argiolas A, Melis MR, Gessa GL. Intraventricular oxytocin induces yawning and penile erection in rats. Eur J Pharmacol. 1985;117:395–6. doi: 10.1016/0014-2999(85)90018-4. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Collu M, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur J Pharmacol. 1988;149:389–92. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol Behav. 2004;83:309–17. doi: 10.1016/j.physbeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bazzani C, Castelli M, Bertolini A. Oxytocin improves male copulatory performance in rats. Horm Behav. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Oxytocin involvement in male and female sexual behavior. Ann N Y Acad Sci. 1992;652:180–93. doi: 10.1111/j.1749-6632.1992.tb34354.x. [DOI] [PubMed] [Google Scholar]
- Arletti R, Calza L, Giardino L, Benelli A, Cavazzuti E, Bertolini A. Sexual impotence is associated with a reduced production of oxytocin and with an increased production of opioid peptides in the paraventricular nucleus of male rats. Neurosci Lett. 1997;233:65–8. doi: 10.1016/s0304-3940(97)00478-3. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Allard J, Wayman C, Douglas AJ. Dopamine-oxytocin interactions in penile erection. Eur J Neurosci. 2009;30:2151–64. doi: 10.1111/j.1460-9568.2009.06999.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Prange AJ, Jr, Pedersen CA. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986;7:175–89. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Binik YM, Pfaus JG. Chronic fluoxetine inhibits sexual behavior in the male rat: reversal with oxytocin. Psychopharmacology (Berl) 1999;144:355–62. doi: 10.1007/s002130051018. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, Meddle SL. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J Neuroendocrinol. 2011;23:1113–24. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353–63. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Brann JH, Gil M, Hull EM. Sexual experience increases nitric oxide synthase in the medial preoptic area of male rats. Behav Neurosci. 2006;120:1389–94. doi: 10.1037/0735-7044.120.6.1389. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior in male rats following d-amphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1999;142:200–8. doi: 10.1007/s002130050880. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994;480(Pt 1):155–61. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M, Bhatt R, Picotte KB, Hull EM. Oxytocin in the medial preoptic area facilitates male sexual behavior in the rat. Horm Behav. 2011;59:435–43. doi: 10.1016/j.yhbeh.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiat. 2008;64:256–8. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiat. 2003;54:1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiat. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hull EM, Rodriguez-Manzo G. Male sexual behavior. In: Pfaff DW, editor. Hormones, Brain, and Behavior. Vol. 1. Elsevier Press; Amsterdam: 2009. pp. 5–65. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoda G, Muschamp JW, Vigdorchik A, Hull EM. A nitric oxide synthesis inhibitor in the medial preoptic area inhibits copulation and stimulus sensitization in male rats. Behav Neurosci. 2004;118:1317–23. doi: 10.1037/0735-7044.118.6.1317. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–44. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacol. 2011;36:2159–68. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Dramatic improvement in sexual function induced by intranasal oxytocin. J Sex Med. 2012;9:1407–10. doi: 10.1111/j.1743-6109.2012.02703.x. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Bell GA, Parrish BP, Hull EM. Dopamine D1 receptors and phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in the medial preoptic area are involved in experience-induced enhancement of male sexual behavior in rats. Behav Neurosci. 2012;126:523–9. doi: 10.1037/a0028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Argiolas A, Gessa GL. Oxytocin-induced penile erection and yawning: site of action in the brain. Brain Res. 1986;398:259–65. doi: 10.1016/0006-8993(86)91485-x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Spano MS, Succu S, Argiolas A. The oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin reduces non-contact penile erections in male rats. Neurosci Lett. 1999;265:171–4. doi: 10.1016/s0304-3940(99)00236-0. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–35. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Sanna F, Boi A, Argiolas A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur J Neurosci. 2009;30:1349–57. doi: 10.1111/j.1460-9568.2009.06912.x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Central control of penile erection: a re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci Biobehav R. 2011;35:939–55. doi: 10.1016/j.neubiorev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behav Neurosci. 2009;123:949–57. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Febo M. Effect of cocaine sensitization prior to pregnancy on maternal care and aggression in the rat. Psychopharmacology (Berl) 2010;209:127–35. doi: 10.1007/s00213-010-1777-z. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–43. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrin. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–50. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–71. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal Oxytocin Blocks Alcohol Withdrawal in Human Subjects. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Academic Press; New York: 1979. [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila GA, Gelez H, Afonso VM, Ismail N, Parada M. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch Sex Behav. 2012;41:31–62. doi: 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- Sato SM, Hull EM. The nitric oxide-guanosine 3′,5′-cyclic monophosphate pathway regulates dopamine efflux in the medial preoptic area and copulation in male rats. Neuroscience. 2006;139:417–28. doi: 10.1016/j.neuroscience.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–86. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–42. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–42. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Melis T, Boi A, Argiolas A, Melis MR. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacology. 2007;52:1034–43. doi: 10.1016/j.neuropharm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Cocco C, Melis T, Boi A, Ferri GL, Argiolas A, Melis MR. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur J Neurosci. 2008;28:813–21. doi: 10.1111/j.1460-9568.2008.06385.x. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Argiolas A, Melis MR. Oxytocin injected into the hippocampal ventral subiculum induces penile erection in male rats by increasing glutamatergic neurotransmission in the ventral tegmental area. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–50. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Witt DM, Insel TR. Increased Fos expression in oxytocin neurons following masculine sexual behavior. J Neuroendocrinol. 1994;6:13–8. doi: 10.1111/j.1365-2826.1994.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–7. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]