Abstract
Background
Physical health status measures have been shown to predict death in heart failure (HF); however, few studies found significant associations after adjustment for confounders and most were not representative of all HF patients.
Methods and Results
HF patients from southeastern Minnesota were prospectively enrolled between 10/2007 and 12/2010, completed a 12-item Short Form Health Survey (SF-12) and a 6-minute walk, and were followed through 2011 for death from any cause. Scores ≤25 on the SF-12 physical component indicated low self-reported physical functioning, and the first question of the SF-12 measured self-rated general health. Low functional exercise capacity was defined as ≤300 meters walked during a 6 minute walk. Over a mean follow-up of 2.3 years, 86 deaths occurred among the 352 participants. A 1.6-fold (95% CI 1.0-2.7) and 1.8-fold (95% CI 1.1-2.9) increased risk of death was observed among patients with low self-reported physical functioning and low functional exercise capacity, respectively. Poor self-rated general health corresponded to a 2.7-fold (95% CI 1.5-4.9) increased risk of death compared to good-excellent general health. All measures equally discriminated between who would die and who would survive (C-statistics: 0.729, 0.750, and 0.740 for self-reported physical functioning, self-rated general health, and functional exercise capacity, respectively).
Conclusions
Three physical health status measures, captured by the SF-12 and a 6 minute walk, equally predict death among community HF patients. Therefore, the first question of the SF-12, which is the least burdensome to administer, may be sufficient to identify HF patients at greatest risk of death.
Keywords: heart failure, mortality, survival, physical functioning, health status
Approximately 5.7 million U.S. adults are living with heart failure (HF), and the median survival among HF patients is only 5 years.1 Physical health status measures have been shown to predict survival among HF patients in several previous studies, although limitations involving the selection of patients and appropriate adjustment for confounding factors limits the ability to draw robust inference. For example, the 6 minute walk, which is an objective measure of physical health used widely in clinical practice, has been shown to predict mortality in HF patients.2, 3 However, these studies were restricted to patients with left ventricular systolic dysfunction, and were thus not representative of the case mix of patients presenting with HF in the community.4 Furthermore, only 1 reported significant associations after adjustment for confounders.2
In addition, self-reported measures of physical health have also been shown to predict mortality in some studies of HF patients, although the results are heterogeneous and not all report significant associations.5 A few cohort studies reported significant associations of physical health status measures with mortality even after multivariable confounder adjustment.6-8 However, many others reporting significant associations were restricted to patients with either severe9 or mild-moderate HF,10 patients hospitalized for HF-related reasons,11 or were among patients enrolled in clinical trials.12, 13 Furthermore, in many others, a significant association of physical health status with death was only apparent in univariate models, and no association was present after adjustment for important clinical predictors of mortality.3, 14-17
Since many studies examining associations of physical health status with survival were performed among subgroups of patients that are not representative of the general HF population and because of the lack of consistent association between physical health status measures and death after adjustment for important confounding variables, further work is warranted to address the utility of physical health status measures in predicting death among HF patients in the community. Thus, the goal of our study was to assess, within community HF patients from southeastern MN (including incident and prevalent HF, inpatients and outpatients, as well as those with both preserved and reduced ejection fraction), the association of various measures of physical health with all-cause mortality.
Methods
Study Setting
This study was conducted in southeastern Minnesota. This area of Minnesota is relatively isolated from other urban centers and only a few providers, including Mayo Clinic, Olmsted Medical Center, and a few other practices, deliver most health care to local residents. The Rochester Epidemiology Project, a record-linkage system, allows the indexing of medical records among residents in southeastern MN, thus enabling the retrieval of all health care related events occurring in this geographic area.18, 19 This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Identification of the Study Cohort
HF diagnoses from October 2007 through December 2010 were identified among residents of Olmsted, Dodge, and Fillmore Counties, MN using natural language processing of electronic medical records.20, 21 Prompt ascertainment of HF diagnoses was possible as documentation from a clinical visit is transcribed and available in the medical record within 24 hours of the encounter. HF diagnoses were validated by trained nurse abstractors by reviewing the medical records to verify that the Framingham criteria was met.22 Both incident and prevalent HF patients identified during either an inpatient or outpatient visit and who met the Framingham criteria were eligible for participation. Patients were then contacted to obtain consent for study participation and a follow-up visit was scheduled within 2 months to obtain a blood sample, an echocardiogram (if one was not available clinically within the past 6 months), a six-minute walk test, and to administer questionnaires related to physical and emotional well-being, social support, and heart failure symptom burden.
Physical Functioning Classification
Cohort participants completed a 12-item Short Form Health Survey (SF-12) and a 6 minute walk as part of the follow-up visit. Self-reported physical functioning was assessed using the physical component of the SF-12 and those scoring ≤25 were categorized as having low self-reported physical functioning, whereas scores >25 indicated moderate-high physical functioning. In addition, the first question of the SF-12 was used as a measure of self-rated general health. Responses to this first question, ‘In general would you say your health is,’ were categorized into poor, fair, and good-excellent general health. Finally, distance walked during a 6 minute walk defined functional exercise capacity as low (≤300 meters) and moderate-high (>300 meters). We excluded those who refused to do the 6 minute walk from the analysis, but those who could not perform the 6 minute walk due to their HF were included in the low category of functional exercise capacity.
Clinical Data Collection
Current (within the past 6 months) cigarette smoking status, marital status, and education were obtained from medical records. Body mass index (BMI) was calculated as weight (in kg) divided by height (in meters) squared. Information on comorbid conditions were abstracted from medical records and a score was calculated using the Charlson comorbidity index.23 A history of hypertension was defined as 2 or more ambulatory blood pressure readings ≥140 mmHg systolic and/or ≥90 mmHg diastolic, or a physician diagnosis of hypertension. Prevalent diabetes was defined according to the American Diabetes Association criteria.24 A clinical diagnosis documented in the medical record identified those with hyperlipidemia, depression, chronic obstructive pulmonary disease (COPD), or a previous myocardial infarction (MI). Treatments for heart failure, including use of statins, beta blockers, and angiotensin converting enzyme inhibitors or angiotensin II receptor blockers were manually abstracted from the medical records.
The closest serum sodium, serum creatinine, and hemoglobin values within 1 year of the HF date were obtained. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease Study (MDRD) equation.25 Anemia was defined by WHO criteria,26 as hemoglobin <13mg/dL in men and <12mg/dL in women. Resting left ventricular ejection fraction (LVEF, %) was determined using values collected from any echocardiogram performed within 6 months prior or 2 months after study enrollment.
Ascertainment of All-Cause Mortality
Participants were followed through December 31, 2011 for deaths from any cause. Deaths were obtained from inpatient and outpatient medical records and death certificates obtained on a yearly basis from the state of Minnesota. In addition, the Mayo Clinic registration office records the obituaries and notices of death in the local newspapers.
Statistical Analysis
Statistical analyses were performed using SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC) and Splus® statistical software, version 8 (TIBCO Software, Inc., Palo Alto, CA). Differences in baseline participant characteristics between low and moderate-high self-reported physical functioning were compared using 2-sample t-tests for normally distributed continuous variables, Wicoxon rank-sum tests for non-normal continuous variables, and chi-square tests or Fisher's exact tests for categorical variables. Person-years of follow-up were calculated from HF date until death, last follow-up visit, or December 31, 2011, whichever came first.
Kaplan-Meier survival plots were constructed to illustrate the association of physical health status measures with all-cause mortality, and differences in survival curves by physical health status level were tested using the log-rank test. The crude rates of death were computed using Poisson regression. Cox proportional hazards regression was used to estimate associations of physical health status with all-cause mortality. Variable selection for the Cox models was implemented by including a set of variables determined as important confounders a priori (age, sex, ejection fraction, incident vs. prevalent HF) with the addition of other potential confounders after backward stepwise elimination using a p<0.10 cutpoint for significance. The fully-adjusted Cox model included adjustment for age, sex, Charlson comorbidity index (log transformed), LVEF (<50% vs. ≥50%), incident vs. prevalent HF, and depression. In addition, a sensitivity analysis was performed further adjusting for anemia, estimated glomerular filtration rate and medications (statins, beta blockers, and angiotensin converting enzyme inhibitors /angiotensin II receptor blockers). The proportional hazards assumption was tested using scaled Schoenfeld residuals and found to be valid.
The concordance (C-statistic), a measure of discrimination, was calculated for the fully-adjusted Cox regression models for each physical health status measure, and 95% confidence intervals for the C-statistics were estimated using approximate jackknife methods.27 To compare the discrimination of a model with 1 physical health status measure to a model with a different physical health status measure, we created 1,000 bootstrap samples, sampling individuals with replacement, and estimated a 95% confidence interval for the difference in concordance between the 2 models. Finally, C-statistics for a fully-adjusted Cox model including both self-reported physical functioning and functional exercise capacity and a second model including both self-rated general health and functional exercise capacity were calculated to determine whether a combination of physical health status measures better predicts death compared to an individual measure of physical health.
Results
A total of 902 HF patients were approached to participate in our study and 519 (58%) consented. Of the 519 HF patients who consented to our study between October 2007 and December 2010, 91 did not complete the questionnaire, 62 refused to do the 6 minute walk, and 14 had missing variables for the covariates in our fully-adjusted model. Thus, 352 cohort participants (mean age 72.7 years, 59.4% men) remained for analysis. Fifty (14%) patients had low self-reported physical functioning and 302 (86%) had moderate-high self-reported physical functioning. In addition, 45 (13%) reported poor, 107 (30%) reported fair, and 200 (57%) reported good-excellent general health based on responses to the first question of the SF-12. Finally, 155 (44%) had low and 197 (56%) had moderate-high functional exercise capacity.
HF patients reporting low physical functioning were more likely to be female, had a higher Charlson comorbidity index, were more likely to have depression, and had slightly higher LVEF compared to those with moderate-high self-reported physical functioning (Table 1). Thirty-four (68.0%) of those with low self-reported physical functioning had prevalent HF and 16 (32.0%) had incident HF. In those with moderate-high self-reported physical functioning, 165 (54.6%) had prevalent HF and 137 (45.4%) had incident HF.
Table 1.
Baseline Participant Characteristics by Self-Reported Physical Functioning
| Overall (N=352) | Low (N=50) | Moderate-High (N=302) | P-value | |
|---|---|---|---|---|
| Age at heart failure onset (years) | 76 (65-83) | 76 (66-84) | 76 (64-83) | 0.52 |
| Male | 209 (59.4) | 23 (46.0) | 186 (61.6) | 0.04 |
| Body mass index (kg/m2) | 29.9 (26.2-34.8) | 31.1 (27.8-36.4) | 29.7 (26.0-34.5) | 0.12 |
| Current smoking status | 28 (8.0) | 3 (6.0) | 25 (8.3) | 0.78 |
| Married | 215 (61.1) | 27 (54.0) | 188 (62.3) | 0.27 |
| >High school education | 164 (48.0) | 18 (37.5) | 146 (50.0) | 0.18 |
| Charlson comorbidity index | 3 (2-5) | 5 (3-7) | 3 (2-5) | <0.001 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 57.8 (22.7) | 54.5 (27.2) | 58.3 (21.9) | 0.30 |
| Serum sodium (mmol/L) | 140 (138-142) | 140 (138-142.6) | 140 (138-142) | 0.61 |
| Hyperlipidemia | 285 (81.0) | 41 (82.0) | 244 (80.8) | 0.84 |
| Hypertension | 318 (90.3) | 48 (96.0) | 270 (89.4) | 0.20 |
| Diabetes | 132 (37.5) | 24 (48.0) | 108 (35.9) | 0.10 |
| Myocardial infarction | 92 (26.1) | 17 (34.0) | 75 (24.8) | 0.17 |
| Chronic obstructive pulmonary disease | 90 (25.6) | 19 (38.0) | 71 (23.5) | 0.03 |
| Depression | 135 (38.4) | 26 (52.0) | 109 (36.1) | 0.03 |
| Ejection fraction <50% | 192 (54.5) | 23 (46.0) | 169 (56.0) | 0.19 |
| Prevalent heart failure | 199 (56.5) | 34 (68.0) | 165 (54.6) | 0.08 |
| Beta blockers | 293 (83.2) | 43 (86.0) | 250 (82.8) | 0.57 |
| Angiotensin converting enzyme inhibitors/angiotensin II receptor blockers | 240 (68.2) | 31 (62.0) | 209 (69.2) | 0.31 |
| Statins | 206 (58.5) | 29 (58.0) | 177 (58.6) | 0.94 |
Values are mean (SD) or median (25th, 75th percentile) for continuous variables and N (%) for categorical variables.
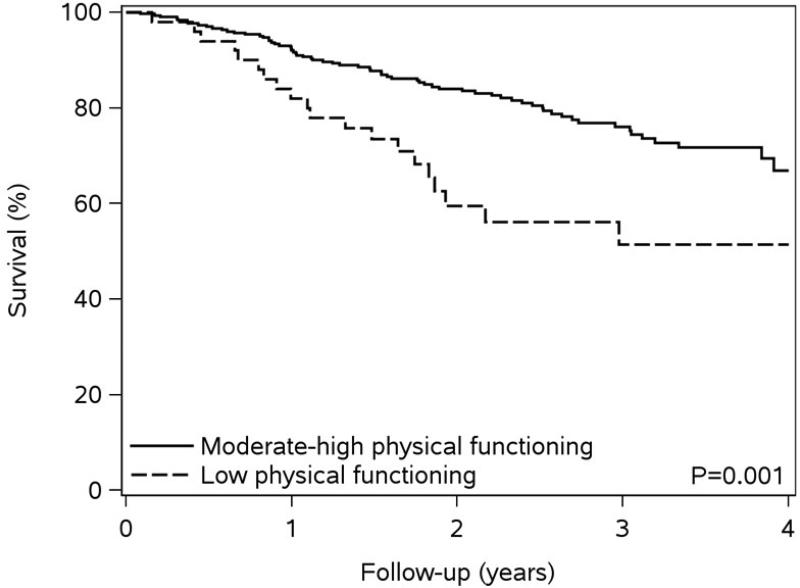
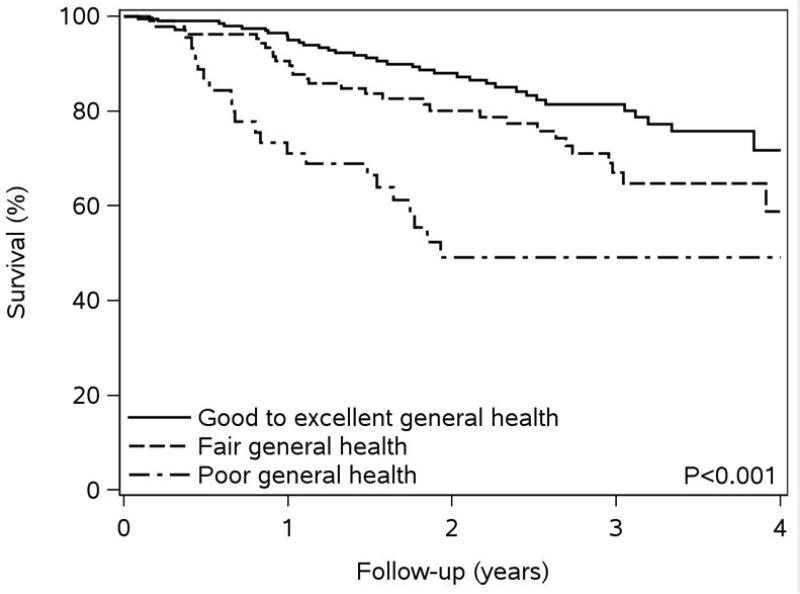
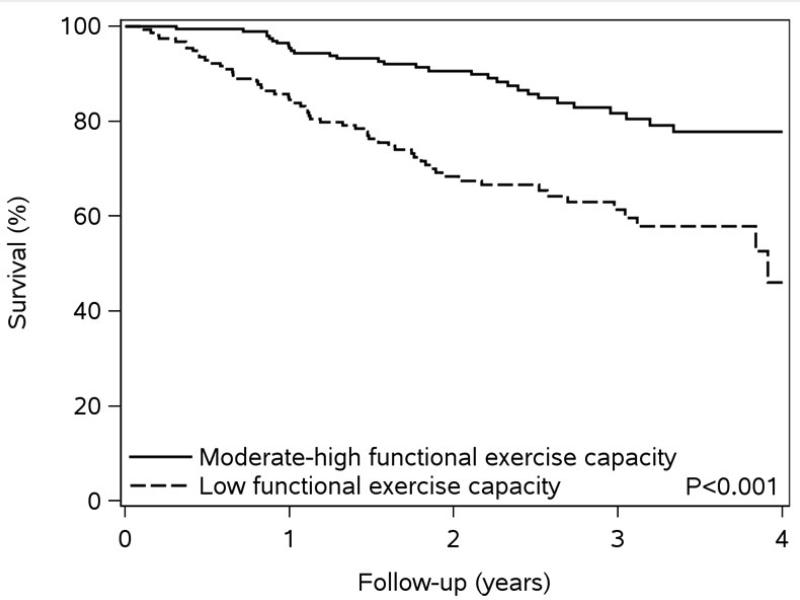
Over a mean follow-up of 2.3 years (maximum of 4.2 years), 86 deaths occurred. Low levels of self-reported physical functioning and functional exercise capacity and poor self-rated general health were associated with worse survival compared to moderate-high levels of physical functioning and exercise capacity and fair or good-excellent self-reported general health (Figure 1). The crude rates of death per 100 person-years, along with unadjusted and fully-adjusted hazard ratios for death also indicate that lower physical functioning measures are associated with a higher risk of death (Table 2). After adjustment, as compared to those with moderate-high self-reported physical functioning, a 1.64-fold (95% CI 0.99-2.74) increased risk of death was found among individuals with low self-reported physical functioning; a similar risk was found for individuals with low vs. moderate-high functional exercise capacity (HR 1.81, 95% CI 1.11-2.94). Furthermore, those who indicated poor self-rated general health exhibited a 2.73-fold (95% CI, 1.52-4.90) increased risk of death compared to those reporting good-excellent general health. Finally, results did not differ after further adjustment for anemia, estimated glomerular filtration rate, statins, beta blockers, and angiotensin converting enzyme inhibitors /angiotensin II receptor blockers for all 3 physical health status measures (data not shown).
Figure 1. Kaplan-Meier Survival Plots by Measure of Physical Health.
Panel A – Kaplan-Meier Survival by Self-Reported Physical Functioning
Panel B – Kaplan-Meier Survival by Self-Rated General Health
Panel C – Kaplan-Meier Survival by Functional Exercise Capacity
Physical health status has been shown to predict death in heart failure (HF) patients; however, community data are lacking. The goal of our study was to assess, within community HF patients (including incident and prevalent HF, inpatients and outpatients, as well as those with both preserved and reduced ejection fraction), the association of various measures of physical health with all-cause mortality. Three measures of physical health, including two self-rated measures based on the SF-12 and an objective measure based on a six minute walk test, independently predicted mortality. HF patients who scored ≤25 on the SF-12 physical component exhibited a 1.6-fold increased risk of death compared to those with scores >25. Patients who responded poor to the first question of the SF-12, ‘In general would you say your health is,’ had a 2.7-fold higher risk of death compared to those who answered good, very good, or excellent. Those who walked ≤300 meters during a six minute walk exhibited a 1.8-fold increased risk of mortality compared to those who walked >300 meters. In addition, all three physical health status measures equally discriminated between who would die and who would survive, and the use of two measures together did not improve the predictive ability beyond a single measure. Therefore, any of these measures may be useful in the management of HF. However, the administration of a single question asking a patient to rate their general health is the least burdensome, yet provides valuable information about the patient's current state of physical health.
Table 2.
Hazard Ratios (95% CI) for All-Cause Mortality by Measure of Physical Health
| N (%) | Death Rate† | Unadjusted HR (95% CI) | Adjusted* HR (95% CI) | |
|---|---|---|---|---|
| Self-Reported Physical Functioning | ||||
| Low | 50 (14) | 20.1 | 2.23 (1.35-3.68), p=0.002 | 1.64 (0.99-2.74), p=0.057 |
| Moderate-High | 302 (86) | 9.1 | 1.00 (ref) | 1.00 (ref) |
| Self-Rated General Health | ||||
| Poor | 45 (13) | 24.3 | 3.48 (2.03-5.99)‡ | 2.73 (1.52-4.90)§ |
| Fair | 107 (30) | 12.0 | 1.65 (1.02-2.69)‡ | 1.48 (0.89-2.46)§ |
| Good to Excellent | 200 (57) | 7.2 | 1.00 (ref) | 1.00 (ref) |
| Functional Exercise Capacity | ||||
| Low | 155 (44) | 17.0 | 2.81 (1.80-4.38), p=0.001 | 1.81 (1.11-2.94), p=0.017 |
| Moderate-High | 197 (56) | 6.1 | 1.00 (ref) | 1.00 (ref) |
Adjusted for age, sex, Charlson comorbidity index, ejection fraction, incident vs. prevalent heart failure, and depression.
Crude rate of all-cause mortality per 100 person-years.
P-value <0.001 for 2 degree of freedom test
P-value=0.005 for 2 degree of freedom test
Each measure of physical health, when added to a model with age, sex, Charlson comorbidity index, LVEF, incident vs. prevalent HF, and depression, had good discrimination for predicting death (Table 3). The C-statistics ranged from 0.729 for the model with self-reported physical functioning to 0.750 for the model with self-rated general health. None of the models were significantly different from each other as judged by the 95% confidence intervals for the difference between 2 models using bootstrapping methods (data not shown). This indicates that all 3 physical health status measures performed equally well in discriminating between those who would die and those who would survive. In addition, when 2 physical health status measures were considered together, the discrimination was very similar to that from each individual model (Table 3). For the model including both self-reported physical functioning and functional exercise capacity, the C-statistic was 0.743; for a model including both self-rated general health and functional exercise capacity, the C-statistic was 0.756.
Table 3.
C-Statistics (95% CI) for Cox Regression Models with Individual and Multiple Measures of Physical Health
| C-Statistic | 95% CI | |
|---|---|---|
| Individual Measures | ||
| Self-Reported Physical Functioning | 0.729 | 0.676-0.781 |
| Self-Rated General Health | 0.750 | 0.697-0.802 |
| Functional Exercise Capacity | 0.740 | 0.688-0.792 |
| Multiple Measures | ||
| Physical Functioning + Exercise Capacity | 0.743 | 0.691-0.794 |
| General Health + Exercise Capacity | 0.756 | 0.704-0.808 |
All models are additionally adjusted for age, sex, Charlson comorbidity index, ejection fraction, incident vs. prevalent heart failure, and depression.
Discussion
In this prospective study of HF patients in the community, 3 physical health status measures predicted all-cause mortality over a mean follow-up of 2.3 years. A 1.6-fold increased risk of death was found for individuals who scored low (≤25 points) on the SF-12 physical component and a 1.8-fold increased risk of death was observed among those who walked ≤300 meters during a 6-minute walk. In addition, compared to those rating their general health as good-excellent, those indicating poor health exhibited a 2.7-fold increased risk of all-cause mortality. Each of these physical health status measures, when added to a model with age, sex, Charlson comorbidity index, LVEF, incident vs. prevalent HF, and depression had equally good discrimination for predicting death. Most importantly, a single question asking a patient to rate his or her general health, which can be very easily administered, performed as well as an objective clinical measure often used in clinical practice, the 6 minute walk. However, the combination of 2 physical health status measures in 1 model did not improve the ability to discriminate who would die and who would survive over a single measure of physical health.
Self-Reported Measures of Health and Mortality
Self-rated measures of health have been shown to predict mortality independently of objective physical measures of health, indicating that patients may have knowledge about their health beyond what is objectively measured by their physician. In HF patients, a single question about perceived health included in the 36-item Short Form (SF-36) and SF-12 questionnaires, ‘In general would you say your health is,’ with responses of excellent, very good, good, fair, and poor, has been shown to be predictive of survival. Fair or poor and good self-rated perceived health were associated with 4.2-fold and 2.3-fold increased risks of all-cause mortality and 4.9-fold and 2.4-fold increased risks of cardiovascular mortality, respectively, compared to those reporting excellent or very good health.6
In addition, the physical component score of the SF-12 or SF-36 have also been predictive of death in 3 studies;11, 13, 16 however, after adjustment for confounders, only 1 of these studies still reported an association of physical functioning with death.13 In that study of patients enrolled in the MADIT II trial, patients scoring below the median on the physical component score of the SF-12 exhibited an 89% increased risk of death, whereas a 42% increased risk of death was observed for each 10-point decrease in the physical component score in multivariable models.13
Our results build on the previous findings adding evidence that measures of physical health are independently associated with all-cause mortality in active HF patients from the community. We observed, even after adjustment for important confounding factors such as demographics, comorbidities, LVEF, incident vs. prevalent HF, and depression, a 2.7-fold increased risk of death among those reporting poor general health compared to good-excellent general health and a 1.6-fold increased risk of death among individuals scoring ≤25 vs. >25 on the physical component of the SF-12. In addition, our study included both inpatients and outpatients, as well as those with both preserved and reduced ejection fraction, and is thus more representative of HF patients in general than most previous studies.
Objective Measures of Health and Mortality
Although self-rated measures of health have been shown to be more predictive of death28 and may also be more predictive of changes in health-related quality of life29 compared to objective measures of health, some objective measures of physical health have been shown to predict death in HF patients. Among HF patients with an LVEF <40%, a 30 meter increase in walk distance during a 6-minute walk was associated with a 16% reduction in death in univariate models; associations were similar after adjustment for age, New York Heart Association (NYHA) class, and C-reactive protein, although they became nonsignificant (HR 0.85, 95% CI 0.71-1.02).3 In the SOLVD Registry Substudy, a 3.7-fold and 2.8-fold increased risk was found among individuals walking <300 meters and between 300-375 meters, respectively, compared to those walking >450 meters during a 6-minute walk test.2 After adjustment for age, sex, cause of HF, NYHA class, and LVEF, a 50% increased risk of death with a decrement of 120 meters walked was reported.2 Within our cohort, a 1.8-fold increased risk of death was found among those who walked ≤300 meters compared to those who walked >300 meters. In addition, we found the 6-minute walk to be equally predictive of death as self-reported physical functioning and self-rated general health, even after controlling for demographics, comorbidity, LVEF, incident vs. prevalent HF, and depression.
Clinical Implications
Our results indicate that measures of physical health, including self-rated measures based on the responses to the SF-12 and an objective measure based on distance walked during a 6-minute walk test, independently predict all-cause mortality equally well in patients with HF. In particular, the independent associations of self-rated measures of health with mortality indicate that patients may have knowledge about their health above and beyond what can be measured by tests, the presence of comorbidities, and physician assessments of patients’ physical state. Thus, the addition of a self-rated measure of health in the management of HF patients may be a useful tool as an indicator of prognosis. The administration of a single question asking a patient to rate their general health, in particular, can be easily administered and is not burdensome to either the patient or the physician, yet provides valuable information about the patient's current state of physical health. This questionnaire data may be useful in streamlining clinical practice in the heart failure clinic, but may also be a useful addition to evaluation in the primary care setting where the use of the 6-minute walk test may be less routine.
Limitations and Strengths
There are some limitations to consider while interpreting these results. First, the relatively small sample size of our cohort and the small number of deaths that occurred among patients in our cohort affected the precision of our estimates and may have limited our ability to detect differences between the different physical health status measures or between a model with 2 physical health status measures compared to a model with only 1 measure of health status. In addition, these factors may have limited our ability to identify potential effect modifiers of the association between physical health status measures and all-cause mortality and to conduct stratified analyses by potential effect modifiers. Second, our study results may have been impacted by differences in patients willing to enroll in the study compared to those who refused. Third, deaths occurring outside of Minnesota may not have been captured; however, we expect very minimal misclassification of the outcome such that our results would not be affected. Finally, the vast majority of participants in our cohort were white and although the results may be generalizable to other whites throughout the U.S., it is possible that our results are not well generalizable to individuals of other race groups or ethnicities.
Our study also has several strengths. Our prospective cohort consists of active HF patients from the community (both inpatients and outpatients with either incident or prevalent HF, including those with both preserved and reduced ejection fraction) enrolled after rigorous validation of each HF event. After enrollment, participants completed the SF-12 and underwent a 6 minute walk, thus allowing the comparison of different physical health status measures, individually or in combination, in the prediction of all-cause mortality in community HF patients.
Conclusions
We have shown that 3 different measures of physical health, captured by responses to the SF-12 and distance walked during a 6 minute walk test, independently predict survival among community patients with HF. Each of these 3 measures had good discrimination and predicted death equally well. Moreover, the use of 2 physical health status measures did not improve predictive ability. Thus, any measure of physical health may be useful in the management of HF, but the first question of the SF-12, which is the least burdensome, may be sufficient to identify HF patients at greatest risk of death. Further research, including developing individual risk prediction models in large cohorts of HF patients, is warranted.
Supplementary Material
Acknowledgements
We thank Kay A. Traverse, RN for assistance in data collection, Jill M. Killian and Ruoxiang Jiang for assistance with statistical analysis, and Deborah S. Russell for secretarial assistance.
Sources of Funding
This work was supported by grants from the National Institutes of Health (R01 HL72435) and the National Institute on Aging (R01 AG034676). Dr. Roger is an Established Investigator of the American Heart Association. The funding sources played no role in the design, conduct, or reporting of this study.
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics--2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, Greenberg B, Woods PA, Bourassa MG. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 3.Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 5.Mommersteeg PMC, Denollet J, Spertus JA, Pedersen SS. Health status as a risk factor in cardiovascular disease: A systematic review of current evidence. Am Heart J. 2009;157:208–218. doi: 10.1016/j.ahj.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Johansson P, Brostrom A, Dahlstrom U, Alehagen U. Global perceived health and ten-year cardiovascular mortality in elderly primary care patients with possible heart failure. Eur J Heart Fail. 2008;10:1040–1047. doi: 10.1016/j.ejheart.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Kato N, Kinugawa K, Seki S, Shiga T, Hatano M, Yao A, Hirata Y, Kazuma K, Nagai R. Quality of life as an independent predictor for cardiac events and death in patients with heart failure. Circulation Journal. 2011;75:1661–1669. doi: 10.1253/circj.cj-10-1308. [DOI] [PubMed] [Google Scholar]
- 8.Murberg TA, Bru E, Svebak S, Tveteras R, Aarsland T. Depressed mood and subjective health symptoms as predictors of mortality in patients with congestive heart failure: A two-years follow-up study. Int J Psychiatry Med. 1999;29:311–326. doi: 10.2190/0C1C-A63U-V5XQ-1DAL. [DOI] [PubMed] [Google Scholar]
- 9.O'Loughlin C, Murphy NF, Conlon C, O'Donovan A, Ledwidge M, McDonald K. Quality of life predicts outcome in a heart failure disease management program. Int J Cardiol. 2010;139:60–67. doi: 10.1016/j.ijcard.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Farkas J, Nabb S, Zaletel-Kragelj L, Cleland JGF, Lainscak M. Self-rated health and mortality in patients with chronic heart failure. Eur J Heart Fail. 2009;11:518–524. doi: 10.1093/eurjhf/hfp038. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, Otero CM, Montes AO, Garcia AN, Conthe P, Chiva MO, Banegas JR, Herrera MC. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165:1274–1279. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 12.Konstam V, Salem D, Pouleur H, Kostis J, Gorkin L, Shumaker S, Mottard I, Woods P, Konstam MA, Yusuf S. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. Am J Cardiol. 1996;78:890–895. doi: 10.1016/s0002-9149(96)00463-8. [DOI] [PubMed] [Google Scholar]
- 13.Piotrowicz K, Noyes K, Lyness JM, McNitt S, Andrews ML, Dick A, Hall WJ, Moss AJ, Zareba W. Physical functioning and mental well-being in association with health outcome in patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial II. Eur Heart J. 2007;28:601–607. doi: 10.1093/eurheartj/ehl485. [DOI] [PubMed] [Google Scholar]
- 14.Alla F, Briançon S, Guillemin F, Juillière Y, Mertès P-M, Villemot J-P, Zannad F, the EPICAL Investigators Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail. 2002;4:337–343. doi: 10.1016/s1388-9842(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 15.Carson P, Tam SW, Ghali JK, Archambault WT, Taylor A, Cohn JN, Braman VM, Worcel M, Anand IS. Relationship of quality of life scores with baseline characteristics and outcomes in the African-American Heart Failure Trial. J Card Fail. 2009;15:835–842. doi: 10.1016/j.cardfail.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Faller H, Stork S, Schowalter M, Steinbuchel T, Wollner V, Ertl G, Angermann CE. Is health-related quality of life an independent predictor of survival in patients with chronic heart failure? J Psychosom Res. 2007;63:533–538. doi: 10.1016/j.jpsychores.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Mejhert M, Kahan T, Persson H, Edner M. Predicting readmissions and cardiovascular events in heart failure patients. Int J Cardiol. 2006;109:108–113. doi: 10.1016/j.ijcard.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 19.St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pakhomov S, Weston SA, Jacobsen SJ, Chute CG, Meverden R, Roger VL. Electronic medical records for clinical research: Application to the identification of heart failure. Am J Manag Care. 2007;13:281–288. [PubMed] [Google Scholar]
- 21.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. J Biomed Inform. 2005;38:145–153. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 27.Kremers WK. Concordance for survival time data: Fixed and time-dependent covariates and possible ties in predictor and time. Mayo Foundation; Rochester, MN: 2007. Technical Report Series #80. [Google Scholar]
- 28.Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 29.Heo S, Moser DK, Riegel B, Hall LA, Christman N. Testing a published model of health-related quality of life in heart failure. J Card Fail. 2005;11:372–379. doi: 10.1016/j.cardfail.2004.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.