Abstract
Elevated oxidative stress is observed more frequently in cancer cells than in normal cells. It is therefore expected that additional exposure to a low level of reactive oxygen species (ROS) will push cancer cells toward death, whereas normal cells might maintain redox homeostasis through adaptive antioxidant responses. We previously demonstrated that parthenolide enhances ROS production in prostate cancer cells through activation of NADPH oxidase. The present study identifies KEAP1 as the downstream redox target that contributes to parthenolide’s radiosensitization effect in prostate cancer cells. In vivo, parthenolide increases radiosensitivity of mouse xenograft tumors but protects normal prostate and bladder tissues against radiation-induced injury. Mechanistically, parthenolide increases the level of cellular ROS and causes oxidation of thioredoxin (TrX) in prostate cancer cells, leading to a TrX-dependent increase in a reduced state of KEAP1, which in turn leads to KEAP1-mediated PGAM5 and Bcl-xL (BCL2L1) degradation. In contrast, parthenolide increases oxidation of KEAP1 in normal prostate epithelial cells, leading to increased Nrf2 (NFE2L2) levels and subsequent Nrf2-dependent expression of antioxidant enzymes. These results reveal a novel redox-mediated modification of KEAP1 in controlling the differential effect of parthenolide on tumor and normal cell radiosensitivity. Further, they show it is possible to develop a tumor-specific radiosensitizing agent with radioprotective properties in normal cells.
Keywords: Keap1, Nrf2, PGAM5, Bcl-xL, parthenolide, radiotherapy, prostate cancer, reactive oxygen species (ROS), redox modification, antioxidant proteins, mitochondrial function
Introduction
It is well-documented that cancer cells are usually under more oxidative stress than normal cells are, in part due to a hyperactive metabolism that fuels their rapid growth (1, 2). Thus, a therapy designed to increase reactive oxygen species (ROS) to a level above the threshold for cancer cell death, but to an adaptable level for normal cells, would be an attractive strategy to selectively kill cancer cells (3, 4). Redox homeostasis is thought to regulate many cellular processes that are essential for maintenance of normal physiological conditions but is aberrantly modulated in cancers (5, 6). The functions of ROS are both beneficial and deleterious due to their dual role in the prosurvival and antisurvival pathways. As a secondary messenger in cell signaling, ROS are required for normal development and can initiate adaptive responses in cellular defense (7, 8). On the other hand, ROS cause structural damage and functional decline in DNA, proteins and lipids, and consequently act as an anti-tumorigenic factor by inducing cell senescence and apoptosis (9, 10). Indeed, ROS-mediated cell death is an important basis for radiotherapy and many chemotherapeutic treatments (11, 12). Currently, these therapeutic strategies are being used to kill cancer cells without benefit of a rational design that exploits the intrinsic differences in the cellular redox status of normal cells and cancer cells.
Antioxidant defense systems are essential for the regulation of ROS levels, which have an important function in the maintenance of cellular redox hemostasis. Mounting evidence demonstrates that a decline in antioxidant function may be involved in tumorigenesis due to prooxidant conditions that result from ROS accumulation. For example, manganese superoxide dismutase (MnSOD) is down-regulated in many types of cancer, and overexpression of MnSOD results in suppression of tumorigenesis (13–15). High levels of antioxidants caused by therapy-mediated activation of pro-survival pathways, such as NF-κB and Nrf2, are thought to protect cancer cells against treatment (16–18). Thus, inhibition of prosurvival pathways has been a traditional strategy to enhance therapeutic efficacy.
Increasing evidence demonstrates that certain mild prooxidant compounds derived from natural herbal medicines might enhance some anticancer treatments by modulating the redox state of cancer cells to high prooxidant levels (19, 20). Parthenolide, an active ingredient derived from the traditional anti-inflammatory medical plant feverfew (Tanacetum parthenium), belongs to the family of sesquiterpene lactones containing an α-methylene-γ-lactone moiety and an epoxide group, which is able to conjugate thiols of proteins through a Michael addition reaction (21). In addition to its anti-inflammatory effect, parthenolide appears to be toxic to a variety of cancer cells (22–25). Importantly, parthenolide has no cytotoxic effect on normal cells (25). Mechanistically, parthenolide has been shown to increase apoptosis in cancer cells through inhibition of multiple prosurvival pathways, such as NF-κB and PI3K-AKT (19, 26). However, these findings do not explain why parthenolide is not toxic to normal cells.
Post-translational modification is a key mechanism by which proteins dramatically increase their functional diversity. Reversible redox modification of protein cysteine residues plays an important role in vital cell signaling pathways related to many physiological and pathogenic processes (27, 28). The Keap1-Nrf2 pathway is one of the main cellular defense mechanisms against oxidative stress (29, 30). The present study examines the role of cysteine modifications in modulating radiation responses in prostate cancer cells versus normal prostate epithelial cells. It elucidates the functional link between redox modulation and cell signaling transduction pathways, and it provides evidence for the differential effect of parthenolide on cellular redox status in normal and cancer cells. The results reveal that parthenolide-mediated redox modification of Keap1 serves as a central regulator of differential responses to radiation therapy in normal and tumor cells. The present study provides a proof-of-concept for utilizing an intrinsic difference in cellular redox conditions to kill tumor cells while protecting normal cells from the unwanted side effects of radiation.
Materials and Methods
Cell culture, cell transfection, treatment, and cell survival analysis
Human prostate carcinoma/adenocarcinoma LNCaP, PC3 and DU145 cell lines as well as human prostate epithelial viral transformed PZ-HPV-7 (PZ) and RWPE-1 cell lines were obtained from American Type Culture Collection (ATCC). Normal prostate epithelial PrEC cells were purchased from Lonza. All cell lines were cultured and maintained in the media recommended in the manufacturer’s protocols. Plasmid cloned Keap1 cDNA and Bcl-xL cDNA (OriGene) and siRNAs for knocking down Keap1, Nrf2, thioredoxin (TrX), PGAM5, and Bcl-xL (Dharmacon) were transfected into cultured cells prior to treatment. Parthenolide and its water soluble prodrug, dimethylamino-parthenolide (DMAPT), were synthesized as previously described (31). The cells were treated with 0–5 μM parthenolide followed by irradiation by a 250 kV X-ray machine (Faxitron X-ray Corp.) with peak energy of 130 kV, 0.05 mm Al filter, at a dose of 0 to 6 Gy. Cell survival rates were quantified by colony survival fraction, Trypan blue exclusion assay, and MTT assay, as previously described (25, 32).
Animals
Four- to five-week-old male NCRNU (nu/nu athymic nude) mice were obtained from Taconic (Hudson). For formation of xenograft tumors, 1.8 × 106 cells mixed in Matrigel (BD Biosciences) were subcutaneously injected into the right flank of the mice. Tumor volumes were routinely measured and their sizes calculated based on a protocol described elsewhere (33). Animals with an average tumor size of 500 mm3 were randomized into several groups for DMAPT and radiation treatments. The tumors were treated five times with 10 mg/kg DMAPT and 3 Gy IR. The tumor tissues were collected, and 100 μg of tissue were lysed to quantify amounts of oxidized or reduced Keap1 and levels of downstream proteins. To determine the protective effect of parthenolide against radiation damage, mice were pretreated with DMAPT followed by radiation treatment (5 × 3Gy). Prostate and bladder tissues were fixed, embedded and processed for routine Electron microscopy (EM). The embedded blocks were sectioned and transferred to copper grids and counterstained with uranyl acetate, followed by lead citrate. Grids were observed in an electron microscope (Hitachi H-600) operated at 75 kV. Mitochondrial damage was quantified by a pathologist (TDO) using identified ultrastructural changes including swelling, vacuolization, myelination, disorganization and loss of cristae, lysosomal degradation, and membrane disruption. Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky, Approval Protocol No. 01077M2006.
Quantification of intracellular superoxide and prooxidant
Dihydroethidium (DHE, Invitrogen), which exhibits blue fluorescence in the cytosol until oxidized, was used to estimate the levels of superoxide after parthenolide treatment. To confirm the level of superoxide induced by parthenolide, the cells were pretreated with 50 μg PEG-SOD (Sigma) for 1 h followed by parthenolide treatment. Antimycin (Sigma) was used as a positive control because it has been shown to increase superoxide in all tested cell lines. A dichlorofluorescein (DCF) assay was used to quantify the levels of intracellular ROS after parthenolide treatment. The cells were labeled by both carboxy-H2DCFDA (sensitive to oxidation, Invitrogen) and carboxy-DCFDA (insensitive to oxidation, Invitrogen). The H2DCFDA:DCFDA ratio was used to optimize the controls of cell number, dry uptake, and ester cleavage. The procedures for both DHE and DCF were performed by the University of Kentucky Flow Cytometer Facility using a FACScan protocol provided by Dr. Douglas R. Spitz (34).
Measurement of oxygen consumption rates (OCR)
To determine how parthenolide changes mitochondrial function in cancer and normal cells, a Seahorse Bioscience XF24 Extracellular Flux Analyzer was used to measure OCR after parthenolide treatment. The XF24 creates a transient, 7-μl chamber in specialized microplates, which allows determination of oxygen and proton concentrations in real time. To allow comparison between experiments, data are expressed as the OCR in pmol/min or the rate of extracellular acidification in mpH/min. Reserve capacity, an important index that indicates capacity of mitochondrial respiration, is calculated by subtracting baseline OCR from maximal OCR.
Detection of oxidized and reduced forms of Keap1 protein
3-N-maleimido-propionyl biocytin was used to selectively label sulfhydryl (SH) and then was detected by biotin-streptavidin integration on the blots, as previously described (35). To quantify disulfide (S-S) bonds, the SH form was stabilized by treating with N-ethylmaleimide and then the S-S bonds were reduced by treating with 2-mercaptoethanol. To identify SH and S-S moieties of Keap1 protein, the labeled proteins were immunoprecipitated by Keap1 antibody (abcam) and subjected to SDS-PAGE, followed by detection with horseradish peroxidase-conjugated streptavidin (Sigma).
Immunoblots and immunoprecipitation
Homogenized cells and tumor tissues were electrophoresed on an 8% (w/v) SDS-PAGE gel, transferred onto a nitrocellulose membrane, and subsequently incubated with primary antibodies against Keap1 (abcam), Nrf2 (abcam), MnSOD (Upstate Biotech), CuZnSOD (eBiosci), Gpx (abcam), catalase (Millipore), TrX (BD Sciences), PGAM5 (Santa Cruz Biotech.), Bcl-xL (Santa Cruz Biotech.), LC3B (cell signaling), β-actin (sigma), and pCNA (Santa Cruz Biotech.). All secondary antibodies were obtained from Santa Cruz Biotech. Immunoblots were visualized using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech.). For immunoprecipitation, cell extracts were incubated overnight with one primary antibody at 4°C and integrated with a protein A/G agarose (Santa Cruz Biotech). Immunocomplexes were precipitated and fractionated on an SDS-PAGE gel. Interacting proteins were detected by immunoblotting with their primary antibodies.
SOD enzymatic assay
MnSOD activities were measured by the nitroblue tetrazolium (NBT)-bathocuproin sulfonate (BCS) reduction inhibition method. Sodium cyanide (2 mM) was used to inhibit CuZnSOD activity (36).
Statistical data analyses
Multiple independent experiments were performed for each set of data presented. Images in Immunoblots were quantified using Carestream Molecular Imaging software (Carestream Health Inc.). Statistical significance was analyzed using one-way ANOVA and Tukey’s Multiple Comparison Test, followed by data analysis with Graphpad Prism.
Results
Parthenolide enhances radiosensitivity of prostate tumors but protects normal tissues from radiation
Our previous studies demonstrated that parthenolide is able to selectively enhance the radiosensitivity of prostate cancer cells without injury to normal prostate epithelial cells (24, 25). To determine whether parthenolide enhances radiotherapy in vivo, human prostate cancer PC3 cells were subcutaneously implanted into the right flank of nude male mice. When tumor size reached a volume of 500 mm3, animals were randomized into groups according to treatment consisting of saline or 10 mg/kg DMAPT. One hour after saline or DMAPT was administered, the tumors were treated with fractionated radiation of 1 Gy or 2 Gy per day for 5 days, followed by routine measurement of tumor volume. The tumor growth curves are shown in Fig. 1A. Mice were humanely killed when a tumor reached the maximum size of 2000 mm3. Tumor growth was clearly delayed in the treatment groups, particularly when the drug and radiation were combined, compared to growth in the untreated group. The tumor growth rates after treatment were compared according to the days needed for tumor volume to reach 2000 mm3. DMAPT significantly enhanced radiotherapeutic efficiency compared to the effects of radiation treatment alone (Fig. 1B). A separate group of nude male mice that had no cancer cell implantation was treated with DMAPT and radiation to determine the toxicity of DMAPT to organs that can be affected by radiation therapy of prostate cancer. Prostates and bladders of the animals were examined by light and EM. At 60 days after irradiation, no gross pathology was observed (data not shown). However, ultrastructural damage was clearly observed by EM (Fig. 1C). Mitochondrial damage was most pronounced and this was morphometrically analyzed. The number of damaged mitochondria in prostate and bladder was proportional to radiation exposure. Pretreatment with DMAPT significantly reduced the number of damaged mitochondria in both organs compared to the group without DMAPT treatment (Fig. 1C and D). These results indicate that DMAPT, the water soluble prodrug of parthenolide, is a promising agent for selectively enhancing the sensitivity of prostate cancer cells to radiation while protecting normal tissues from damage caused by radiation.
Figure 1. The effect of parthenolide on radiosensitivities of prostate cancer and normal cells.
(A) Prostate cancer PC3 cells were injected into the flanks of nude male mice. The resulting tumors were treated with DMAPT and IR. Tumor volume was measured and tumor growth was calculated. (B) Time needed for tumor growth to reach 2000 mm3 volume after treatment was calculated and plotted. (C) Mice without cancer were treated with radiation alone (5 × 3 Gy) and DMAPT (10 mg/kg) with radiation. Prostate and bladder tissues were removed for pathological analysis using EM. Asterisks indicate normal mitochondria and arrows indicate mitochondria with myelin figures. M, normal mitochondria; Ly, lysosome; and V, mitochondria with vacuoles. (D) Quantification of mitochondrial damage in mice prostate and bladder tissues.
Parthenolide differentially modulates cellular ROS levels in cancer and normal cells
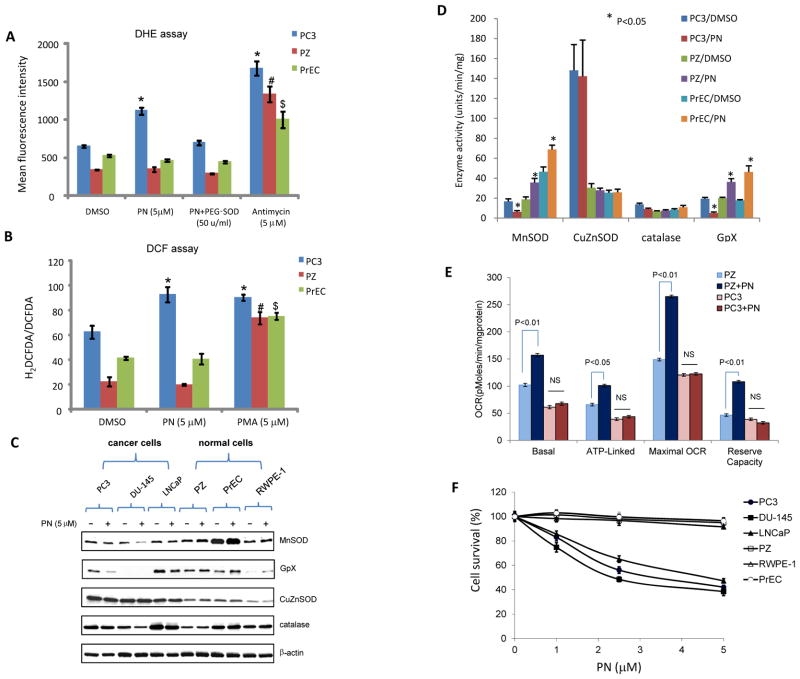
To determine the effect of parthenolide on redox homeostasis in cancer and normal cells, the levels of superoxide and total ROS after parthenolide treatment were measured using flow cytometry. The mean fluorescence intensity of DHE and the H2DCFDA:DCFDA ratio were higher in prostate cancer PC3 cells than in normal prostate PZ and PrEC cells, indicating higher basal levels of superoxide and total ROS, respectively (Fig. 2A–B). Following parthenolide treatment, DHE and DCF levels increased further in PC3 cells but declined slightly in both types of non-cancerous cells. Combining PEG-SOD with parthenolide treatment restored the basal level of DHE fluorescence. As positive controls, addition of equivalent concentrations of the ROS stimulating agents antimycin (Fig. 2A) and PMA (Fig. 2B) caused high levels of ROS in all the tested cell lines.
Figure 2. The effect of parthenolide on redox homeostasis in prostate cancer and normal cells.
(A–B) Cells were treated with parthenolide and then labeled with DHE or DCF. The mean fluorescence intensity of DHE (A) and the ratio of H2DCFDA to DCFDA (B) were determined using flow cytometry. Concentrations of cellular superoxide and total ROS were estimated by quantification of fluorescence intensity. Antimycin and PMA were used as positive controls for generation of ROS. PEG-SOD was used as a control to remove superoxide generated by DHE. PN, parthenolide. (C) The levels of antioxidant proteins were quantified by western blots. (D) The activities of the corresponding enzymes were measured. (E) Quantification of the basal oxygen consumption, ATP-linked oxygen consumption, the maximal OCR after the addition of FCCP, and the reserve capacity of the cells. NS, not significant. (F) Three cancer cell lines and three non-cancer cell lines were treated with parthenolide (PN) at the indicated concentrations. Cell survival fraction was determined by MTT assay.
The levels of antioxidant proteins were also quantified (Fig. 2C). Parthenolide altered the protein level of the antioxidant enzymes, in particular mitochondria-localized antioxidant enzymes. MnSOD and glutathione peroxidase (GpX) were significantly reduced in all three parthenolide-treated prostate cancer cell lines. Intriguingly, parthenolide had the opposite effect in the three normal prostate cell lines. However, neither cancer nor normal cells showed any obvious changes after parthenolide treatment for the major cytosolic superoxide removal protein, copper and zinc-containing SOD (CuZnSOD). This observation was confirmed by quantification of the corresponding enzyme activity (Fig. 2D). These results suggest that parthenolide-mediated alteration of cellular redox status is mediated, at least in part, by changing the activities of antioxidant enzymes in mitochondria.
To probe whether altering cellular redox status is associated with a change in mitochondrial respiration, the OCR in the parthenolide-treated cells was measured using a Seahorse Bioscience FX OxygenFlux Analyzer. The basal and maximal OCR in normal cells was higher than in cancer cells (Fig. 2E). Importantly, parthenolide was able to increase the OCR and reserve capacity in PZ cells, whereas parthenolide had no effect on PC3 OCR. Finally, the cytotoxicity of parthenolide was tested in all the cell lines using an MTT assay, which requires active mitochondria. As shown in Fig. 2F, parthenolide was toxic to all the cancer cells but not to the normal cell lines. Taken together, these results suggest that changes in cellular redox status and mitochondrial function may be a cause for the differential biological effects of parthenolide on cancer and normal cells.
Keap1 is susceptible to parthenolide-mediated redox modification
Keap1, a redox sensitive protein, has been reported to play an important role in cell survival under oxidative stress (29). To investigate whether parthenolide modifies Keap1 function, a Keap1 antibody linked to biotin was used to immunoprecipitate redox-modified Keap1 protein, and the presence of oxidized (-S-S-) and reduced (-SH) cysteine residues was detected using a secondary antibody linked to streptavidin. In the three normal cell lines, parthenolide increased the oxidized form of Keap1 but decreased the reduced form of Keap1 (Fig. 3A). Interestingly, the results from the three cancer cell lines appeared to be completely opposite to results observed in normal cells treated with parthenolide: the level of the oxidized form was decreased, but the level of the reduced form was increased (Fig. 3B). To verify that the observed increase in reduced Keap1 also occurred in vivo, mouse xenograft tumor tissues with and without DMAPT treatment were also used for determination of Keap1 redox status. Consistent with data obtained from cultured tumor cells treated with parthenolide, the in vivo results show that parthenolide decreased the oxidized form of Keap1 but increased the reduced form of Keap1 in the tumors (Fig. 3C). Changes in antioxidant proteins in mouse xenograft tumor tissues treated with DMAPT are also consistent with the result obtained from in vitro studies (Fig. 3D), indicating that parthenolide decreases the level of mitochondrial antioxidant proteins in prostate tumors.
Figure 3. Keap1 redox modification by parthenolide.
(A–B) Three normal cell lines (A) and three prostate cancer cell lines (B) were treated with saline or parthenolide (PN). Keap1 was immunoprecipitated with its antibody and SH- and S-S moieties of Keap1 were demonstrated by ECL detection. (C) Tumor tissues were homogenized and incubated with MPB. Reduced (SH-) and oxidized (S-S) forms of Keap1 in the tissues were detected. (D) Tumor tissues were homogenized and antioxidant proteins were quantified by western blots. The right panel shows representative blots and the left panel shows the average of multiple blots.
Oxidization of Keap1 leads to activation of the Nrf2 pro-survival pathway in normal cells
Activation of the Nrf2 signaling pathway through dissociation with Keap1 resulting in Nrf2 nuclear translocation is considered to be a primary pro-survival pathway in response to oxidative stress (30, 37). To examine whether parthenolide changes Nrf2 nuclear translocation, the levels of Nrf2 in nuclei were measured. As shown in Fig. 4A, the nuclear levels of Nrf2 were increased in the three normal cell lines treated with parthenolide, but no changes were observed in the three cancer cell lines. To examine whether activation of the Nrf2 pathway is a major mechanism by which parthenolide protects normal cells against radiation injury, Keap1 and Nrf2 were silenced in PZ cells by transfecting their siRNA (Fig. 4B, left panel). Cell survival decreased when Nrf2 was silent. IR significantly reduced cell survival but the cell survival was restored when Keap1 was silenced (Fig. 4B, right panel). These results suggest that oxidation of Keap1 and subsequent activation of Nrf2 by parthenolide are essential for normal cell survival after radiation treatment.
Figure 4. Activation of Keap1-Nrf2 pathway by parthenolide in normal cells.
(A) Parthenolide (PN) increases nuclear levels of Nrf2 in normal cells but not in cancer cells. Nuclear proteins extracted from the parthenolide-treated cell lines were immunoblotted to quantify nuclear levels of Nrf2 using PCNA as loading control. (B) The effect of Keap1 and Nrf2 in normal cells. PZ cells were transfected with siRNAs to knock down Keap1 or Nrf2, respectively. After treatment with parthenolide (PN) and IR, the cell survival fraction was quantified using Trypan blue exclusion assay (right), and the knocked-down Keap1 and Nrf2 were confirmed by western blots (left).
Thioredoxin is necessary for parthenolide-mediated reduction of Keap1 in cancer cells
TrX is highly expressed in cancer cells and stimulates cell growth. We previously reported that parthenolide decreases the reduced form of TrX but increases the oxidized form of TrX in prostate cancer cells (25). In the present study, we verify that TrX was expressed at a high level in all three cancer cell lines whereas a low level was observed in the three non-cancer cell lines (Fig. 5A). Immunoprecipitation of Keap1 protein from PC3 cell extracts using a TrX antibody suggests an interaction between Keap1 and TrX that is increased by parthenolide (Fig. 5B). To detect whether the parthenolide-influenced reduction of Keap1 in cancer cells is dependent on TrX, we selectively silenced TrX by transfecting its siRNA prior to parthenolide treatment (Fig. 5C, left panel). As expected, the reduced form of Keap1 was decreased, but the oxidized form of Keap1 was increased when TrX was silent (Fig. 5C, middle and right panels). The results suggest that TrX is interacting with Keap1 to keep Keap1 in a reduced state in parthenolide-treated cells. To further confirm that the function of Keap1 leads to cell death in parthenolide-treated cancer cells, a Keap1 expression construct was transfected into PC3 cells, followed by parthenolide and IR treatments. Overexpression of Keap1 resulted in increases in cell death in both treated and untreated cells (Fig. 5D, top panel). The levels of mitochondrial phosphoglycerate mutase 5 (PGAM5), a protein serine/threonine phosphatase that interacts with Bcl-xL in the mitochondrial membrane (38), and Bcl-xLwere clearly decreased in the Keap1 transfected cells, but no changes were observed in Nrf2, Ikkα and IkBα (Fig. 5D, bottom panel). These results suggest that the parthenolide-increased reduced form of Keap1facilitates Keap1-mediated ubiquitin/proteasome-dependent degradation of PGAM5 and Bcl-xL, which is an established mechanism for parthenolide-mediated cell death in cancer cells.
Figure 5. TrX-dependent Keap1 reduction by parthenolide in prostate cancer cells.
(A) The levels of TrX in normal and cancer cells before and after treatment with parthenolide (PN) were detected by western blots. (B) After the indicated treatment, Keap1 was immunoprecipitated using a TrX antibody. (C) PC3 cells were transfected with TrX siRNA prior to the indicated treatment (top). SH- and S-S bands in Keap1 protein were detected as described in Fig. 3 (bottom). (D) A Keap1 cDNA construct was transfected into PC3 cells. The levels of related proteins were detected by western blots (bottom). The effect of Keap1 on radiosensitivity was analyzed using a Trypan blue exclusion assay (top).
Keap1 triggers PGAM5-mediated Bcl-xL ubiquitin degradation in parthenolide-treated cancer cells
To further investigate the mechanism by which parthenolide enhances the radiosensitivity of prostate cancer cells, we determined the interactions between Keap1, PGAM5 and Bcl-xL. The results demonstrate that a reduced form of Keap1, which is increased in parthenolide-treated PC3 cells, enhanced interaction between Keap1 and PGAM5, as detected by immunoprecipitation using a PGAM5 antibody (Fig. 6A). Bcl-xL, a prosurvival mitochondrial protein, was also increased in the pulled down complex (Fig. 6A). Interestingly, the proteins that are associated with Keap1 were decreased in whole cell extracts (Fig. 6B). A time course of parthenolide treatment shows that PGAM5 and Bcl-xL proteins were slightly increased at 12 h but decreased at 24 and 48 h after treatment (Fig. 6C). Proteins in different cellular fractions were also quantified (Fig. 6D). The mitochondria-associated proteins PGAM5 and Bcl-xL were reduced by the parthenolide treatment, but no change was observed in Hsp75, a control for mitochondrial protein. Parthenolide had no major effect on the levels of Nrf2 and Ikkα in treated cells. These results suggest that parthenolide enhances Keap1-mediated ubiquitin/proteasome-dependent degradation of PGAM5 and Bcl-xL (39). In addition, parthenolide increased the level of mitochondria-associated autophagic protein LC3B, suggesting that parthenolide may enhance the radiation sensitivity of prostate cancer cells partially through triggering the autophagy pathway.
Figure 6. Degradation of PGAM5-Bcl-xL caused by parthenolide-mediated reduction of Keap1 in prostate cancer cells.
(A) PC3 cells were treated with parthenolide (PN). Keap1 and Bcl-xL were immunoprecipitated using a PGAM5 antibody. (B) The total levels of Nrf2, PGAM5, and Bcl-xL were quantified by western blots. (C) The levels of mitochondria- associated proteins after parthenolide treatments. (D) Proteins in various cellular fractions were identified with antibody specific for each protein. (E) PC3 cells were transfected with siRNA to knock down PGAM5, Bcl-xL and Nrf2, respectively (bottom). Cell survival fraction was quantified by Trypan blue exclusion assay (top). (F) A Bcl-xL expression construct was transfected into PC3 cells and the expression of Bcl-xL was monitored by western blots (bottom). Cell survival fraction was quantified by Trypan blue exclusion assay (top).
Because Keap1 interacts with PGAM5/Bcl-xL/Nrf2, we decided to determine the effect of PGAM5/Bcl-xL/Nrf2 in mediating parthenolide’s effect on cancer cells. PGAM5, Bcl-xL and Nrf2 were silenced using their siRNAs, followed by parthenolide treatment (Fig. 6E, bottom panel). The cell survival fraction was decreased when PGAM5 or Bcl-xL was silent, which is similar to the effect of parthenolide (Fig. 6E, top panel). No significant additive effects were observed when parthenolide was combined with PGAM5 or Bcl-xL siRNA. In contrast, a significant effect was observed when Nrf2 was silent. These results suggest that Keap1-mediated PGAM5/Bcl-xL degradation, but not Nrf2 degradation, is important for parthenolide-induced cancer cell death.
To further determine whether the function of Bcl-xL plays a major role in protecting cancer cells against parthenolide-induced cell death, a plasmid carrying Bcl-xL cDNA was transfected into PC3 cells followed by parthenolide treatment (Fig. 6F, bottom panel). The results show that expression of Bcl-xL efficiently protects cells from cytotoxicity caused by parthenolide (Fig. 6F, top panel). Taken together, these results suggest that parthenolide enhances the radiosensitivity of prostate cancer cells, in part, by triggering ubiquitin/proteasome-based degradation of Bcl-xL.
In summary, parthenolide provides radiosensitization in prostate cancer cells but radioprotection in normal cells, and the observed differential effects are mediated, in part, by redox modification of Keap1, i.e., reducing Keap1 in cancer cells but oxidizing Keap1 in normal cells. The distinct redox modification of Keap1 initiates different signaling pathways that affect mitochondrial function, leading to cell survival or cell injury in response to radiation, as illustrated in Fig. 7.
Figure 7. A proposed mechanistic model for parthenolide-mediated inverse therapeutic effects on radiosensitivity of prostate cancer and radioresistance of normal cells.

Parthenolide sensitizes cancer cells to radiation, in part, by maintaining Keap1 in a reduced state and enhancing its interaction with PGAM5 and Bcl-xL, resulting in degradation of Bcl-xL in mitochondria. In contrast, parthenolide protects normal cells against radiation via oxidation of Keap1 and release of the Nrf2 transcription factor for activation of mitochondrial antioxidant enzymes.
Discussion
The majority of anticancer therapies fail because cancers develop phenotypes that are treatment resistant and because treatments cause unwanted and/or detrimental side effects to normal cells or to untargeted tissues. While conventional adjuvant therapies improve tumor response to radiation therapy, they generally cause additional damage to normal tissues. Thus, the focus of the present study is to identify adjuvant therapeutics that can reduce the side effects of radiation therapy. Our study provides a proof-of-concept for improving the efficacy of radiation therapy while protecting against injury to normal tissues. It has been demonstrated that parthenolide, the anti-inflammatory phytochemical, is able to suppress tumor growth in many organs (22–25). In addition, parthenolide appears to synergically enhance chemotherapeutic efficiency when it is combined with taxol or cisplatin to treat lung and gastric cancer cells (23, 40). Parthenolide also sensitizes radioresistant osteosarcoma cells to radiotherapy (41). Here, we demonstrate that DMAPT, a parthenolide prodrug, sensitized prostate cancer cells to radiotherapy in vivo and protected normal prostate and bladder against radiation-induced tissue injury. These results extend our previous survival studies in prostate cancer cell lines and normal prostate epithelial cells.
ROS, as products of cell metabolism, play a dual role in tumorigenesis and tumor suppression. The “two-faced” character of ROS has emerged as a potential source for discovering anticancer drugs. Redox homeostasis is frequently deregulated in cancers as it is constantly exposed to high levels of ROS compared to normal counterparts. Our data demonstrate that constitutively elevated levels of oxidative stress in cancer cells represent a specific vulnerability that can be selectively targeted by direct- or indirect-acting prooxidants and antioxidants or redox modulators. Theoretically, the differential redox status of cancer cells compared to normal cells should provide a therapeutic window for selective redox intervention via additional increases in ROS. In this context, normal and cancer cells should respond differently to the same level of prooxidant action generated either by direct production of oxidative species or by modulation of specific cellular targets involved in redox regulation. In this study we demonstrate that parthenolide serves as a prooxidant and displays a selective redox modification capability that differentially modulates cellular redox signals and targets. The Michael acceptor reacts with a thiol group of target proteins through covalent adduction (21). Parthenolide contains electrophilic α-methylene-γ-lactone, a bisfunctional Michael acceptor, and displays a potential for bifunctional target alkylation and crosslinking. The present study demonstrates the inverse effects of parthenolide on redox modification in cancer cells compared to normal cells. Remarkably, observations of the cytotoxic and cytoprotective effects of parthenolide are consistent with its action in the modulation of ROS levels in both cancer and normal cells. Alteration of cellular ROS by parthenolide is attributed to functionally up- or down-regulating antioxidant enzymes in mitochondria, which consequently regulates mitochondrial respiration. Parthenolide is able to selectively reduce the activity of several enzymes involved in oxidative stress removal in cancer cells, which in turn can cause ROS levels to rise above the threshold for cell death. This finding predicts that antioxidant proteins and mitochondria are feasible therapeutic targets.
It has been reported that parthenolide is a potent inhibitor of NF-κB, which is a ROS-responsive transcriptional factor involved in both tumor progression and tumor resistance to treatment through upregulation of anti-apoptotic genes, such as Bcl-2, Bcl-xL, survivin, and XIAP (42). We previously demonstrated that NADPH oxidase-mediated inactivation of the Foxo 3 signaling pathway is involved in the parthenolide-enhanced radiosensitivity of prostate cancer (25). However, previous studies did not explain how parthenolide exerts such an opposing effect in tumor and normal cells. The present study identifies Keap1 as a redox signaling sensor that plays a pivotal role in the differential regulation of the downstream signaling targets in response to radiation-mediated cytotoxicity in prostate cancer and normal cells. Keap1, an adaptor protein for ubiquitin-based processing by the CUL3/RBX1-dependent E3 ubiquitin ligase complex, functions as a sensor for thiol-reactive redox modification (43). The present study demonstrates that stabilization of Nrf2 by oxidation of Keap1 serves as a major mechanism by which parthenolide protects normal tissues against radiotoxicity through up-regulation of antioxidant enzymes in mitochondria. However, Nrf2 transcriptional activation did not play a major role in parthenolide-treated prostate cancer cells. Thus, it is interesting to note that unlike traditional chemotherapeutic agents, parthenolide is unable to enhance resistance of prostate cancer to radiation treatment by stimulating Nrf2 target genes.
In addition to regulating the Nrf2 signaling pathway, Keap1 is able to bind other proteins such as p62 and PGAM5 (44). Interaction between Keap1 and p62 facilitates release of Nrf2 from the complex, which is considered to be a noncanonical cysteine-independent mechanism for the autophagy deficiency-activated Nrf2 pathway (45). The N-terminus of PGAM5 interacts with the Kelch domain of Keap1 and its C-terminus binds to Bcl-xL. Keap1-dependent ubiquitination results in proteasome-dependent degradation of PGAM5 and Bcl-xL (38). Bcl-xL, an important member of the Bcl-2 family, is a potent antiapoptotic factor that plays a crucial role in cell survival by maintaining the electrochemical and osmotic homeostasis of mitochondria (46). The present study demonstrates that parthenolide increases the level of reduced Keap1 and consequently induces Keap1-dependent degradation of PGAM5 and Bcl-xL in cancer cells, suggesting that formation of the Keap1-PGAM5-Bcl-xL complex is a mechanism underlying the effect of parthenolide on radiosensitization of prostate cancer cells.
Although a high rate of aerobic glycolysis in tumors, known as the Warburg effect, has been observed in various types of cancer, cancers have functional mitochondria, and mitochondrial respiration is necessary for cancer cell proliferation (47). Cancer cells depend on a hyperactive metabolism to fuel their rapid growth and also on antioxidative enzymes to quench potentially toxic ROS generated by such a high metabolic demand (48). Our results demonstrate that parthenolide not only suppresses MnSOD and GpX, two major antioxidant enzymes in mitochondria, but also activates Bcl-xL degradation in cancer cells, which suggests that mitochondria are a feasible target for anticancer treatment. The present study also shows that parthenolide may maintain normal cell survival through induction of MnSOD and GpX activity. Thus, a more efficient and safe therapy may involve modification of cellular redox signaling by alteration of the antioxidant response coupled to selective degradation of prosurvival members of the Bcl2 family in cancer cells, because conventional anticancer therapy mainly causes cell growth arrest or cell death by raising cellular ROS, which oxidizes and damages DNA, proteins and lipids. Optimizing prototype redox chemotherapeutics from natural sources provides an exciting opportunity to further develop even better candidates to enhance therapeutic efficacy with less off-target toxicity.
Acknowledgments
This work was initiated by a previous graduate student, Yulan Sun, and was supported by National Institutes of Health grants CA49797, CA115801, and CA143428 to Daret K. St. Clair and William St. Clair. Additional support was provided by the Edward P. Evans Foundation and by the resources and facilities of the William S. Middleton Veterans Administration Hospital (Madison, WI).
Footnotes
The authors disclose no potential conflicts of interest
References
- 1.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–52. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–83. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 3.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Tew KD, Townsend DM. Redox platforms in cancer drug discovery and development. Curr Opin Chem Biol. 2011;15:156–61. doi: 10.1016/j.cbpa.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Autreaux B, Toledano MB. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–24. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 6.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–30. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 7.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Cabello CM, Bair WB, 3rd, Wondrak GT. Experimental therapeutics: Targeting the redox Achilles heel of cancer. Curr Opin Investig Drugs. 2007;8:1022–37. [PubMed] [Google Scholar]
- 10.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Yi J. Cancer cell killing via ROS to increase or decrease, that is the question. Cancer Biol Ther. 2008;7:1875–84. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 12.Spyratou E, Makropoulou M, Mourelatou EA, Demetzos C. Biophotonic techniques for manipulation and characterization of drug delivery nanosystems in cancer therapy. Cancer Lett. 2012 Jan 17; doi: 10.1016/j.canlet.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Oberley LW, Oberley TD. Role of antioxidant enzymes in cell immortalization and transformation. Mol Cell Biochem. 1988;84:147–53. doi: 10.1007/BF00421049. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Chaiswing L, Oberley TD, Batinic-Haberle I, St Clair W, Epstein CJ, et al. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–5. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 15.Dhar SK, Tangpong J, Chaiswing L, Oberley TD, St Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011;71:6684–95. doi: 10.1158/0008-5472.CAN-11-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josson S, Xu Y, Fang F, Dhar SK, St Clair DK, St Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene. 2006;25:1554–59. doi: 10.1038/sj.onc.1209186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan M, Ahmed KM, Coleman MC, Spitz DR, Li JJ. Nuclear factor-kappaB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007;67:3220–28. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- 18.Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–32. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 19.Hassane DC, Sen S, Minhajuddin M, Rossi RM, Corbett CA, Balys M, et al. Chemical genomic screening reveals synergism between parthenolide and inhibitors of the PI-3 kinase and mTOR pathways. Blood. 2010;116:5983–90. doi: 10.1182/blood-2010-04-278044. [DOI] [PubMed] [Google Scholar]
- 20.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–34. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Hwang DR, Wu YS, Chang CW, Chang CW, Lien TW, Chen WC, et al. Synthesis and antiviral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorg Med Chem. 2006;14:83–91. doi: 10.1016/j.bmc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Pajak B, Gajkowska B, Orzechowski A. Molecular basis of parthenolide-dependent proapoptotic activity in cancer cells. Folia Histochem Cytobiol. 2008;46:129–35. doi: 10.2478/v10042-008-0019-2. [DOI] [PubMed] [Google Scholar]
- 23.Gill KK, Kaddoumi A, Nazzal S. Mixed micelles of PEG(2000)-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: enhanced chemosensitization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur J Pharm Sci. 2012;46:64–71. doi: 10.1016/j.ejps.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, St Clair DK, Fang F, Warren GW, Rangnekar VM, Crooks PA, et al. The radiosensitization effect of parthenolide in prostate cancer cells is mediated by nuclear factor-kappaB inhibition and enhanced by the presence of PTEN. Mol Cancer Ther. 2007;9:2477–86. doi: 10.1158/1535-7163.MCT-07-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, St Clair DK, Xu Y, Crooks PA, St Clair WH. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010;70:2880–90. doi: 10.1158/0008-5472.CAN-09-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of Nuclear Factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res. 2007;13:59–67. doi: 10.1158/1078-0432.CCR-06-1559. [DOI] [PubMed] [Google Scholar]
- 27.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–73. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen HH, Hamilton EJ, Liu CC, Figtree GA. Reversible oxidative modification: implications for cardiovascular physiology and pathophysiology. Trends Cardiovasc Med. 2010;20:85–90. doi: 10.1016/j.tcm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–45. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–37. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neelakantan S, Nasim S, Guzman ML, Jordan CT, Crooks PA. Aminoparthenolides as novel anti-leukemic agents: Discovery of the NF-kappaB inhibitor, DMAPT (LC-1) Bioorg Med Chem Lett. 2009;19:4346–9. doi: 10.1016/j.bmcl.2009.05.092. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Fang F, St Clair DK, Josson S, Sompol P, Spasojevic I, et al. Suppression of RelB-mediated manganese superoxide dismutase expression reveals a primary mechanism for radiosensitization effect of 1alpha, 25-dihydroxyvitamin D(3) in prostate cancer cells. Mol Cancer Ther. 2007;6:2048–56. doi: 10.1158/1535-7163.MCT-06-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Josson S, Fang F, St Clair DK, Wan XS, Sun Y, et al. RelB enhances prostate cancer growth: implications for the role of the NF-κB alternative pathway in tumorigenicity. Cancer Res. 2009;69:3267–71. doi: 10.1158/0008-5472.CAN-08-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slane BG, Aykin-Burns N, Smith BJ, Kalen AL, Goswami PC, Domann FE, et al. Mutation of succinate dehydrogenase subunit C results in increased O2·−, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–20. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- 35.Bayer EA, Safars M, Wilchek M. Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal Biochem. 1987;161:262–71. doi: 10.1016/0003-2697(87)90450-7. [DOI] [PubMed] [Google Scholar]
- 36.Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–97. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu RP, Hayashi T, Cottam HB, Jin G, Yao S, Wu CC, et al. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2010;107:7479–84. doi: 10.1073/pnas.1002890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo SC, Hannink M. PGAM5, a Bcl-xL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J Biol Chem. 2006;281:37893–903. doi: 10.1074/jbc.M606539200. [DOI] [PubMed] [Google Scholar]
- 39.Niture SK, Jaiswal AK. Inhibitor of Nrf2 (INrf2 or Keap1) protein degrades Bcl-xL via phosphoglycerate mutase 5 and controls cellular apoptosis. J Biol Chem. 2011;286:44542–56. doi: 10.1074/jbc.M111.275073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Sohma I, Fujiwara Y, Sugita Y, Yoshioka A, Shirakawa M, Moon JH, et al. Parthenolide, an NF-κB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics. 2011;8:39–47. [PubMed] [Google Scholar]
- 41.Zuch D, Giang AH, Shapovalov Y, Schwarz E, Rosier R, O’Keefe R, et al. Targeting radioresistant osteosarcoma cells with parthenolide. J Cell Biochem. 2012;113:1282–91. doi: 10.1002/jcb.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs. 2011;71:1385–96. doi: 10.2165/11592590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Sekhar KR, Rachakonda G, Freeman ML. Cysteine-based regulation of the CUL3 adaptor protein Keap1. Toxicol Appl Pharmacol. 2010;244:21–26. doi: 10.1016/j.taap.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–85. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusten TE, Stenmark H. p62, an autophagy hero or culprit? Nat Cell Biol. 2010;12:207–9. doi: 10.1038/ncb0310-207. [DOI] [PubMed] [Google Scholar]
- 46.Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–37. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg F, Chandel NS. Mitochondrial metabolism and cancer. Ann NY Acad Sci. 2009;1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x. [DOI] [PubMed] [Google Scholar]
- 48.Pavlides Stephanos, Vera Iset, Gandara Ricardo, et al. Warburg Meets Autophagy: Cancer-Associated Fibroblasts Accelerate Tumor Growth and Metastasis via Oxidative Stress, Mitophagy, and Aerobic Glycolysis. Antioxidants & Redox Signaling. 2012:1264–84. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]