Abstract
The non-histone chromatin binding protein HMGA2 is expressed predominantly in the mesenchyme prior to its differentiation, but it is also expressed in tumors of epithelial origin. Ectopic expression of HMGA2 in epithelial cells induces epithelial-mesenchymal transition (EMT), which has been implicated in the acquisition of metastatic characters in tumor cells. However, little is known regarding in vivo modulation of HMGA2 and its effector functions in tumor metastasis. Here we report that HMGA2 loss-of-function in a mouse model of cancer reduces tumor multiplicity. HMGA2-positive cells were identified at the invasive front of human and mouse tumors. Additionally, in a mouse allograft model, HMGA2 overexpression converted non-metastatic 4TO7 breast cancer cells to metastatic cells that homed specifically to liver. Interestingly, expression of HMGA2 enhanced TGFβ signaling by activating expression of the TGFβ type II receptor (TGFβRII), which also localized to the invasive front of tumors. Together our results argued that HMGA2 plays a critical role in EMT by activating the TGFβ signaling pathway, thereby inducing invasion and metastasis of human epithelial cancers.
Keywords: High-mobility group A2 (HMGA2), TGFβ type II receptor (TGFβRII), epithelial-mesenchymal transition (EMT), invasive front, metastasis
Introduction
HMGA2 is an architectural transcription factor predominantly expressed in the mesenchyme prior to its differentiation and a regulator of mesenchymal proliferation and differentiation (1, 2). Because of its endogenous expression pattern, HMGA2 is not detected in normal adult tissues (3), but is misexpressed and disrupted in several mesenchymal benign tumors, most predominantly lipomas (1). In mice, the Hmga2 null reveals a pygmy phenotype due to the decreased number of mesenchymal cells (3).
Despite its exquisite and specific expression pattern in the mesenchyme during normal development and its causal role in benign mesenchymal tumorigenesis, there have also been a number of intriguing observations with regards to HMGA2 in malignant epithelial tumors over the past decades. Interestingly, high HMGA2 expression is correlated to late stage of Dukes’ classification in patients with colon cancer (4) and associated with the high histologic grade of invasive ductal type of breast cancer (5). Misexpression of HMGA2 occurred in parallel with reduced survival rates of patients with breast cancer (6), colorectal cancer (CRC) (7), lung cancer (8) and tumor recurrence in oral carcinoma (9).
The epithelial-mesenchymal transition (EMT) occurs during critical phases of embryonic development and tumor progression. Epithelial cells lose their characteristics and exhibit a molecular profile indicative of mesenchymal cells as well as a profound change in morphology. These include being loosely embedded in an extracellular matrix and an increased motile and invasive behavior. During this transition, mesenchymal cells acquire a morphology appropriate for migration in an extracellular environment and settlement in areas that are involved in organ formation. This important concept is also required during tumor progression and several signaling pathways have been uncovered that are common to EMT in both development and tumor progression (10). At the invasive front of the tumor, neoplastic cells are found that differ morphologically from other cells in the bulk of the tumor. The invasive front is defined as the one-cell thick layer of the primary tumor juxtaposing, and small clusters of cancer cells that have advanced into the host stroma. These tumor cells are incohesive, lack polarity and are dedifferentiated, acquiring a mesenchymal phenotype by the process of EMT (10). The dedifferentiation of epithelial cells to fibroblastoid, migratory, and more malignant cells also reveal a profoundly altered, mesenchymal gene expression program (11). It is these mesenchymal-like capabilities of the cells that are said to be necessary for metastasis (10).
The TGFβ signaling pathway is a major inducer of the EMT during tumor development. In humans, the TGFβ superfamily represents a diverse set of growth factors (12). The TGFβ cytokines signal by bringing together two pairs of receptor serine/threonine kinases, the type I and type II receptors. On binding these ligands, active type II receptor contacts and phosphorylates the type I receptor (13) which leads to the activation of the canonical Smad pathway. Smad2 and Smad3, which are known as the receptor-associated Smads (R-Smads), are then phosphorylated by the type I receptor and released to propagate the signal with Smad4 (12).
In the present study, in order to analyze the complexity of the process of metastasis, we have identified the architectural transcription factor, HMGA2, as being essential for tumor progression, EMT and metastasis in vitro and in vivo. Furthermore, we demonstrate for the first time that the molecular mechanism of HMGA2 in tumor pathogeneisis is mediated through the activation of the TGFβ signaling pathway in epithelial carcinomas.
Materials and Methods
Cell culture and Invasion Assay
The SW480, SW620, SW403, HCT116 and HT29 human colon cancer, MCF-7, MDA-MB231, human breast cancer cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA) and propagated and maintained according to protocols supplied by ATCC. The 4TO7, 4T1 mouse breast cancer cell lines were provided as a kind gift from Fred Miller (Wayne State University) and were grown in DMEM (Gibco) containing 10% fetal bovine serum (FBS) supplemented with 4 mM L-glutamine, penicillin/streptomycin (Gibco). The transgene construct was made by cloning the human HMGA2 cDNA into pcDNA3.1(+) plasmid (Invtrogen, Carlsbad, CA). Transfection of the pcDNA-HMGA2 or the vector alone (Mock) into the SW480 and MCF-7 cells was performed with the Lipofectamine 2000 (Invitrogen) method. The pCMV6-HMGA2-GFP plasmid and pCMV6-AC-GFP (shuttle vector) plasmid were purchased from OriGene (Rockville, MD) and transfected into 4TO7 cells with electroporation. Twenty-four hours post-transfection, these cells were passaged into fresh growth medium containing the G418 sulfate (1mg/ml) and maintained for a week to select stable transfected cells. Matrigel invasion assays were performed as described (14). Specific shRNAs of Hmga2 were designed as 5′-GGTTAACAGTACCCAATGA-3′, 5′-TGGGCTTAATCAGTCACTA-3′ and 5′-CACAACAAGTCGTTCAGAA-3′ and integrated into a SMARTvector 2.0 lentiviral shRNA (Thermo Scientific Dharmacon, Lafayette, CO, USA). 4T1 mouse breast cancer cells were transduced with the Lentiviral Hmga2-shRNA particles and Non-targeting control-shRNA (NTC-shRNA) particles in the presence of Polybrene (4μg/ml). Eighteen hours post-transduction, these cells were passaged into fresh growth medium containing puromycin (2 μg/ml) and maintained for a week so as to select stable transduced cells.
Immunocytochemistry
For immunocytochemistry, 105 cells were seeded onto CC2-coated chamber slides (Nalge Nunc, Naperville, IL) the day before staining. The primary antibodies for E-cadherin, β-catenin, Vimentin (Sigma, St. Louis, MO) and Smad3 (Cell Signaling Technology, Danvers, MA), ZEB1 (Cell Signaling Technology, Danvers, MA, USA) and Fibronectin (Abcam, Cambridge, MA, USA) were used at a dilution of 1:300, and appropriate rhodamine-tagged secondary antibodies were used at 1:500 dilution. The fluorescence was observed under an appropriately equipped Nikon microscope.
Immunohistochemistry and Western blotting
Paraffin-embedded murine and human samples were sectioned at 4 μm, and stained with polyclonal anti-HMGA2 antibody (3) and monoclonal anti-β-catenin (1:100 dilution, BD Biosciences, Bedford, MA), anti E-cadherin (1:50, Dako Cytomation, Carpinteria, CA), anti-Ki67 (1:200, Dako Cytomation, Carpinteria, CA), anti-TGFβ type II receptor (1:400, Abcam, Cambridge, MA), and anti-insulin-like growth factor 2 binding protein (IGF2BP2) (1:400, Santa Cruz Biotechnology, Santa Cruz, CA) antibodies using an immunoperoxidase technique (Vectastain ABC Elite kit, Vector Laboratories, Burlingame, CA, or Histomouse-SP kit, Zymed Laboratories, San Francisco, CA). At least 3 sections from each tumor were stained for each antibody study performed. Tissues from Hmga2 null mice were used as negative controls for the anti-Hmga2 antibody. Western blotting was performed as described (9). Ten micrograms of total protein was run per lane and antibodies were used as described above.
Gene expression analysis
Total RNA was isolated from tissues and cells using RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. All mRNA expression analyzes were performed with real-time quantitative RT-PCR using a TaqMan Gene Expression Assay (Applied Biosystems) in an ABI Prism 7300HT Sequence Detection System (PE Biosystems, Foster City, CA). The reverse transcription and PCR reactions were performed using TaqMan Reverse Transcriptase Reagents (Applied Biosystems). The relative expression data was calculated by the comparative CT method as described elsewhere (15).
Mice
All mice were housed and handled according to the Institutional Animal Care and Use Committee guidelines. Hmga2 specific knockout mice have been described (3). Seventh generation C57BL/6J-backcrossed Hmga2+/− female mice were mated with C57BL/6J-Wnt1 male mice (The Jackson Laboratory, Bar Harbor, Maine). F1 Wnt1 transgenic, Hmga2+/− male mice were then mated with F1 Wnt transgenic, Hmga2+/− female littermates to obtain the F2 transgenic mice in the Hmga2+/+, Hmga2+/−, and Hmga2−/− genetic backgrounds. PCR-based genotyping for the Wnt (16) and Hmga2 (2) loci have been described. For the mouse tumors from the Wnt mice we examined 44 samples (3 sections each) for all antibody stains described and for the in vivo metastasis study we examined 10 mice with three sections for each tissue type studies.
Human tissue samples
De-identified human tissue samples were obtained from the Surgical Pathology archives of Columbia Presbyterian Hospital (New York, NY) from patients with breast and colorectal neoplasms that were staged by the Dukes’ classification (17), The study was carried out in compliance with HIPAA criteria. In the case of the colon cancer samples the number of tumors examined is documented in Table 1 following the protocol as described in the Supplemental Materials and Methods utilizing images as represented in Supplementary Fig. 1. For the human breast cancer studies there were 100 samples with at least 3 sections from each tumor stained for HMGA2 with a minimum of 19 samples (3 sections each) stained for TGFβRII and IGF2BP2.
Table 1. Human Colorectal Cancer (CRC).
Immunohistochemical expression indices for HMGA2 and Ki67 in diffferent types of human colorectal tissue. Indices for nuclear HMGA2 and Ki67 (i.e. proliferation) accumulation for human colorectal neoplasms are expressed as percent (%) of positive nuclei per total nuclei of colonic epithelial cells ± SD.
| Tissue | Entire Neoplasm | ^Dysplasia or ◆Invasive Front | |||
|---|---|---|---|---|---|
|
| |||||
| n | HMGA2 | Ki67 | HMGA2 | Ki67 | |
| Non- neoplastic |
14 | 8.5±3.6 | 13.8±5.1 | NA | NA |
| Adenoma | 11 | 3.6±2.2 | 51.1 ±7.4* | NA | NA |
| Carcinoma in situ^ |
4 | ND | ND | 30.8±12.4+ | 42.3±8.9 |
| Dukes’ A◆ | 9 | 28.7±21.5* | 52.3±16.8* | 72.7±26.3++ | 54.0±24.7 |
| Dukes’ B◆ | 12 | 32.8±26.7* | 55.5±16.5* | 78.3±14.1++ | 48.5±20.8 |
| Dukes’ C/D■ | 9 | 65.7±17.3*#§ | 42.9±18.0* | 69.3±17.9 | 45.3±13.5 |
| Metastases■ | 13 | 66.5±20.8*#§ | 37.4±15.1* | 69.4±18.4 | 44.6±13.6 |
n=number of patient tumors examined
P<0.01 vs. non-neoplastic.
P<0.001 vs. Dukes’A.
P<0.01 vs. Dukes’B.
P<0.01 vs. adenoma.
P<0.01 vs. entire neoplasm.
All P-values are from Student’s t-test.
= Margin of tumor
Tumor implantation
Twelve-week old female BALB/cJ mice (Taconic Farms) were implanted subcutaneously into the right 4th mammary gland with 2×105 of 4TO7, 4TO7-HMGA2-GFP, 4T1 and 4T1-Hmga2-shRNA mouse breast cancer cells (or a mixture of each 1×105 of 4TO7 cells and 4TO7-HMGA2-GFP cells) in 100μl PBS using 28-gauge needle. Tumor size was measured 3 times a week by callipers and tumor volumes were calculated as Volume (mm3) = L×W2×0.5. Mice were sacrificed when the tumor size exceeded 20 mm in diameter in either direction or at the end of the observation period (25 days).
Statistical Analyses
All statistical analyzes were performed with PRISM v. 5.0d (Avenida De La Jolla, San Diego, CA). If statistically significant, comparisons between individual groups were performed by Student’s t-test. The analysis of tumor free percentage was performed by log-rank test. P < 0.05 was considered significant.
Results
HMGA2 Directly Induces EMT and Invasiveness in Epithelial Tumor Cells
Our initial experiments to understand the function of HMGA2 in epithelial tumorigenesis examined the expression of HMGA2 in a number of human cancer cell lines. The selected cell lines were confirmed to exhibit anchorage independent growth characteristics as previously defined (18) and interestingly, while the expression levels of HMGA1a and HMGA1b remained unchanged, the level of HMGA2 expression was found to be directly proportional to the in vitro anchorage independent growth characteristics of the colon cancer cell lines (Fig. 1A, Supplementary Fig. 2) (18). Additionally, it was found that HMGA2 expression was inversely related to expression of E-cadherin (Fig. 1A). Although HMGA2 is identified in the SW480 line by the sensitive technique of qRT-PCR, it is below the levels required for conversion to a mesenchymal and invasive phenotype (Fig. 1A, B).
Figure 1.
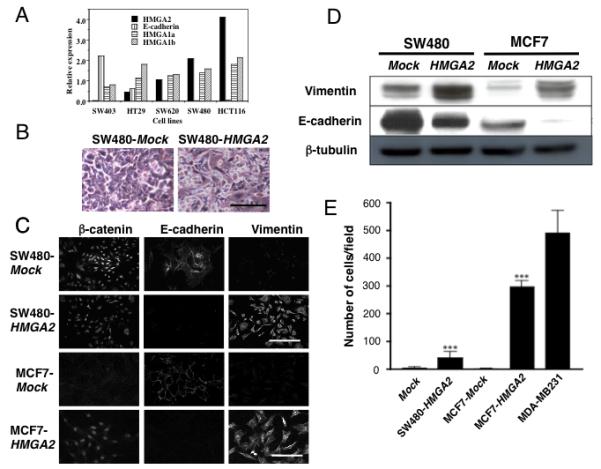
Ectopic expression of HMGA2 induces epithelial-mesenchymal transition and invasiveness in epithelial cancer cells. (A) Five different colon cancer cell lines tested for the mRNA expression level of HMGA2, E-cadherin, HMGA1a, and HMGA1b. (B) SW480 colon cancer cells transfected with empty vector (Mock) or HMGA2 three days post-confluence. Scale bars represent 50 μm. (C) Immunofluorescence analysis of β-catenin (left), E-cadherin (center), and vimentin (right) expression in SW480 and MCF-7 cells. Scale bars represent 50 μm. (D) Western blot analysis of Vimentin and E-cadherin expression in SW480-Mock (lane 1), SW480-HMGA2 (lane 2), MCF-7-Mock (lane 3), and MCF7-HMGA2 (lane 4) cells. β-tubulin expression is shown as loading control. (E) A matrigel invasion assay. The number of “invaded” cells was counted per field (at 100× magnification) in at least 10 different fields per filter. The numbers are shown as mean±SD from two experiments performed in triplicate. ***=P<0.001.
In order to directly test if HMGA2 was sufficient for the EMT in epithelial tumor cells, stable clones derived from the SW480 colorectal cancer and MCF-7 breast cancer epithelial cell lines were established that ectopically expressed HMGA2 under the control of the CMV promoter. Whereas Mock-transfected SW480 (SW480-Mock) cells maintained a regularly aligned pattern consistent with an epithelial morphology three days post-confluence, SW480 HMGA2-transfected (SW480-HMGA2) cells revealed a pronounced elongated, spindle-shaped mesenchymal phenotype (Fig. 1B). To confirm the morphological appearance of the cells, molecular markers of the mesenchymal phenotype were examined utilizing immunocytochemistry and western blotting in the HMGA2- and Mock-transfected cells. Consistent with the mesenchymal phenotype, the SW480-HMGA2 cells exhibited an enhanced expression of the mesenchymal marker Vimentin (19) and a marked reduction in the expression of the epithelial marker E-cadherin (20), as compared to the Mock-transfected controls (Fig. 1C, D). Similar results were obtained for cells of the MCF-7 breast cancer cell line with the MCF7-HMGA2 cells (Fig. 1C, D) exhibiting enhanced relocalization of β-catenin expression predominantly in the nucleus (Fig. 1C), a characteristic of the EMT (10). Additional studies were performed with other classic EMT-associated genes including ZEB1 and fibronectin with similar results (Supplementary Fig. 3). Finally, the HMGA2-transfected SW480 and MCF-7 clones exhibited invasion in the matrigel assay (Fig. 1E) and closely matched the behaviour of the control breast cancer cell line MDA-MB231, which is highly invasive. These results suggest that ectopic HMGA2 expression is sufficient as a “driver” for multiple cancer cell types to undergo the epithelial to mesenchymal transition, which leads to an increase in the invasive behaviour of these cells.
HMGA2 Is Expressed in Tumor Cells Located at The Invasive Front of The Primary Tumor and in Secondary Metastatic Lesions
The direct relevance of the mechanistic studies to human tumors was investigated. In colorectal tumors expression of HMGA2 was relatively absent in non-neoplastic (8.5 ± 3.6) and early adenomas (3.6 ± 2.2)(Table 1, Fig. 2). In contrast, the carcinomas of increasing severity (Dukes’ A through D) showed a progressively increased proportion of HMGA2 positive neoplastic cells (Table 1, Fig. 2) in the entire neoplasm. However, the number of HMGA2 positive cells at the invasive front remained constant showing a preferential distribution of HMGA2 positive cells at the invasive front. Indeed, there was no statistical difference between the number of cells expressing HMGA2 in the entire neoplasm and invasive front in the most severe grade of tumors (Dukes C/D) since these tumors are mostly of a dedifferentiated phenotype (17, 21), but also contain well differentiated areas (22) (Supplementary Fig. 1). Additionally, HMGA2 staining can clearly be seen in the metastatic lesion with increased β-catenin staining and loss of E-cadherin (Fig. 2).
Figure 2.
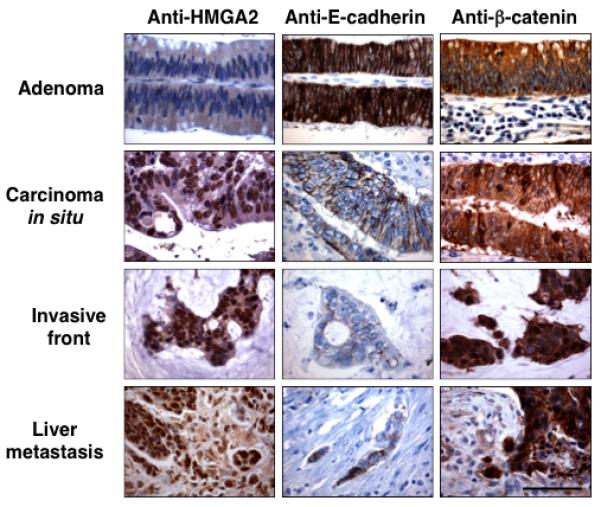
High HMGA2 expression correlates with loss of E-cadherin expression and nuclear relocalization of β-catenin in adenoma-carcinoma-metastasis sequence of human CRC. Immunohistochemical analyzes of HMGA2, E-cadherin, and β-catenin expression are shown on tissue sections from the same tumors. Scale bars represent 50 μm. These are representative sections from staining performed on patient tumor samples described in Table 1.
Also, the proliferation index (PI) for colonic epithelial cells significantly increased in adenomas as compared to non-neoplastic tissue, while at the same time the fraction of HMGA2 positive cells decreased (Table 1). The fact that proliferation remained constant and independent of tumor location in all tumor stages indicates that HMGA2 expression does not occur concomitant with expansion of non-invasive neoplastic colonic cells, but correlates with the invasive cancer cells that exhibit EMT.
In addition, cells at the invasive front in CRC have undergone EMT (21), where the overwhelming majority of cells are HMGA2 positive. Two well established markers for EMT in CRC are membrane-to-nucleus relocalization of β-catenin (21), and loss of E-cadherin expression along the cell membrane (10). Importantly, the mesenchymal-like cells at the invasive front, which exhibited the strongest nuclear HMGA2 accumulation, also demonstrated nuclear relocalization of β-catenin and loss of membrane E-cadherin expression (Fig. 2).
HMGA2 Activates the TGFβRII and Enhances TGFβ Signaling Pathway
In order to define the molecular mechanism of HMGA2 related EMT, we decided to investigate the possible relationship between the HMGA2 and TGFβ signalling pathway which is well established to play a role in the EMT (20) and invasion (23). Consistent with the expression of HMGA2 and its bona fide downstream target gene, Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2)(24, 25), the TGFβRII was highly expressed in MCF7-HMGA2 and MDA-MB231 cells compared to MCF7-Mock cells (Fig. 3A, B). TGFβ type I receptor (TGFβRI) expression level was unchanged (data not shown). Depletion of HMGA2, on HMGA2 siRNA-treated MDA-MB231 cells down regulated TGFβRII mRNA expression (Fig. 3C) and protein (Fig. 3D).
Figure 3.
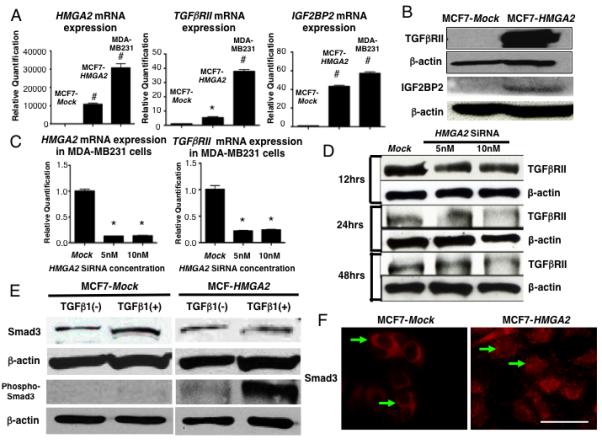
TGFβRII expression in human breast cancer cell lines. (A) HMGA2, TGFβRII and IGF2BP2 mRNA expression in MCF7-Mock, MCF7-HMGA2, MDA-MB231 cells. *=P<0.05, #=P<0.001. (B) TGFβRII and IGF2BP2 protein levels in MCF7-Mock and MCF7-HMGA2 transfected cells. β-actin is shown as a loading control. (C) HMGA2 and TGFβRII mRNA expression in Mock and HMGA2 siRNA (5 and 10nM) transfected MDA-MB231 cells. *=P<0.05. (D) TGFβRII protein levels after 12, 24 and 48 hours HMGA2 siRNA transfection in MDA-MB231 cells. (E) Western blot of Smad3 and phos-Smad3 in TGFβ1 (10ng/ml) treated cells. β-actin is shown as a loading control. (F) Immunofluorescence of Smad3 in MCF7-HMGA2 compared to MCF7-Mock transfected cells (arrows) without the addition of TGFβ1. Scale bars represent 25 μm.
Next, the activation of the TGFβRII by HMGA2 was examined through the phosphorylation status of Smad3. Whereas Smad3 is typically localized in the cytoplasm, activation of the TGFβ pathway causes Smad3 to be phosphorylated and migrate to the nucleus (26). Although Smad3 expression levels were unchanged in MCF7 or MCF7-HMGA2 cells even after TGFβ1 was added (Fig. 3E), in the MCF7-HMGA2 cells, the levels of phospho-Smad3 increased as compared to the MCF7-Mock cells with a dramatic enhancement observed in phospho-Smad3 expression levels in the presence of exogenous ligand, TGFβ1. Just as importantly, Smad3 is observed exclusively in the cytoplasm of MCF7-Mock cells but in the MCF7-HMGA2 cells, expression is most prominent in the nucleus (Fig. 3F). Taken together, these results suggest that HMGA2 increases the susceptibility to the TGFβ ligand through the activation of TGFβRII and strongly suggests that HMGA2 is upstream of the TGFβ pathway.
In order to elucidate the direct relevance of the in vitro mechanistic studies, human breast and colorectal cancer tissues were investigated. In breast cancer tumors, a coincident expression pattern was observed for HMGA2 and TGFβRII (Fig. 4 upper, left two lines). Dukes’ C/D CRC, the most severe grade of tumors (17) with a prominent invasive front were also analyzed for the expression of HMGA2 and TGFβRII. Expression was coincident for the two proteins within the cells of CRC tumor tissue at the invasive front (Fig. 4 lower, left two lanes), but not in the well-differentiated area of the tumor (Fig. 4 middle, left two lanes). IGF2BP2 was also strongly expressed at the invasive front of human advanced breast cancer tumors (Fig. 4 upper, right two lines) and severe grade of human CRC tumors (Fig. 4 lower, right two lines), but not in the well-differentiated area of CRC tumors (Fig. 4 middle, right lines). In conclusion, the expression of the TGFβ type II receptor was coincident with HMGA2 and IGF2BP2, which is expressed in the cells that undergo the EMT at the invasive front.
Figure 4.
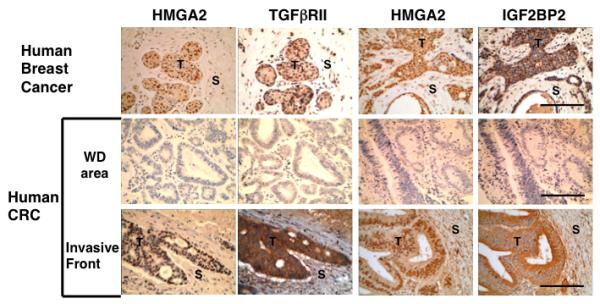
TGFβRII and IGF2BP2 expression at the invasive front consistent with the HMGA2 expression in human breast and colon cancer. HMGA2, TGFβRII and IGF2BP2 expression at the invasive front of advanced human breast cancer and human CRC (Dukes’ C/D), but not in the well differentiated (WD) area of human CRC. Scale bars represent 100 μm. T;Tumor, S; Stroma. Representative sections from 19 stained samples are shown.
HMGA2 and TGFβRII Expression and Loss of Hmga2 in the MMTV-Wnt1 Transgenic Mouse Breast Cancer Model
In the MMTV-Wnt1 transgenic mouse model, the expression of Wnt1 induces hyperplasia of breast at two weeks of age and by one year, 90% develop mammary adenocarcinomas (16, 27). Therefore, the MMTV-Wnt1 transgenic mouse was used as an animal model of epithelial tumors. Interestingly, Hmga2 was expressed in the hyperplastic mammary glands and mammary adenocarcinomas of the Wnt1 transgenic mice (Fig. 5A) but not in the normal breast tissue of the wild-type littermates.
Figure 5.
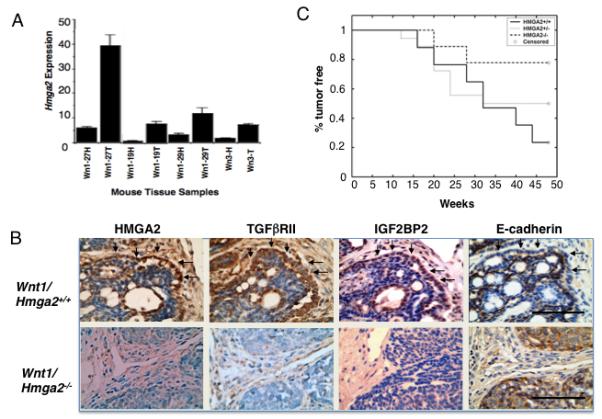
Loss of HMGA2 protects Wnt1 mice from tumor development through the TGFβ signaling pathway. (A) The levels of Hmga2 mRNA expression in the hyperplastic (H) mammary glands or tumor (T) of Wnt1 transgenic mice as a fold increase over the normal breast tissue of wild-type mice. (B) TGFβRII, IGF2BP2 and loss of E-cadherin expression at the same invasive front consistent with HMGA2 expression in Hmga2+/+, but not in Hmga2−/− genotypic backgrounds. Scale bars represent 50 μm. (C) Comparison of the Wnt1-mediated tumor incidence in Hmga2+/+ and Hmga2−/− genotypic backgrounds (P<0.022, log-rank test). The age at which 50% mice were found to have tumors (T50) was 32 weeks for Hmga2+/+ and Hmga2+/− mice. In contrast, <25% of Hmga2−/− mice developed tumors up until the 50th week.
Mammary tumors in the MMTV-Wnt1 mouse develop as multiple small tumors that are expansive and infiltrate the stroma. The edge of each of the multiple small tumors was defined as the invasive front. The majority of HMGA2 expressing cells are detected at the invasive front of individual tumors on the Hmga2+/+ genotypic background (Fig. 5B, upper). Interestingly, the TGFβRII and and IGF2BP2 expression were observed at the same invasive front on the Hmga2+/+ genotypic background (Fig. 5B upper). In addition, the loss of E-cadherin (Fig. 5B upper) and relocalization of β-catenin (Supplementary Fig. 4) were also detected at the same invasive front of Wnt1-mediated tumors on the Hmga2+/+ genetic background.
Having obtained the encouraging result that Hmga2 was expressed in the MMTV-Wnt1 tumors, the next step was to determine if this correlative result could be extended. Therefore, to examine the in vivo effect of Hmga2 on the initiation and progression of Wnt1-mediated tumorigenesis, the Hmga2 mutation (3) was bred onto the background of the MMTV-Wnt1 transgenic mouse for two generations (the Hmga2−/− null mice are sterile) to obtain MMTV-Wnt1 transgenic mice with Hmga2+/+ (n=17), Hmga2+/− (n=18), and Hmga2−/− (n=9) genetic backgrounds. There was no difference in the timing and size of the tumors on the different HMGA2 backgrounds. Remarkably, there were 70% fewer mice with tumors on the Hmga2−/− background as compared to the Hmga2+/+ background (Fig. 5C). Interestingly, although Wnt1-mediated tumor formation typically results in a heterogeneous glandular structures with frequent blood- or secretion-filled cysts (27), no obvious glandular and cystic structures were observed on the Hmga2−/− genotypic background (Fig. 5B lower).
Interestingly, a small number of tumors did develop on the null background which were further analyzed by immunohistochemistry. Unlike in the wild-type tumors, TGFβRII and IGF2BP2 expression were absent at the edge of tumor on the Hmga2−/− genotypic background (Fig, 5B lower). In addition, β-catenin did not relocalize to the nucleus and was detected both at the edge and throughout the tumor (Supplementary, Fig. 4). Interestingly, E-cadherin was present both throughout the tumor and at the edge of the tumor (Fig. 5B lower). These results suggest that HMGA2 is required in mammary tumorigenesis for tumor development and progression that is initiated through the Wnt1 pathway, via the TGFβ signaling pathway.
HMGA2 and Metastasis
The next series of studies took advantage of two well-characterized mouse mammary tumor cell lines derived from a single spontaneously arising, mammary tumor in a BALB/cJ mouse (28) to further investigate direct role of HMGA2 in metastasis in vivo. 4TO7 cells disseminate from the primary tumor and can enter the bloodstream but cannot proliferate at their secondary site in the distant organs (28, 29). On the other hand, 4T1 cells have full metastatic properties where the cells from the primary tumor disseminate into the bloodstream and form macrometastasis in the liver and lung (29). A third cell line was generated which was a derivative of the 4TO7 cell line but expressed HMGA2-GFP (4TO7-HMGA2-GFP)(see Material and Methods). As was the case for the cell line described in Figure 1B, the 4TO7 cell attained a mesenchymal phenotype upon transfection of HMGA2 exhibiting spindle cell metaplasia and loss of E-cadherin. Interestingly, in our study TGFβRII was up-regulated in 4T1 cells as compared to in 4TO7 cells (Supplementary, Fig. 5).
Mice were inoculated with three different cell lines and formed palpable primary tumors within 6 days. HMGA2 was enhanced in the primary tumors injected with the 4T1 cells (Fig. 6A). Remarkably, 4TO7-HMGA2-GFP cells formed primary tumors more rapidly than the 4TO7-Mock cells after 9 days (Fig. 6B) and grew at the same rate as the 4T1 cells. In the 4T1 metastatic tumors, HMGA2 expressing cells were detected at the edge of the lung tumors and liver stroma (Glisson’s capsule) into the parenchyma (Supplementary Fig. 6A).
Figure 6.
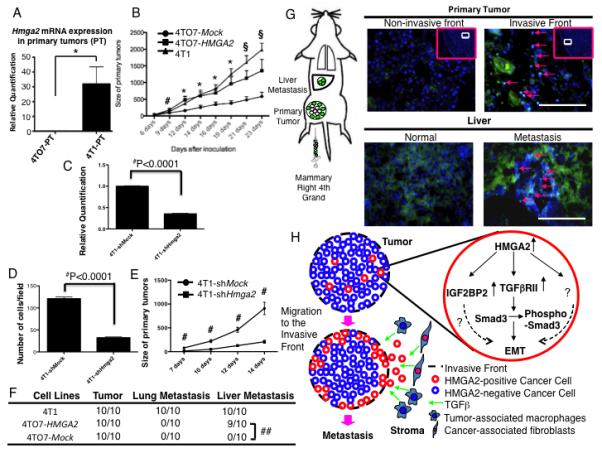
4TO7 cells stably over-expressing HMGA2 form primary tumors more rapidly and can metastasize to the liver. (A) Hmga2 mRNA expression in the primary tumor of 4T1 and 4TO7 derived cells. (B) Primary tumors derived from 4TO7-HMGA2 cells rapidly developed similar to 4T1 cells until 16 days after implantation when compared to 4TO7-Mock cells. #=P<0.05 (4TO7-Mock vs. 4TO7-HMGA2 cells). *=P<0.05 (4TO7-Mock vs. 4TO7-HMGA2 cells and 4TO7-Mock vs. 4T1 cells). §=P<0.05 (4TO7-Mock vs. 4TO7-HMGA2 cells) and P<0.001 (4TO7-Mock vs. 4T1 cells). (C) Hmga2 mRNA expression in 4T1-NTC-shRNA and 4T1-Hmga2-shRNA cells. ##=P<0.0001 (D) A matrigel invasion assay (4T1-NTC-shRNA and 4T1-Hmga2-shRNA cells). The number of “invaded” cells was counted per field (at 100× magnification) in at least 10 different fields per filter. The numbers are shown as mean±SD from two experiments performed in triplicate. ##=P<0.0001. (E) Primary tumors derived from 4T1-Hmga2-shRNA cells slowly developed 7 days after implantation when compared to 4T1-NTC-shRNA cells. #=P<0.001. (F) Liver metastases (arrows) were induced in 4TO7 cells stably over-expressing HMGA2 (4TO7-HMGA2), but not in 4TO7 Mock-transfected (4TO7-Mock) cells (##=P<0.0001). (G) 4TO7-HMGA2 cells were localized to the invasive front (arrows), but not at the non-invasive front of the primary tumor developed from the mixture between 4TO7-Mock and 4TO7-HMGA2 cells. 4TO7-HMGA2 cells were detected in the metastatic liver lesion (liver parenchyma, arrows). Scale bars represent 100 μm. (H) Schematic of HMGA2-induced metastasis. HMGA2 induces expression of the TGFβRII. These cells then undergo EMT, migrate and localize to the invasive front of the tumor where they interact with TGFβ from the stroma facilitating invasion and metastasis.
In order to determine if the loss of Hmga2 reduced the invasive properties of the 4T1 mouse invasive breast cancer cells and subsequent tumor development, we generated 4T1 cells stably expressing Hmga2 shRNA (4T1-Hmga2-shRNA cells)(see Material and Methods). Hmga2 mRNA levels were remarkably reduced in the 4T1-Hmga2-shRNA cells (Fig. 6C) and these 4T1-Hmga2-shRNA cells exhibited marked reduction of invasion in a matrigel invasion assay (Fig. 6D). Most remarkably, 7 days after injection the 4T1-Hmga2-shRNA cells formed primary tumors more slowly than the 4TO7-NTC-shRNA cells in vivo (Fig. 6E). Since the primary tumors were smaller no assessment of metastasis could be formulated for the 4T1-hmga2-shRNA cells.
Interestingly, metastases developed in 9 of 10 mice injected with 4TO7-HMGA2-GFP cells (##=P<0.0001)(Fig. 6F, Supplementary Fig. 6B) but exclusively in the liver and no metastasis was observed in the lung. So, the 4TO7-HMGA2-GFP cells are converted to a metastatic form in their ability to invade and colonize the liver parenchyma.
Finally, the HMGA2-GFP fusion protein was traced after implantation of a mixture of equal numbers of 4TO7-Mock and 4TO7-HMGA2-GFP cells. At the primary site, 4TO7-HMGA2-GFP cells were localized at the invasive front and were not detected in the centre of the primary tumors (Fig. 6G upper). Similarly, in the liver metastases, the majority of the 4TO7-HMGA2-GFP cells were detected especially in the liver parenchyma invaded by cancer cells emanating from the blood vessel into the stroma (Fig. 6G lower, Supplementary Fig. 7).
These results suggest that when HMGA2 is expressed in cells during tumor development, the cells have the ability to localize to the invasive front of the primary tumor and HMGA2 can directly induce liver metastasis in the mouse allograft model.
Discussion
The described studies define a fundamental pathway in the EMT and tumor metastasis. Our initial in vitro experiments established that HMGA2 converts non-invasive cell types into their invasive counterparts through the induction of the EMT. Importantly, the cells at the invasive front of human tumors preferentially express HMGA2 where the tumor cells exhibit the EMT and it is these cells that are said to be destined to become metastatic and progress further onto malignancy (10). The genetic studies with the mouse model of tumorigenesis reveal that the HMGA2 pathway is essential for tumor pathogenesis for the majority of tumors. Furthermore, the molecular mechanism by which HMGA2 mediates the induction of EMT was determined to go through the TGFβ canonical pathway, and specifically through the activation of the TGFβRII. Most decisively, the expression of TGFβRII was absent in the HMGA2 independent tumors leading to the conclusion that HMGA2 dependent tumor pathogenesis is mediated via the TGFβRII. Consistent with the distribution of the expression pattern of HMGA2 and its activation of the TGFβRII, we demonstrated that the TGFβRII is also expressed exclusively at the invasive front of human tumors.
The hypothesis for a relationship between genes that are involved in mesenchymal proliferation and differentiation and the EMT and hence invasion and metastasis is further strengthened by studies of these genes in human tumors. For HMGA2, the present study demonstrated that the protein is localized to the invasive front. Consistent with these findings, there were a number of studies that suggested a positive correlation between HMGA2 expression and severity of tumor grade for a variety of tumor cell types (5, 9). Furthermore, the presence of HMGA2 in the blood (presumably due to metastasizing circulating tumor cells) correlates with either tumor recurrence or mortality (6). Where analyzed, there are a number of similar observations as for HMGA2 on the expression of Snail, Slug and Twist in human tumors. Both Snail and Twist are maximally expressed in multiple tumor types of the most severe grade (30, 31) and expression in breast cancer is related to patients survival (32). Pertinently, Snail was mainly expressed at the invasive front in human oesophageal cancer (33) and both Snail and Twist are also found at the invasive front of the tumor in malignant parathyroid neoplasia (34). In addition, Slug is predominantly expressed at the invasive front of pancreatic tumor (35). We would predict that future cellular localization studies would reveal that these genes are also expressed at the invasive front in cells that have undergone EMT. The correlative human studies in conjunction with the in vitro and in vivo studies described in the results section clearly define the functional significance of HMGA2 expression in the EMT and metastasis. Although all three of these mesenchyme-specific genes induce EMT, this does not imply that they are in the same molecular pathway. For example, Twist does not induce Snail expression in carcinoma cells (36) and and our studies show there was not a statistically significant difference in twist or snail expression by HMGA2 in MCF7 cells (Supplementary Fig. 8). It should be noted that HMGA2 was said to induce the expression of Snail and Twist in normal murine mammary gland epithelial (NMuMG) cells (37) (see below). Cumulatively, these results suggest that in various cell types mesenchyme-specific molecular pathways are activated in tumor cells in order for cells to ultimately metastasize and potentially the particular EMT driver directs metastasis to a specific tissue.
The in vitro studies strongly indicate a role for HMGA2 in the EMT and the clinical studies suggest a role for HMGA2 in metastasis. Therefore, in order to understand the function of HMGA2 in metastasis directly in vivo, the two closely related mouse breast cancer cell lines, 4TO7 and 4T1 were utilized. The 4TO7-HMGA2-GFP cells localized to the invasive front of the primary tumor. Therefore, expression of the HMGA2 pathway causes the cells to migrate to the exterior of the tumor within a relatively short amount of time in tumor development (20 days). This would suggest that HMGA2 expression in tumor cells requires few, if any, additional genetic events to attain the capability to migrate to the invasive front of a tumor. Compared to the parental 4TO7 cells which are detected only within the blood vessels of Glisson’s capsule, the 4TO7-HMGA2-GFP cells are converted to a metastatic form in their ability to invade and colonize the liver parenchyma, similar to the 4T1 cells. Whether these 4TO7-HMGA2-GFP cells at the exterior of the primary tumor require additional genetic events to metastasize to distant organs remains to be elucidated. Interestingly, the 4TO7-HMGA2-GFP cells did not metastasize to the lung twenty-five days after implantation, as do the 4T1 cells. This tissue-specificity of colonization could be a function of HMGA2 or due to the loss of the 4TO7 cells capability to metastasize to the lung. Such a finding warrants further investigation and is an area of future study.
In the present study, ectopic HMGA2 expression mediates EMT through an interaction via the TGFβ signaling pathway that has been strongly implicated in EMT induction in vitro for a number of cell types (20, 38). Specifically, ectopic HMGA2 expression induced EMT and invasiveness through up-regulation of the TGFβRII in MCF-7 breast cancer cells (Fig. 1,3) without the activation of TGFβ1 expression (Supplementary Fig. 9). Also, in our study, the endogenous HMGA2 expression was not increased by TGFβ1 treatment in the MCF-7 breast cancer cells (Supplementary Fig. 10). It should be noted that with NMuMG cells, although Thuault et al. (2006) did observe EMT induced by the ectopic expression of HMGA2, treatment of these cells by TGFβ1 was found to increase HMGA2 expression from only the endogenous locus. This apparent discrepancy may lie in the difference between the two breast cancer cell types that were utilized which may represent tumor cells at variant competencies to the ligand. Most importantly, the function of HMGA2 in the EMT via the TGFβ pathway and more specifically, TGFβRII, derived from the in vitro studies were directly demonstrated to be relevant to in vivo tumors from both human and mouse studies. TGFβRII was highly expressed at the invasive front of the human breast (Fig. 4 upper) and colon cancer (Fig. 4 lower) tissue consistent with the expression of HMGA2 in vivo as well as in the mammary carcinomas of the MMTV-Wnt1 mouse model. In conclusion, HMGA2 activates the expression of the TGFβRII, directly or indirectly, in tumor cells and allows these tumor cells to respond to TGFβ which may be secreted from the tumor microenviroment (39, 40) during invasion and metastasis (Fig. 6H).
In addition, IGF2BP2 identified as a target gene of HMGA2 both during development (24) and in tumorigenesis (25), was detected in the cells at the invasive front of the MMTV-Wnt1 tumor. Studies demonstrate that secreted IGF2BP2 from metastatic cells allows for the recruitment of endothelial cells during metastasis (41). This suggests that HMGA2 could regulate metastasis not only through the TGFβ pathway but also by increasing endothelial cell recruitment through the activation of the downstream pathway. Therefore, HMGA2 can be considered a “driver” of the EMT and metastasis and delineation of the HMGA2 pathway will potentially define a series of genes specific for invasion and metastasis of epithelial cancer cells.
Supplementary Material
Acknowledgements
We thank F. Miller for the kind gift of the 4TO7 and 4T1 cells and Tina Zelonina for technical assistance.
Grant Support: Columbia University LAM Center and the National Heart, Lung, and Blood Institute (NHLBI), contracts R01-HL086936 and RC1HL100384 (JMD).
Financial Support: Grant support for the present study was provided by grants from the Columbia University LAM Center and the National Heart, Lung, and Blood Institute (NHLBI), contracts R01-HL086936 and RC1HL100384 (JMD).
Footnotes
Conflict of Interest: No potential conflict of interest.
References
- 1.Ashar HR, Fejzo MS, Tkachenko A, Zhou X, Fletcher JA, Weremowicz S, et al. Disruption of the architectural factor HMGI-C: DNA-binding AT hook motifs fused in lipomas to distinct transcriptional regulatory domains. Cell. 1995;82:57–65. doi: 10.1016/0092-8674(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 2.Anand A, Chada K. In vivo modulation of Hmgic reduces obesity. Nat Genet. 2000;24:377–80. doi: 10.1038/74207. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Benson KF, Ashar HR, Chada K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature. 1995;376:771–4. doi: 10.1038/376771a0. [DOI] [PubMed] [Google Scholar]
- 4.Huang ML, Chen CC, Chang LC. Gene expressions of HMGI-C and HMGI(Y) are associated with stage and metastasis in colorectal cancer. Int J Colorectal Dis. 2009;24:1281–6. doi: 10.1007/s00384-009-0770-7. [DOI] [PubMed] [Google Scholar]
- 5.Rogalla P, Drechsler K, Kazmierczak B, Rippe V, Bonk U, Bullerdiek J. Expression of HMGI-C, a member of the high mobility group protein family, in a subset of breast cancers: relationship to histologic grade. Mol Carcinog. 1997;19:153–6. doi: 10.1002/(sici)1098-2744(199707)19:3<153::aid-mc2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Langelotz C, Schmid P, Jakob C, Heider U, Wernecke KD, Possinger K, et al. Expression of high-mobility-group-protein HMGI-C mRNA in the peripheral blood is an independent poor prognostic indicator for survival in metastatic breast cancer. Br J Cancer. 2003;88:1406–10. doi: 10.1038/sj.bjc.6600935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Liu X, Li AY, Chen L, Lai L, Lin HH, et al. Overexpression of HMGA2 Promotes Metastasis and Impacts Survival of Colorectal Cancers. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarhadi VK, Wikman H, Salmenkivi K, Kuosma E, Sioris T, Salo J, et al. Increased expression of high mobility group A proteins in lung cancer. J Pathol. 2006;209:206–12. doi: 10.1002/path.1960. [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa J, Mitoro A, Kawashiri S, Chada KK, Imai K. Expression of mesenchyme-specific gene HMGA2 in squamous cell carcinomas of the oral cavity. Cancer Res. 2004;64:2024–9. doi: 10.1158/0008-5472.can-03-1855. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 11.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–7. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Liu Z, Shao C, Gong Y, Hernando E, Lee P, et al. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res. 71:349–59. doi: 10.1158/0008-5472.CAN-10-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–9. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 17.Fleming ID, Phillips JL, Menck HR, Murphy GP, Winchester DP. The National Cancer Data Base report on recent hospital cancer program progress toward complete American Joint Committee on Cancer/TNM staging. Cancer. 1997;80:2305–10. [PubMed] [Google Scholar]
- 18.Yang JL, Seetoo D, Wang Y, Ranson M, Berney CR, Ham JM, et al. Urokinase-type plasminogen activator and its receptor in colorectal cancer: independent prognostic factors of metastasis and cancer-specific survival and potential therapeutic targets. International journal of cancer Journal international du cancer. 2000;89:431–9. doi: 10.1002/1097-0215(20000920)89:5<431::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Kirschmann DA, Seftor EA, Nieva DR, Mariano EA, Hendrix MJ. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat. 1999;55:127–36. doi: 10.1023/a:1006188129423. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–36. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GD, Dixon MF, Smeeton NC, Williams NS. Observer variation in the histological grading of rectal carcinoma. Journal of clinical pathology. 1983;36:385–91. doi: 10.1136/jcp.36.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–52. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- 24.Brants JR, Ayoubi TA, Chada K, Marchal K, Van de Ven WJ, Petit MM. Differential regulation of the insulin-like growth factor II mRNA-binding protein genes by architectural transcription factor HMGA2. FEBS Lett. 2004;569:277–83. doi: 10.1016/j.febslet.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 25.Cleynen I, Brants JR, Peeters K, Deckers R, Debiec-Rychter M, Sciot R, et al. HMGA2 regulates transcription of the Imp2 gene via an intronic regulatory element in cooperation with nuclear factor-kappaB. Mol Cancer Res. 2007;5:363–72. doi: 10.1158/1541-7786.MCR-06-0331. [DOI] [PubMed] [Google Scholar]
- 26.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–25. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 28.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 29.Dykxhoorn DM, Wu Y, Xie H, Yu F, Lal A, Petrocca F, et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS One. 2009;4:e7181. doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida J, Horiuchi A, Kikuchi N, Hayashi A, Osada R, Ohira S, et al. Changes in the expression of E-cadherin repressors, Snail, Slug, SIP1, and Twist, in the development and progression of ovarian carcinoma: the important role of Snail in ovarian tumorigenesis and progression. Med Mol Morphol. 2009;42:82–91. doi: 10.1007/s00795-008-0436-5. [DOI] [PubMed] [Google Scholar]
- 31.Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 32.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–96. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Franz M, Spiegel K, Umbreit C, Richter P, Codina-Canet C, Berndt A, et al. Expression of Snail is associated with myofibroblast phenotype development in oral squamous cell carcinoma. Histochem Cell Biol. 2009;131:651–60. doi: 10.1007/s00418-009-0559-3. [DOI] [PubMed] [Google Scholar]
- 34.Fendrich V, Waldmann J, Feldmann G, Schlosser K, Konig A, Ramaswamy A, et al. Unique expression pattern of the EMT markers Snail, Twist and E-cadherin in benign and malignant parathyroid neoplasia. Eur J Endocrinol. 2009;160:695–703. doi: 10.1530/EJE-08-0662. [DOI] [PubMed] [Google Scholar]
- 35.Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–76. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Owens RB. Glandular epithelial cells from mice: a method for selective cultivation. J Natl Cancer Inst. 1974;52:1375–8. doi: 10.1093/jnci/52.4.1375. [DOI] [PubMed] [Google Scholar]
- 38.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–83. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karamitopoulou E, Zlobec I, Panayiotides I, Patsouris ES, Peros G, Rallis G, et al. Systematic analysis of proteins from different signaling pathways in the tumor center and the invasive front of colorectal cancer. Hum Pathol. 42:1888–96. doi: 10.1016/j.humpath.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


