Abstract
The emergence of antiestrogen resistance in breast cancer is an important clinical phenomenon affecting long-term survival in this disease. Identifying factors that convey cell survival in this setting may guide improvements in treatment. Estrogen (E2) can induce apoptosis in breast cancer cells that have been selected for survival after E2 deprivation for long periods (MCF-7:5C cells), but the mechanisms underlying E2-induced stress in this setting have not been elucidated. Here, we report that the c-Src kinase functions as a key adapter protein for the estrogen receptor (ER, ESR1) in its activation of stress responses induced by E2 in MCF-7:5C cells. E2 elevated phosphorylation of c-Src which was blocked by 4-hydroxytamoxifen (4-OHT), suggesting that E2 activated c-Src through the ER. We found that E2 activated the sensors of the unfolded protein response (UPR), IRE1α (ERN1) and PERK kinase (EIF2AK3), the latter of which phosphorylates eukaryotic translation initiation factor-2α (eIF2α). E2 also dramatically increased reactive oxygen species (ROS) production and up-regulated expression of heme oxygenase HO-1 (HMOX1), an indicator of oxidative stress, along with the central energy sensor kinase AMPK (PRKAA2). Pharmacological or RNAi-mediated inhibition of c-Src abolished the phosphorylation of eIF2α and AMPK, blocked E2-induced ROS production, and inhibited E2-induced apoptosis. Together, our results establish that c-Src kinase mediates stresses generated by E2 in long-term E2-deprived cells that trigger apoptosis. This work offers a mechanistic rationale for a new approach in the treatment of endocrine-resistant breast cancer.
Keywords: c-Src, endoplasmic reticulum stress, oxidative stress, apoptosis, estrogen receptor
Introduction
Developing drugs that target the estrogen receptor (ER) either directly (tamoxifen) or indirectly (aromatase inhibitors) has improved the prognosis of breast cancer (1,2). Although aromatase inhibitors show considerable advantages over tamoxifen with respect to patient disease free survival and tolerability, acquisition of resistance to all forms of endocrine treatments is inevitable (3,4). Multiple mechanistic changes are involved in antihormone resistance which provides the scientific rationale for the clinical development of additional targeted therapies (5,6). It is well known that the biological actions of E2 are mediated through the ER, which functions in the nucleus as ligand-dependent transcription factors to promote gene transcription and stimulation of cell growth (7). Paradoxically, laboratory evidence demonstrates that E2 can induce apoptosis in sensitive antihormone-resistant cells in vivo (8–10). This new targeted strategy provides novel therapeutic approaches to endocrine resistant breast cancer. A recent phase II clinical trial reports that E2 provides a clinical benefit for patients with aromatase inhibitor-resistant advanced breast cancer (11). Additionally, the laboratory results on E2-induced apoptosis using antihormone treated MCF-7 cells have been used to explain the reduction of breast cancer and the reduction in mortality observed in postmenopausal hysterectomized women in their 60’s treated with conjugated equine estrogen (CEE) when compared to a placebo treated control (12). The antitumor action of CEE is observed, not only during CEE treatment but for 6 years after treatment. These data suggest a cidal effect for CEE and has been noted recently (13). These encouraging clinical results prompted us to investigate the mechanisms underlying E2-induced apoptosis, to increase the therapeutic benefits of E2 in aromatase inhibitor resistant breast cancer.
Experimental evidence has established the oncogene, c-Src, as a critical component of multiple signaling pathways that regulate proliferation, survival, angiogenesis and metastasis (14,15). Increased c-Src activity is believed to play an important role in the development and progression of breast cancer (16), and c-Src has been considered as a survival signal for endocrine resistant breast cancer cells (17). Therefore, a c-Src inhibitor administered as a single agent or in combination with other anti-hormone therapy has the potential to enhance the inhibitory effects of antihormones and delay antihormone resistance (18). These observations highlight c-Src as an important therapeutic target for the treatment of human breast cancer.
Mitochondria are important intracellular organelles involved in apoptosis via an intrinsic pathway (19). Although the molecular mechanisms of E2-induced apoptosis are not fully understood, evidence indicates that mitochondrial related caspase pathways are involved (20, 21). Similarly, a variety of events in apoptosis focus on mitochondria, including the loss of mitochondrial transmembrane potential, release of cytochrome c, and participation of pro- and antiapoptotic Bcl-2 family proteins (22,23). However, accumulating evidence suggests that the endoplasmic reticulum where members of the Bcl-2 family of proteins localize, is also a major point of integration of pro-apoptotic signaling or damage sensing (24,25). The endoplasmic reticulum senses local stress such as unfolded protein through a set of pathways known as the unfolded protein response (UPR) (26), which activates three transmembrane sensors PRK-like endoplasmic reticulum kinase (PERK), inositol-requiring 1 alpha (IRE-1α), and activating transcription factor 6 (ATF-6) in endoplasmic reticulum (26). Depending on the duration and degree of stress, the UPR can provide either survival signals by activating adaptive and antiapoptotic signals, or death signals by inducing cell death programs (27, 28).
We have found that E2 changes the cell number according to the treatment period in long-term E2 deprived breast cancer cell lines MCF-7:5C and MCF-7:2A (25). E2 has the capacity to decrease around 80 percentage of cell number in MCF-7:5C cells after 7 days treatment whereas in MCF-7:2A cells after two weeks treatment (29). Unexpectedly, the c-Src inhibitor effectively rescues the decreasing of cell number by E2 in two long-term E2 deprived cell lines (29). The goal of this study is to identify the mechanisms underlying the early stage of E2-induced apoptosis and the function of c-Src in the process of E2-initiated apoptosis. To that end, we demonstrate that E2 triggers endoplasmic reticulum stress and oxidative stress which activate two main apoptotic pathways, the mitochondrial ('intrinsic') and death receptor ('extrinsic') pathways, whereas c-Src plays an essential role in mediating stress responses induced by E2 in MCF-7:5C cells. These findings have important clinical implications for the appropriate application of combination therapies in advanced aromatase inhibitor resistant breast cancer.
Materials and Methods
Materials
Estradiol was purchased from Sigma-Aldrich (St. Louis, MO). c-Src inhibitor PP2 was purchased from CalBiochem (San Diego, CA). ERα antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Total MAPK, phosphorylated MAPK, phosphorylated c-Src, phosphorylated eIF2α, total eIF2α, IRE1 α antibodies were from Cell Signaling Technology (Beverly, MA). Total c-Src mouse antibody was from Millipore (Temecula, CA). Estrogen dendrimer conjugate (EDC) was a kind gift by Dr. Katzenellenbogen (University of Illinois at Urbana-Champaign).
Cell culture conditions and cell proliferation assays
Estrogen-deprived MCF-7:5C cells were maintained in estrogen-free RPMI 1640 medium supplemented with 10% dextran-coated charcoal-stripped fetal bovine serum as previously described (20). The DNA fingerprinting pattern of cell line is consistent with the report by the ATCC (29). The DNA content of the cells, a measure of proliferation, was determined by using a DNA fluorescence Quantitation kit (29).
Cell cycles analysis
Briefly, MCF-7:5C cells were treated with vehicle (0.1% EtOH) and E2 (10−9 mol/L) respectively. Cells were harvested and gradually fixed with 75% EtOH on ice. After staining with propidium iodide (PI), cells were analyzed using a FACSort flow cytometer (Becton Dickinson, San Jose, CA), and the data were analyzed with ModFit software.
Annexin V analysis of apoptosis
The FITC Annexin V Detection Kit I ( BD Pharmingen, San Diego, CA) was used to quantify apoptosis by flow cytometry according to the manufacturer's instructions. In brief, MCF-7:5C cells were treated with different compounds respectively. Cells were suspended in 1× binding buffer and 1 × 105 cells were stained simultaneously with FITC-labeled annexin V (FL1-H) and propidium iodide (PI) (FL2-H). Cells were analyzed using FACSort flow cytometer (Becton Dickinson, San Jose, CA).
Mitochondrial/Transmembrane potential (Δψm) detection
Mitochondrial membrane potential was measured by flow cytometry using the cationic lipophilic green fluorochrome rhodamine-123 (Rh123) (Molecular Probes) as previously described (20). Disruption of ΔΨm is associated with a lack of Rh123 retention and a decrease in fluorescence.
Detection of oxidative stress
Intracellular ROS were detected by fluorescent dye 2',7'-dichlorofluorescein diacetate (H2DCFDA, Invitrogen) (30). Briefly, MCF-7:5C cells were treated with E2 for different time points using vehicle (0.1% EtOH) cells as control. Cells were loaded with 1 µM CM-H2DCFDA for 10 min and washed with PBS twice. Then, cells were monitored at fluorescence 530 nm and an excitation wavelength of 488 nm through flow cytometry.
Immunoblotting
Proteins were extracted in cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with Protease Inhibitor Cocktail (Roche, Indianapolis, IN) and Phosphatase Inhibitor Cocktail Set I and Set II (Calbiochem, San Diego, CA). The immunoblotting was performed as previously described (29).
Transient transfection reporter gene assays
Transient transfection assay was performed using a dual-luciferase system (Promega, Madison, WI). To determine ER transcriptional activity, cells were transfected with an estrogen response element (ERE)-regulated (pERE (5x) TA-ffLuc plus pTA-srLuc) dual-luciferase reporter gene sets. The cells were treated with E2 for 24 hours following the transfection. Then, the cells were harvested and processed for dual-luciferase reporter activity, in which the firefly luciferase activity was normalized by renilla luciferase activity.
Quantitative real-time RT-PCR
Total RNA, isolated with an RNeasy Micro kit (Qiagen, Valencia, CA), was converted to first-strand cDNA using a kit from Applied Biosystem (Foster City, CA). Quantitative real-time PCR assays were done with the SYBR Green PCR Master Mixes (Applied Biosystems, Foster City, CA) and a 7900HT Fast Real-time PCR System (Applied Biosystems, Foster City, CA). All primers were synthesized in Integrated DNA Technologies (San Diego, CA). The sequence of primers was shown in the Supplementary table S1. All the data were normalized by 36B4.
RNA sequencing (RNA-seq) analysis
MCF-7:5C cells were treated with different compounds for 72 hours. Cells were harvested in TRIzol. Total RNA was isolated with an RNeasy Micro kit. These long RNA samples were first converted into a library of cDNA fragments. Sequencing adaptors were subsequently added to each cDNA fragment and a 2×100 bp paired-end sequence was obtained from each cDNA using high-throughput sequencing technology (Illumina GAII). An average of 73.8 million such reads was produced for each sample. The resulting sequence reads were aligned to reference genome build hg19 using TopHat 1.3.0 (31), a splice junction aligner. Transcript abundance were estimated as Fragments Per Kilobase of exon per Million fragments mapped (FPKM), using Cufflinks 1.0.3 (32). Additional analysis was performed with the alternative expression analysis by sequencing (Alexa-seq) software package as previously described (33). Gene expression measures were compared between Cufflinks and Alexa-seq for the set of 17993 overlapping genes. Correlations were excellent with Spearman correlations of 0.955 to 0.971 for the six samples. Pathway analysis was performed with DAVID (34) on lists of differentially expressed gene lists.
Statistical analysis
All reported values are the means ± SE. Statistical comparisons were determined with two-tailed Student's t tests. Results were considered statistically significant if the P value was <0.05.
Results
c-Src mediated estrogen-activated growth pathways in long-term estrogen deprived breast cancer cells MCF-7:5C
It is well documented that E2 stimulates growth and prevents apoptosis in wild-type breast cancer cells and estrogen-responsive osteoblast cells (35,36). In contrast, physiological concentrations of E2 induce apoptosis in long-term E2 deprived breast cancer cells (20,21). c-Src plays a critical role in relaying ER signaling pathways in breast cancer cells (37). To investigate the function of E2 and c-Src in long-term E2-deprived breast cancer cells MCF-7:5C, a specific c-Src tyrosine kinase inhibitor, PP2, was utilized to block phosphorylation of c-Src (Fig. 1A). It also effectively abolished the growth pathways including the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT pathways in MCF-7:5C cells (Fig. 1A). E2 activated c-Src through ER since 4-hydroxytamoxifen (4-OHT) completely suppressed phosphorylation of c-Src (Fig. 1B). Although our previous finding showed that E2 initiates apoptosis in MCF-7:5C cells (20), E2 was able to activate non-genomic (Supplementary Fig. S1A) and genomic pathways in MCF-7:5C cells (Fig. 1C). These actions were blocked by the c-Src inhibitor, PP2 (Fig. 1C and Supplementary Fig. S1A). Even though the characteristic E2-induced apoptosis occurs after 72 hours treatment (20), cell numbers were initially increased by E2 with a high percentage in S phase (Fig.1D). All of these results suggested that E2 caused an imbalance between growth and apoptosis in MCF-7:5C cells.
Figure 1. c-Src mediated estrogen-activated growth pathways in MCF-7:5C cells.
A, MCF-7:5C cells were treated with vehicle (0.1% DMSO) and PP2 (5×10−6mol/L) for different durations. Phosphorylated c-Src, MAPK, and Akt were detected by immunoblotting. Total c-Src, MAPK, and Akt were used for loading controls. B, MCF-7:5C cells were treated with vehicle (0.1% DMSO), E2 (10−9mol/L), 4-OHT (10−6mol/L), E2 (10−9mol/L) plus 4-OHT (10−6mol/L), PP2 (5×10−6mol/L), E2 (10−9mol/L) plus PP2 (5×10−6mol/L) respectively for 48 hours. Phosphorylated c-Src was detected by immunoblotting. Total c-Src was used for loading control. C, MCF-7:5C cells were treated with E2 or combined with PP2 respectively for 24 hours. Phosphorylated MAPK and Akt were examined by immunoblotting. Total MAPK and Akt were used for loading controls. D, MCF-7:5C cells were treated with vehicle and E2 for different durations. Total DNA was determined using a DNA fluorescence quantitation kit. As a parallel experiment, MCF-7:5C cells were treated with vehicle and E2 for 72 hours. Cells were fixed for cell cycles analysis. P<0.05, * compared with respective control.
Inhibition of c-Src suppressed estrogen-induced apoptosis in MCF-7:5C cells
We have shown that long-term E2 deprivation increases c-Src activity (29). Therefore, we addressed the question of whether the c-Src inhibitor, PP2, in combination with E2 would enhance apoptosis in MCF-7:5C cells. Unexpectedly, the c-Src inhibitor blocked apoptosis initiated by E2 (Fig. 2A and Supplementary Fig. S1D). To confirm that inhibition of c-Src could block E2-induced apoptosis, a specific siRNA was utilized to knock down c-Src in MCF-7:5C cells (Fig. 2B), which reduced the percentage of Annexin V binding induced by E2 (Fig. 2C). Further experiments showed that E2 disrupted mitochondrial membrane potential (ΔΨm) after 48 hours treatment which was measured by flow cytometry using rhodamine 123 (Rh123) (Fig. 2D). The c-Src inhibitor PP2 and 4-OHT both prevented reduction of Rh123 retention induced by E2 (Fig. 2D). These data demonstrated that E2-triggered apoptosis utilize the c-Src tyrosine kinase pathway. To evaluate the role of the non-genomic pathway in E2-induced apoptosis, studies were completed with a synthetic ligand, estrogen dendrimer conjugate (EDC), that only activates the non-genomic pathway at certain concentration (38). The results demonstrated that EDC (10−8 mol/L) activated the non-genomic pathway incorporating c-Src (Supplementary Fig. S2). Importantly, EDC had no capacity to activate endogenous E2 target gene pS2 and did not induce apoptosis in MCF-7:5C cells (Supplementary Fig. S2). All of these findings suggested that the non-genomic pathway does not play a critical role in triggering E2-induced apoptosis.
Figure 2. Inhibition of c-Src suppressed estrogen-induced apoptosis in MCF-7:5C cells.
A, MCF-7:5C cells were treated with different compounds respectively as above for 72 hours and Annexin V binding assay was used to detect apoptosis. B, MCF-7:5C cells were transfected with siRNA of c-Src for 72 hours using non-target siRNA as control. c-Src was detected by immunoblotting. The β-actin was used for loading control. C, MCF-7:5C cells were transfected with c-Src siRNA and non-target siRNA as above. Then, they were treated with vehicle (0.1% EtOH) and E2 (10−9mol/L) respectively for 72 hours. Apoptosis was detected through Annexin V binding assay. P<0.05, * compared with control. D, MCF-7:5C cells were treated with different compounds respectively as above for 48 hours and cells were harvested to detect mitochondrial potential through Rh123. P<0.001, ** compared with control.
Suppression of E2-induced apoptosis by the c-Src inhibitor was independent of the classical estrogen response element (ERE) regulated transcriptional genes in MCF-7:5C cells
The ER is the initial site for E2 to induce apoptosis since anti-estrogens ICI 182,780 and 4-OHT completely block apoptosis triggered by E2 (reference 20 and Supplementary Fig. S3A). In addition to the mediation of ER growth pathways, c-Src is involved in the process of ligand-activated ER ubiquitylation (39). Therefore, blockade of c-Src tyrosine kinase with PP2 further increased ERα protein and mRNA expression levels in MCF-7:5C cells (Fig. 3A). E2 activated estrogen response element (ERE) activity which could be blocked by 4-OHT but not by PP2 (Fig. 3B). It was interesting to find that the c-Src inhibitor alone could up-regulate E2 inducible gene pS2 and was additive with E2 to elevate pS2 mRNA level (Fig. 3C). Another important ER target gene progesterone receptor (PR) has been regarded as an indicator of a functional ER pathway, since expression of PR is regulated by E2. Although the c-Src inhibitor alone did not elevate PR expression, it dramatically synergized with E2 to up-regulate PR mRNA (Fig. 3D). All of these results demonstrated that blockade of c-Src increased expression of classical ER target genes. It also implied that classical ER pathway might not directly involve in the E2-induced apoptosis.
Figure 3. Suppression of E2-induced apoptosis by the c-Src inhibitor was independent of the classical ERE regulated transcriptional genes in MCF-7:5C cells.
A, MCF-7:5C cells were treated with vehicle (0.1% DMSO) and PP2 (5×10−6mol/L) respectively for 24 hours. ERα protein was detected by immunoblotting. ERα mRNA was quantified with qPCR. P<0.05, * compared with control. B, MCF-7:5C cells were transfected with ERE firefly luciferase plasmid plus renilla luciferase plasmid. Then, cells were treated with different compounds respectively for 24 hours to detect ERE activity. P<0.001, ** compared with control. C, MCF-7:5C cells were treated with different compounds respectively for 24 hours. The pS2 mRNA was quantified with qPCR. P<0.001, ** compared with control. D, MCF-7:5C cells were treated with different compounds respectively for 72 hours. The PR mRNA was quantified with qPCR. P<0.001, ** compared with control.
c-Src was involved in the process of triggering apoptosis-related genes by E2 in MCF-7:5C cells
To further investigate the mechanisms of the suppression of E2-induced apoptosis by PP2, RNA-seq analysis was performed to examine the genes regulated by E2 to trigger apoptosis in MCF-7:5C cells. A wide range of apoptosis-related genes were activated by E2 (Fig.4A), which were functionally classified into three groups: TP53-related genes (such as TP63, PMAIP1, and CYFIP2), stress-related genes (such as HMOX1, PPP1R15A, ZAK, NUAK2 etc.), and inflammatory response-related genes (such as LTB, FAS, TNFRSF21, and CXCR4 etc.). Most were stress-related genes (Supplementary Fig. S3B). Consistent with the biological experiments, 4-OHT and PP2 both blocked apoptosis-related genes induced by E2 but to a different extent in MCF-7:5C cells (Fig.4A). The majority of these apoptosis-related genes were confirmed by real-time PCR with similar changes noted as in RNA-seq analysis (Fig. 4B, 4C, 4D and Supplementary Fig. S4). E2 dramatically increased p63 mRNA levels (Fig. 4B) but did not arrest cells in the G1 phase. In fact, S phase was markedly elevated in MCF-7:5C cells (Fig. 1D). Heme oxygenase 1 (HMOX1) which is active at high concentrations of heme, catalyzes the degradation of heme and is thought to function as an oxidative stress indicator (40). In breast cancer cells, cytochrome c is a major source of heme protein found in the inner membrane of the mitochondrion. E2 markedly increased HMOX1 in MCF-7:5C cells (Fig. 4C) thereby confirming that E2 may damage the mitochondria and caused cytochrome c release. In contrast to MCF-7:5C cells, E2 decreased HMOX1 levels in wild-type MCF-7 cells (Supplementary Fig. S5A) and clearly did not change HMOX1 expression in another long-term E2 deprived cell line MCF-7:2A (Supplementary Fig. S5B), both of MCF-7 and MCF-7:2A do not undergo apoptosis after exposure to E2 in the first three days. Additionally, E2 upregulated tumor necrosis factor (TNF) family members (such as TNFα, LTA, and LTB), which were abolished by 4-OHT and PP2 (Fig. 4D and Supplementary Fig. S6A and S6B). Low dose of TNFα activated pro-apoptotic pathways in MCF-7:5C cells and inhibited cell growth (Supplementary Fig. S6C and S6D). All of these data suggested that E2 widely activated intrinsic and extrinsic apoptosis pathways and c-Src was directly involved in mediating apoptosis.
Figure 4. c-Src was involved in the process of triggering apoptosis-related genes by E2 in MCF-7:5C cells.
A, MCF-7:5C cells were treated with vehicle and different compounds respectively as above for 72 hours. Cells were harvested in TRIzol for RNA-seq analysis. B, MCF-7:5C cells were treated with different compounds as above. TP63 mRNA was quantified with qPCR. P<0.001, ** compared with control. C, HMOX1 mRNA was quantified with qPCR. P<0.001, ** compared with control. D, TNFα mRNA was quantified with qPCR. P<0.001, ** compared with control.
The c-Src inhibitor blocked estrogen-induced oxidative stress in MCF-7:5C cells
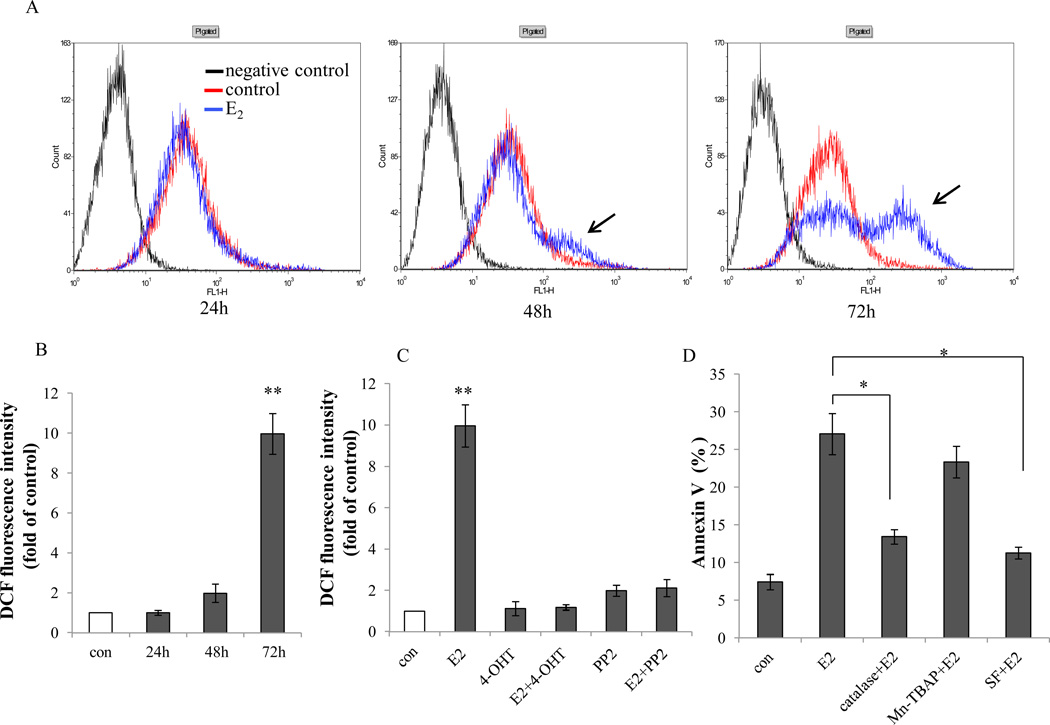
Reactive oxygen species (ROS) are the product of oxidative stress by mitochondria, whereas an increase in ROS contributes to degenerative changes in mitochondrial function (41). Under physiological conditions, cellular ROS levels are tightly controlled by low-molecular-weight radical scavengers and by a complex intracellular network of enzymes such as catalases (CAT) and superoxide dismutases (SOD). Under conditions of lethal stress, ROS are considered as key effectors of cell death (42). Intracellular ROS were detected by CM-H2DCFDA through flow cytometry (Fig.5A). Detectable ROS appeared after 48 hours of treatment with E2. The production of ROS reached a peak after 72 hours treatment (Fig.5A and 5B). Blocking ER (by 4-OHT) and c-Src (by PP2) abolished ROS generation induced by E2 (Fig.5C), indicating that both ER and c-Src were upstream signals of ROS. Free radical scavengers Mn-TBAP, catalase, and sodium formate (SF) which respectively act on superoxide radical (O2−), H2O2, and hydroxyl radical (OH−), were utilized to suppress the production of ROS. Our results suggested that H2O2 and OH− were the major sources of ROS induced by E2. This conclusion was based on the observation that catalase and sodium formate inhibited E2-induced apoptosis, whereas Mn-TBAP was less effective (Fig. 5D). The RNA-seq analysis demonstrated that E2 did not significantly regulate antioxidant enzymes such as catalases (CAT) and superoxide dismutases (SOD) in MCF-7:5C cells (data not shown). Our results suggest that E2 has the potential to damage mitochondria to cause oxidative stress.
Figure 5. The c-Src inhibitor blocked estrogen-induced oxidative stress in MCF-7:5C cells.
A, MCF-7:5C were treated with vehicle and E2 for different durations. ROS was detected through flow cytometry. B, Quantification of ROS production induced by E2 was compared with control. P<0.001, ** compared with control. C, MCF-7:5C cells were treated with different compounds as above. ROS production was detected through flow cytometry. P<0.001, ** compared with control. D, MCF-7:5C cells were treated with vehicle (0.1% EtOH), E2 (10−9mol/L), catalase (5000U/mL) plus E2 (10−9mol/L), Mn-TBAP(5×10−5mol/L) plus E2 (10−9mol/L), sodium formate (2×10−3mol/L ) plus E2 (10−9mol/L) for 72 hours. Apoptosis was detected through Annexin V binding assay. P<0.05, * compared with E2 treated group.
c-Src was involved in estrogen-induced endoplasmic reticulum stress in MCF-7:5C cells
Our previous global gene array data show that E2 activates genes related to endoplasmic reticulum stress in MCF-7:5C cells (25). To relieve stress, sensors of unfolded protein responses (UPR) are activated as initial responses (43). In this study, a significant induction of UPR sensors, inositol-requiring protein 1 alpha (IRE1α) and PERK/eukaryotic translation initiation factor-2α (eIF2α), by E2 occurred after 24 hours of treatment and was further increased by prolonging treatment times in MCF-7:5C cells (Fig. 6A). The antiestrogen 4-OHT completely abolished the response (Fig. 6A). The PERK inhibitor blocked phosphorylation of eIF2α and prevented E2-induced apoptosis (Fig. 6B and 6C), confirming that endoplasmic reticulum stress was important in the apoptosis initiated by E2. Phosphorylated eIF2α closely associates with an important cellular energy sensor, adenosine monophosphate (AMP)-activated protein kinase (AMPK), to regulate protein translation and apoptosis (44). AMPK, which phosphorylates many metabolic enzymes to stimulate catabolic pathways and increases the capacity of cells to produce ATP (45), was significantly activated after 48 hours treatment with E2 (Fig. 6D). The c-Src inhibitor, PP2, blocked the phosphorylation of eIF2α but not IRE1α induced by E2 (Fig. 6E). PP2 also prevented the activation of AMPK after E2 treatment (Fig. 6F). All of these data indicate that c-Src acts as an important transducer in the protein kinase pathways (eIF2α and AMPK) of stress response (Fig. 6E and 6F) that result in apoptosis.
Figure 6. c-Src was involved in estrogen-induced endoplasmic reticulum stress in MCF-7:5C cells.
A, MCF-7:5C were treated with E2 (10−9mol/L) or combined with 4-OHT (10−6mol/L) for different durations. IRE1α and phosphorylated eIF2α were used as indicators of UPR activation. B, MCF-7:5C cells were treated with vehicle (0.1% DMSO), E2 (10−9mol/L), PERK inhibitor (1×10−5mol/L), E2 (10−9mol/L) plus PERK inhibitor (1×10−5mol/L) respectively for 24 hours. Phosphorylated eIF2α was examined as the downstream of PERK. Total eIF2α was determined for loading control. C, MCF-7:5C cells were treated with E2 or combined with PERK inhibitor respectively for 72 hours. Apoptosis was detected through Annexin V binding assay. D, MCF-7:5C cells were treated with E2 or combined with 4-OHT as above. Phosphorylated AMPK was examined by immunoblotting. Total AMPK was determined for loading control. E, MCF-7:5C cells were treated with E2 or combined with PP2 for 24 hours. IRE1α and phosphorylated eIF2α were examined by immunoblotting. Total eIF2α and β-actin were determined for loading controls. F, MCF-7:5C cells were treated with E2 or combined with PP2 for 48 hours. Phosphorylated AMPK and total AMPK were examined by immunoblotting.
Discussion
We have previously investigated the inhibitory effects of E2 on long-term endocrine resistant breast cancer tumor growth in vivo (8–10). And we have confirmed this therapeutic effect is related with the apoptosis induced by E2 (20). This scientific discovery has been used in the clinical trials to treat aromatase inhibitor resistant breast cancer patients and 30% of patients receive benefit (11). The potential limitation on translational research in the treatment of hormone responsive breast cancer is that only four ER positive breast cancer cell lines are available to use routinely (46). Only MCF-7 of the four produces the phenotype of E2-induced apoptosis observed clinically (20,21). The purpose of establishing long-term E2 deprivation in vitro models is to mimic administration of an aromatase inhibitor that reduces levels of circulating estrogen in clinical studies (47). After a period of proliferative quiescence lasting a few months, the return of proliferation is similar to the relapses observed 12–18 months after primary hormonal therapy in patients. Multiple pathways are involved in the adaptive response to the pressure of E2 deprivation (48). Although MCF-7 cells grown long term have been shown to differ substantially in various properties depending upon the number of passages and geographic source of the cell lines, induction of apoptosis by physiological concentrations of E2 is the common characteristic of these in vitro model systems (20,21). Nevertheless, how E2 induces apoptosis is at present unclear. Our new observation (29) that a c-Src inhibitor paradoxically can block E2-induced apoptosis naturally demands further study. We examined this aspect of c-Src pharmacology to describe fully this phenomenon and gain an insight into the convergence of ER and c-Src pathways for the modulations of an apoptotic trigger in breast cancer. Here, for the first time we document that c-Src participates in the mediation of stress responses induced by E2 to widely activate apoptosis-related genes involved in the intrinsic and the extrinsic apoptosis pathways.
The ER is the initial point for E2 to induce apoptosis since anti-estrogens ICI 182,780 and 4-OHT completely block apoptosis triggered by E2 (reference 20 and Supplementary Fig. S3A). Contradictory to the traditional apoptosis mechanism caused by cytotoxic chemotherapy with cell cycles arrest, E2-induced apoptotic cells simultaneously undergo proliferation with an increased S phase of cell cycle resulting in increased cell number despite p53 family members being up-regulated (Fig.1D and Fig.4B). E2 exerts a dual function on MCF-7:5C cells, with both initial proliferation and the apoptosis. In other words, the initial response of E2 to stimulate growth is the up-regulating of classical transcriptional activity by ER (Fig. 3B) without any detected apoptotic changes in the first 24 hours. Activation of apoptotic genes appeared after 48 hours treatment with E2 (data not shown), and reached a peak by 72 hours (Fig. 4B, 4C, and 4D). Consistently, characteristic apoptosis occurred at 72 hours (Fig. 2A). These data suggest that the higher rate of proliferation by E2 might activate other pathways to trigger apoptosis. Our data demonstrate that E2 caused endoplasmic reticulum stress which activated the unfolded protein response (UPR) within 24 hours (Fig. 6A). The initial aim of UPR is to restore normal function of the cell, however, if the damage is too severe to repair, the UPR ultimately initiates cell death through activation of the apoptotic pathway (49).
c-Src functioned as an important downstream signal of ER in MCF-7:5C cells, which was activated by E2 (Fig.1B, Supplementary Fig. S1A, S1B, and S1C) and demonstrated multiple levels of association with ER (Fig. 1B, 1C, Fig. 2A, Fig. 3A, 3C, and 3D). An important finding in this study is that c-Src tyrosine kinase is critical for E2-induced apoptosis (Fig. 2A, 2C, and 2D). This, therefore, raised the question of the actual role played by c-Src in the process of apoptosis induced by E2. c-Src mediated PI3K/AKT and MAPK growth pathways by E2 (Fig. 1C). However, specific inhibitors of PI3K/Akt (LY294002) and MAPK (U0126) could inhibit cell growth but not prevented E2-induced apoptosis in MCF-7:5C cells (Supplementary Fig. S7), which imply that MAPK/Akt growth pathways are not directly involved in the apoptosis-induced by E2. In MCF-7:5C cells, E2 activated the non-genomic pathway after 10 minutes treatment and the c-Src inhibitor blocked the non-genomic pathway (Supplementary Fig. S1A and S1B). Detectable elevation of c-Src phosphorylation appeared after 30 mins treatment with E2 (Supplementary Fig. S1B). Consistent stimulation of c-Src appeared after 24 hours treatment and gradually increased when extending to 48 hours (Fig. 1B and Supplementary Fig. S1C). All of these data suggest that c-Src activation is a direct effect resulting from E2. To further explore the function of the non-genomic pathway in the process of E2-induced apoptosis, EDC was used to treat MCF-7:5C cell which is very ineffective in stimulating transcription of endogenous E2 target genes (38). The EDC (10−8 mol/L) activated the non-genomic pathway but without capacity to activate genomic pathway and did not induce apoptosis in MCF-7:5C cells (Supplementary Fig. S2). All of these results suggest that the non-genomic pathway does not play a critical role in the E2-induced apoptosis. Interestingly, the EDC could continuously activate c-Src and Akt but without any effect on MAPK after 24 hours treatment (Supplementary Fig. S2E), which may be resulted from enhanced association between ERα and membrane growth factor receptor (48).
Additionally, E2 activated classical ERE activity but the c-Src inhibitor could not block the response (Fig. 3B). Furthermore, the c-Src inhibitor collaborated with E2 to up-regulate endogenous ER target genes pS2 and PR (Fig. 3C and 3D). All of these results imply that classical ER transcriptional pathways are not directly involved in E2-induced apoptosis. Similarly, Zhang et al reported that the inhibitory effects of E2 on cell growth are independent of the classical ERE regulated transcriptional genes (50). Our global gene array data suggest that E2 signaling can occur through a non-classical transcriptional pathway involving the interaction of ER with other transcription factors such as activator protein-1 (AP-1) and Sp1, which may regulate stress responses (25). In the present study, E2 initiated UPR (Fig. 6A), increased ROS production (Fig. 5A), and widely activated apoptosis related genes (Fig. 4A). The c-Src was involved in the stress responses and inhibition of c-Src decreased the expression of apoptosis related genes induced by E2, which are critical mechanisms for the blockade of c-Src to prevent E2-induced apoptosis.
Overall, E2 induces endoplasmic reticulum and mitochondrial stresses in MCF-7:5C cells, which subsequently up-regulates apoptosis-related genes to activate intrinsic and extrinsic apoptotic pathways. Unexpectedly, c-Src tyrosine kinase plays a critical role in the stress response induced by E2. These data clearly raise a concern regarding the ubiquitous use of c-Src inhibitors to treat patients with advanced aromatase inhibitor-resistant breast cancer, thereby undermining the beneficial effects of E2-induced apoptosis.
Supplementary Material
Acknowledgments
Grants support
VCJ is supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409; the Susan G Komen For The Cure Foundation under Award number SAC100009; GHUCCTS CTSA (Grant # UL1RR031975) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. JWG is supported by the SU2C (AACR) Grant (SU2C-AACR-DT0409). JAK is supported by NIH grant (PHS 5R37DK015556). OLG was supported by a fellowship from the Canadian Institutes of Health Research (CIHR). The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
There are no conflicts to disclose.
References
- 1.Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69:1243–1254. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009;69:1416–1428. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan VC, O'Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25:5815–5824. doi: 10.1200/JCO.2007.11.3886. [DOI] [PubMed] [Google Scholar]
- 5.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 6.Sabnis G, Goloubeva O, Jelovac D, Schayowitz A, Brodie A. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway improves response of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res. 2007;13:2751–2757. doi: 10.1158/1078-0432.CCR-06-2466. [DOI] [PubMed] [Google Scholar]
- 7.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 8.Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O’Regan RM, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–2036. [PubMed] [Google Scholar]
- 9.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–1608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 11.Ellis MJ, Gao F, Dehdashti F, Jeff DB, Marcom PK, Carey LA, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obiorah I, Jordan VC. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause. 2013 doi: 10.1097/GME.0b013e31828865a5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Ann Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 15.Pascoe D, Oursler MJ. The Src signaling pathway regulates osteoclast lysosomal enzyme secretion and is rapidly modulated by estrogen. J Bone Miner Res. 2001;16:1028–1036. doi: 10.1359/jbmr.2001.16.6.1028. [DOI] [PubMed] [Google Scholar]
- 16.Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA. A Src/AbI Kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res. 2007;67:1580–1588. doi: 10.1158/0008-5472.CAN-06-2027. [DOI] [PubMed] [Google Scholar]
- 17.Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–1360. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- 18.Hiscox S, Jordan NJ, Smith C, James M, Morgan L, Taylor KM, et al. Dual targeting of SRC and ER prevents acquired antihormone resistance in breast cancer cells. Breast Cancer Res Treat. 2009;115:57–67. doi: 10.1007/s10549-008-0058-6. [DOI] [PubMed] [Google Scholar]
- 19.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 21.Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17 beta-estradiol. J Natl Cancer Inst. 2001;93:1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 22.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 23.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 25.Ariazi EA, Cunliffe HE, Lewis-Wambi JS, Slifker MJ, Willis AL, Ramos P, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci USA. 2011;108:18879–18886. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakrabarti A, Chen AW, Varner JD. A review of the mammalian unfolded protein response. Biotechnol Bioeng. 2011;108:2777–2793. doi: 10.1002/bit.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai YC, Weissman AM. The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer. 2010;1:764–778. doi: 10.1177/1947601910383011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts CG, Gurisik E, Biden TJ, Sutherland RL, Butt AJ. Synergistic cytotoxicity between tamoxifen and the plant toxin persin in human breast cancer cells is dependent on Bim expression and mediated by modulation of ceramide metabolism. Mol Cancer Ther. 2007;6:2777–2785. doi: 10.1158/1535-7163.MCT-07-0374. [DOI] [PubMed] [Google Scholar]
- 29.Fan P, McDaniel RE, Kim HR, Clagett D, Haddad B, Jordan VC. Modulating therapeutic effects of the c-Src inhibitor via oestrogen receptor and human epidermal growth factor receptor 2 in breast cancer cell lines. Eur J Cancer. 2012;48:3488–3498. doi: 10.1016/j.ejca.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ. 2011;18:282–292. doi: 10.1038/cdd.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Baren MJ, et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffith M, Griffith OL, Mwenifumbo J, Goya R, Morrissy AS, Morin RD, et al. Alternative expression analysis by RNA sequencing. Nat Methods. 2010;7:843–847. doi: 10.1038/nmeth.1503. [DOI] [PubMed] [Google Scholar]
- 34.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 35.Fan P, Yue W, Wang JP, Aiyar S, Li Y, Kim TH, et al. Mechanisms of resistance to structurally diverse antiestrogens differ under premenopausal and postmenopausal conditions: evidence from in vitro breast cancer cell models. Endocrinology. 2009;150:2036–2045. doi: 10.1210/en.2008-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, et al. Nongenotropic, sex-onspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 37.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–7989. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 38.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 39.Chu I, Arnaout A, Loiseau S, Sun J, Seth A, McMahon C, et al. Src promotes estrogen-dependent estrogen receptor alpha proteolysis in human breast cancer. J Clin Invest. 2007;117:2205–2215. doi: 10.1172/JCI21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araujo JA, Zhang M, Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda S, Umeda M, Uchida H, Kato H, Araki T. Alterations of oxidative stress markers and apoptosis markers in the striatum after transient focal cerebral ischemia in rats. J Neural Transm. 2009;116:395–404. doi: 10.1007/s00702-009-0194-0. [DOI] [PubMed] [Google Scholar]
- 43.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 44.Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2a in adipocytes Biochem Biophys Res Commun. 2006;340:43–47. doi: 10.1016/j.bbrc.2005.11.159. [DOI] [PubMed] [Google Scholar]
- 45.Birnbaum MJ. Activating AMP-activated protein kinase without AMP. Mol Cell. 2005;19:289–290. doi: 10.1016/j.molcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Sweeney EE, McDaniel RE, Maximov PY, Fan P, Jordan VC. Models and Mechanisms of Acquired Antihormone Resistance in Breast Cancer: Significant Clinical Progress Despite Limitations. Horm Mol Biol Clin Investig. 2012;9:143–163. doi: 10.1515/hmbci-2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang SY, Wolf DM, Yingling JM, Chang C, Jordan VC. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol Cell Endocrinol. 1992;90:77–86. doi: 10.1016/0303-7207(92)90104-e. [DOI] [PubMed] [Google Scholar]
- 48.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura S, et al. Adaptive hypersensitivity to estrogen: Mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95:155–165. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Zhao H, Asztalos S, Chisamore M, Sitabkhan Y, Tonetti DA. Estradiol-induced regression in T47D:A18/PKCalpha tumors requires the estrogen receptor and interaction with the extracellular matrix. Mol Cancer Res. 2009;7:498–510. doi: 10.1158/1541-7786.MCR-08-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.