Abstract
High‐grade astrocytomas (HGAs), corresponding to World Health Organization grades III (anaplastic astrocytoma) and IV (glioblastoma; GBM), are biologically aggressive, and their molecular classification is increasingly relevant to clinical management. PDGFRA amplification is common in HGAs, although its prognostic significance remains unclear. Using fluorescence in situ hybridization (FISH), the most sensitive technique for detecting PDGFRA copy number gains, we determined PDGFRA amplification status in 123 pediatric and 263 adult HGAs. A range of PDGFRA FISH patterns were identified and cases were scored as non‐amplified (normal and polysomy) or amplified (low‐level and high‐level). PDGFRA amplification was frequent in pediatric (29.3%) and adult (20.9%) tumors. Amplification was not prognostic in pediatric HGAs. In adult tumors diagnosed initially as GBM, the presence of combined PDGFRA amplification and isocitrate dehydrogenase 1 (IDH1)R132H mutation was a significant independent prognostic factor (P = 0.01). In HGAs, PDGFRA amplification is common and can manifest as high‐level and focal or low‐level amplifications. Our data indicate that the latter is more prevalent than previously reported with copy number averaging techniques. To our knowledge, this is the largest survey of PDGFRA status in adult and pediatric HGAs and suggests PDGFRA amplification increases with grade and is associated with a less favorable prognosis in IDH1 mutant de novo GBMs.
Keywords: astrocytoma, FISH, IDH1, isocitrate dehydrogenase 1, PDGFRA, prognosis
Introduction
High‐grade astrocytomas (HGAs), including anaplastic astrocytoma (AA), World Health Organization (WHO) Grade III, and glioblastoma (GBM), WHO Grade IV, occur in both adults and children and are among the deadliest forms of cancer. Current practice stratifies HGAs based upon clinical, histopathologic and limited molecular features. As our understanding of gliomagenesis and tumor response to therapy improves, this stratification will likely undergo multiple revisions with incorporation of additional molecular markers. As such, the continued development and validation of robust methods to assess molecular alterations in HGA is critical.
HGAs are characterized by alterations in receptor tyrosine kinase (RTK) signaling, and abnormal platelet‐derived growth factor (PDGF) signaling has been demonstrated in a significant subset of both adult and pediatric tumors. In adult HGAs, PDGF receptor alpha (PDGFRA) is the second most commonly altered RTK receptor after epidermal growth factor receptor (EGFR), with amplification of the PDGFRA locus being the most common mechanism 7, 9, 18, 25, 27, 30. While estimates vary, in a large study using array‐based comparative genomic hybridization (CGH), amplification of PDGFRA was identified in 11% of patients 27. Increased PDGF pathway activity, however, has been reported in up to 33% of adult GBM 3. Indeed, PDGF signaling pathway alterations are a characteristic feature of many tumors designated as “proneural” based on genomic, transcriptomal and proteomic features 3, 21, 30. However, while tumors with a proneural phenotype may have an improved overall survival 21, other studies have suggested that PDGFRA copy number gain/amplification may be associated with worse overall survival in astrocytoma 1, 29.
In children, increased PDGF signaling is also thought to be an important driver of HGAs and PDGFRA amplification is similarly considered a common mechanism, with frequencies ranging from 3.4 to 12% 16, 20, 23. In specific clinical subsets of HGA, such as diffuse intrinsic pontine glioma, up to 25% of tumors may have amplification 32, potentially corresponding to its distinctive biologic properties 22. Despite the relatively high frequency of PDGFRA amplification in both pediatric and adult HGAs, the prognostic significance of this alteration remains largely unclear.
Mutations in isocitrate dehydrogenase 1 (IDH1) are common in adult AA and in subsets of adult GBM, including GBM that have progressed from a lower‐grade astrocytoma (secondary GBM); they are also found in a small subset of tumors diagnosed initially as GBM (de novo GBM) 5, 8, 19, 31. IDH1 mutant tumors, with R132H being the most common mutation, exhibit unique spatial, temporal and biologic characteristics, including enhanced overall survival relative to IDH1 wild‐type tumors of a similar grade 15, 17. While infrequent in de novo GBM, IDH1 mutations are enriched in the proneural subtype, which is also characterized by alterations in PDGFRA signaling 21, 30.
Currently, there are no established criteria for the assessment of PDGFRA copy number gain/amplification in clinical samples. While many studies have relied on copy number averaging techniques, such as polymerase chain reaction (PCR) and single nucleotide polymorphism arrays, these methods may underestimate the frequency of PDGFRA amplifications when only scattered cells are amplified or the degree of amplification is low level. This is particularly true given the tremendous intratumoral heterogeneity of HGAs as recently illustrated for EGFR and PDGFR 24, 25. As such, the simplest and most sensitive technique for detecting copy number gains in routinely processed pathology specimens is fluorescence in situ hybridization (FISH). Using this technique, we assess a large series of HGAs, provide practical interpretive guidelines for the clinical assessment of PDGFRA copy numbers, and examine the prognostic significance of amplification in both adult and pediatric cohorts.
Materials and Methods
Cohort
Formalin‐fixed, paraffin‐embedded (FFPE) tumor tissue from a total of 123 pediatric HGAs and 307 adult HGAs, including 103 adult AAs, 187 de novo GBMs (i.e. tumors initially diagnosed as GBM), and 17 IDH1 mutant secondary GBMs (i.e. GBM documented progression from a lower‐grade astrocytoma) were obtained from 10 institutions: UCSF Brain Tumor Research Center (BTRC) Tissue Bank; Department of Pathology, Newcastle General Hospital; Department of Pathology, Children's Hospital, Los Angeles; Department of Lab Medicine and Pathobiology and Department of Surgery, University of Toronto; Department of Pathology & Laboratory Medicine, University of Kentucky College of Medicine; Department of Pathology and Laboratory Medicine, Emory University; Department of Pathology, Gemelli Hospital, University of Sacred Heart, Rome Italy; Department of Pathology and Laboratory Medicine, The Children's Hospital of Philadelphia, Philadelphia, PA; Department of Pathology and Laboratory Medicine, University of Pennsylvania; and Brain Tumor Translational Resource, Department of Pathology and Laboratory Medicine, University of California Los Angeles. These included both whole tissue and tissue microarray (TMA) sections, the latter obtained from ten previously generated HGA TMAs. Clinical characteristics are summarized in Table 1. Pediatric (0.1–20 years of age) and adult (25.7–83 years of age) astrocytoma patients were diagnosed with either AA, WHO grade III, or GBM, WHO grade IV. Clinical and molecular characteristic of the tumor were obtained when available from the respective institutions and included survival from time of initial surgery, age at initial diagnosis, sex, and IDH1 mutation status (IDH1R132H) using IDH1(R132H) immunohistochemistry (H09, Dianova GmbH, Hamburg, Germany) or sequencing 12.
Table 1.
Clinical and molecular characteristics of 123 pediatric and 263 adult HGAs
| Pediatric | Adult | |||
|---|---|---|---|---|
| AA (n = 66) | GBM (n = 57) | AA (n = 103) | GBM (n = 160) | |
| Mean age (years ± SD) | 9.06 ± 5.16 | 10.1 ± 5.0 | 50.6 ± 13.8 | 55.1 ± 13.1 |
| Sex ratio (M : F) | 1.0 | 1.0 | 8.1 | 1.6 |
| Location† (supra‐ vs. infra‐tentorial) (%) | 86.0% | 72.3% | 96.7% | N/A |
| Location (frontal vs. other) | 14.0% | 6.38% | 47.2% | N/A |
| IDH1 mutant | N/A | N/A | 62.5% (n = 96) | 7.50% (n = 160) |
| Median survival (days) | 490 (n = 34) | 596 (n = 29) | 2070 (n = 69) | 450 (n = 148) |
AA = anaplastic astrocytoma; GBM = glioblastoma; IDH1R132H = isocitrate dehydrogenase 1; N/A = data are not available; n = number analyzed; SD = standard deviation.
†Data on tumor location was available for 43 pediatric AA, 47 pediatric GBM, and 91 adult AA.
FISH (PDGFRA)
Dual‐color FISH analysis was performed on 5‐μm thick FFPE whole and TMA sections as previously described 12, 14. Briefly, sections were deparaffinized, digested with pepsin, heat denatured and allowed to hybridize with probe sets overnight at 37°C in a humidified oven. A SpectrumOrange (SO)‐labeled home brew probe for PDGFRA (BAC clone RP11‐231C18, CHORI BACPAC Resources Center, Oakland, CA, USA; previously reported in ref 2) diluted 1:10 in DenHybe (Insitus, Albuquerque, NM) was paired with SpectrumGreen (SG)‐labeled centromere enumerating probe (CEP4) 4p11‐q11 (reference probe) (Abbott, Downers Grove, IL, USA). Following washes to remove excess unbound probe, the nuclei were counterstained with 10 μL DAPI (Insitus, Albuquerque, NM, USA) and slides were coverslipped. The fluorescent signals were enumerated under an Olympus BX41 fluorescent microscope with appropriate filters (Olympus; Melville, NY, USA). For each hybridization, green and orange signals were enumerated in 100 non‐overlapping nuclei. Slides were scanned for regional variability and were considered abnormal regardless of whether the alteration appeared focal or diffuse. Hybridizations were considered non‐informative if the FISH signals were either lacking or too weak to interpret.
Statistical analysis
A two‐tailed t‐test was used to compare mean values except where noted. P < 0.05 was considered statistically significant. For Kaplan–Meier survival analysis, groups were compared using the log‐rank (Mantel–Cox) test. Overall survival was truncated at 750 days for pediatric AAs, as there was a single death after 750 days, and at 4500 days for adult AAs, as there was a single censored patient after 4500 days. Contingency analysis was performed using Fisher's exact test, two‐sided. Multivariate Cox proportional hazard regression was used to model survival; while, 10‐fold cross‐validation and an integrated Brier score 6, 13 were used to compare the predictive error scores. All statistical analyses were carried out using GraphPad software (GraphPad Software Inc., La Jolla, CA, USA) and R 26.
Study approval
All procedures were performed according to protocols approved by the University of California Committee on Research (San Francisco, CA, USA). De‐identified FFPE sections of human HGAs and TMAs were obtained from the participating institutions including the UCSF Brain Tumor Research Center Tissue Bank, Department of Pathology, Newcastle General Hospital; Department of Pathology, Children's Hospital, Los Angeles; Department of Lab Medicine and Pathobiology, University of Toronto; Department of Pathology & Laboratory Medicine, University of Kentucky; Department of Pathology and Laboratory Medicine, Emory University; Department of Pathology, Gemelli Hospital, University of Sacred Heart, Rome Italy; The Children's Hospital of Philadelphia; Department of Pathology and Laboratory Medicine, University of Pennsylvania; and Brain Tumor Translational Resource, Department of Pathology and Laboratory Medicine, University of California Los Angeles.
Results
Patient characteristics
The study cohort included 123 pediatric and 263 adult HGAs whose clinical characteristics are summarized in Table 1. For pediatric HGAs, a total of 71 AAs, mean age 9.06 years (range 0.2–20), and 57 GBMs mean age 10.1 years (range 0.1–19) were analyzed. For adult HGAs, 103 AAs, mean age 50.6 years (range 25.7–80) and 160 de novo GBMs, mean age 55.1 years (range 27–79) were analyzed. Based on these data, an additional 27 de novo GBM with mutations in IDH1 and a mean age of 46 years (range 21–83) and 17 IDH1 mutant secondary GBM, with documented progression from a lower‐grade astrocytoma, with a mean age of 39.9 years (range 26–55) were analyzed.
Detection of PDGFRA copy number gain/amplification by FISH
A wide range of FISH patterns was identified, reflecting both inter‐ and intra‐tumoral heterogeneity. Based on this heterogeneity we devised a scoring system that reflected both the relative number of tumor cells with copy number gain and the magnitude of copy number gain (Figure 1). Cases were scored as: normal (no increase or <10% cells with <6 PDGFRA signals); polysomy (>10% cells with 2–6 signals); low‐level amplification (<10% of cells with >12 or innumerable signals or >40% cells with 6–12 signals); or high‐level amplification (>10% cells with >12 or innumerable signals). Both low‐level and high‐level amplification were considered as “PDGFRA amplified.” These definitions were based on similar definitions for amplification of other oncogenes using clinical FISH assays 28. For instance, the definition of low‐level amplification with >40% cells containing ≥6 signals is nearly identical to that utilized for EGFR amplification in lung cancer 10. Although biologically, this is probably more accurately described as a “high‐level polysomy” rather than true gene amplification, the definition nonetheless correlates strongly with clinical outcome, including therapeutic response to tyrosine kinase inhibitors 10, 28. It was similarly felt that the more common pattern of gene amplification by FISH (>12 or innumerable signals) found in <10% of cells would have a roughly equivalent overall increase in dosage to that of a lower level of gain in larger numbers of cells (i.e., low‐level amplification). Often, these two patterns of low‐level amplification could be seen together in the same tumor. The decision not to use the PDGFRA/CEP4 ratio as part of the definition was based on the finding of several cases in which co‐amplification of the centromeric region was found (Figure 1F) wherein a ratio near 1.0 would falsely exclude an interpretation of gene amplification.
Figure 1.
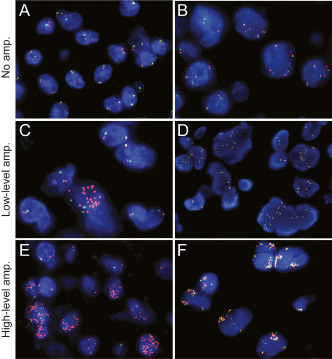
Determination of PDGFRA copy number gain/amplification by fluorescence in situ hybridization. Fluorescent images illustrating the different patterns of PDGFRA amplification in high‐grade astrocytomas. A. Normal, no increase in PDGFRA signals. B. Polysomy, >10% of cells with >2, but <6 signals for both PDGFRA and centromere enumerating probe (CEP4). C. The most frequent pattern of low‐level amplification, innumerable PDGFRA signals in <10% of cells. D. Another pattern of low‐level amplification, >40% cells with ≥6 signals (a few signals are beyond the plane of focus). E. High‐level amplification, > 10% cells with >12 or innumerable PDGFRA signals. F. Rare tumors demonstrated high‐level co‐amplification of PDGFRA and CEP4. Amp. denotes PDGFRA amplification. PDGFRA probe (red) and CEP4 (green), magnification ×1000.
Pediatric HGAs
FISH analysis of 123 pediatric HGAs demonstrated that 36 of 123 (29.3%) tumors had PDGFRA amplification, including 19 (15.4%) high‐level and 17 (13.8%) low‐level examples. A high frequency of PDGFRA amplification has been reported in a subset of diffuse intrinsic pontine gliomas (DIPGs) 22, 32. In our cohort we did not observe an increase in PDGFRA amplification in the brainstem/cerebellum vs. other brain regions (P = 0.53); however, our cohort included only 12 HGAs that involved the brainstem or cerebellum. To examine potential clinical differences between patients with PDGFRA‐amplified vs. non‐amplified tumors, we analyzed GBM and AA separately.
In pediatric GBM, FISH analysis demonstrated a striking 22 of 57 (38.6%) tumors with amplification of PDGFRA, including 11 (19.3%) with low‐level and 11 (19.3%) with high‐level amplification. Stratification of clinical variables based on PDGFRA amplification is shown in Table 2. Patients with PDGFRA‐amplified tumors tended to be older than patients without amplification; however, this difference was not significant (P = 0.13). There was no difference in overall survival between patients with and without PDGFRA copy number gain/amplification (Figure 2A).
Table 2.
Clinical characteristics of 57 pediatric patients with glioblastoma.
| Patient characteristics | No amplification | PDGFRA amplification | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Number (%) | 35 (61.4%) | 22 (38.6%) | ||
| Mean age (years ± SD) | 9.23 ± 5.49 | 11.40 ± 3.82 | 0.13 | |
| Sex ratio (M : F) | 1.1 | 1.0 | 1.067 (0.3464–3.285) | 1.0 |
| Median survival (days) | 596 | 494 | 0.97 | |
| Location: BS/CB vs. other | 24.1% (n = 29) | 11.2% (n = 18) | 2.545 (0.4656–13.92) | 0.45 |
BS/CB = brainstem/cerebellum; CI = confidence interval; SD = standard deviation.
Figure 2.

Pediatric high‐grade astrocytomas patients have similar overall survival with and without PDGFRA amplification. A. Pediatric patients with glioblastoma (GBM) with (n = 13) and without (n = 16) PDGFRA amplification have no significant difference in overall survival based on Kaplan–Meier survival analysis, P = 0.97. B. Kaplan–Meier survival analysis examining overall survival for pediatric patients with anaplastic astrocytoma with (n = 9) and without (n = 25) amplification of PDGFRA, P = 0.21. AA = anaplastic astrocytoma.
In pediatric AA, we identified a slightly lower percentage of PDGFRA‐amplified cases than in GBM (Table 3). A total of 14 of 66 (21.2%) tumors had amplification, including 6 (9.09%) with high‐level and 8 (12.1%) with low‐level amplification. Although not statistically significant, the age of patients with PDGFRA‐amplified tumors tended to be older (P = 0.08). PDGFRA‐amplified tumors also tended to be more common in women than men (P = 0.18). Similar trends in age and sex in the PDGFRA‐amplified tumors was noted in the pediatric GBM cohort. There was no significant difference in overall survival between PDGFRA‐amplified and non‐amplified tumors, although there was a trend toward worse survival with amplification (log‐rank, P = 0.21; Figure 2B).
Table 3.
Clinical characteristics of 66 pediatric patients with anaplastic astrocytoma
| Patient characteristics | No amplification | PDGFRA amplification | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Number (%) | 52 (78.8%) | 14 (21.2%) | ||
| Mean age (years ± SD) | 8.16 ± 5.49 | 11.2 ± 3.76 | 0.08 | |
| Sex ratio (M : F) | 1.38 | 0.45 | 3.115 (0.7859–12.35) | 0.18 |
| Median survival (days) | 513 | 403 | 0.21 | |
| Location: BS/CB vs. other | 6.67% (n = 30) | 7.69% (n = 13) | 0.8571 (0.0707–10.38) | 1.0 |
BS/CB = brainstem/cerebellum; CI = confidence interval; SD = standard deviation.
Adult HGAs
FISH analysis in adult HGAs demonstrated 55 of 263 (20.9%) PDGFRA‐amplified tumors, including 25 (9.50%) with high‐level and 30 (11.4%) with low‐level amplification. Similar to pediatric HGAs, PDGFRA amplification was more frequent in de novo GBM than AA. In adults, 36 of 160 (22.5%) GBM had PDGFRA amplification, including 17 (10.6%) high‐level and 19 (11.9%) low‐level (Table 4). While the patient's age, sex and overall survival were similar between PDGFRA‐amplified and non‐amplified tumors, there was a significant association between PDGFRA amplification and mutations in IDH1 (P = 0.028; Table 4 and Figure 3A).
Table 4.
Clinical and molecular characteristics of 160 adult glioblastoma† patients
| Patient and tumor characteristics | No amplification | PDGFRA amplification | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Number (%) | 124 (77.5%) | 36 (22.5%) | ||
| Mean age (years ± SD) | 55.7 ± 13.3 | 53.6 ± 12.7 | 0.56 | |
| Sex ratio (M : F) | 1.9 | 1.1 | 0.5806 (0.2091–1.612 | 0.31 |
| % IDH1 mutant | 5.08% (n = 124) | 16.7% (n = 36) | 3.933 (1.184–13.07) | 0.028 |
| Median survival (days) | 450 (n = 114) | 455 (n = 34) | 0.45 |
BS/CB = brainstem/cerebellum; CI = confidence interval; IDH1R132H = isocitrate dehydrogenase 1; SD = standard deviation.
†All tumors were de novo GBM and diagnosed at initial presentation as GBM.
Figure 3.
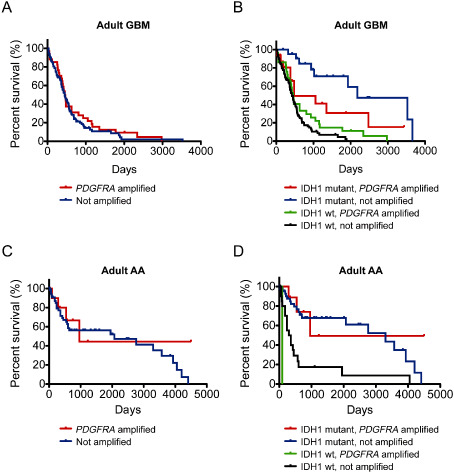
PDGFRA amplification is associated with worse overall survival in adult isocitrate dehydrogenase 1 (IDH1R132H) mutant glioblastoma (GBM). (A) Kaplan–Meier survival analysis of patients with de novo GBM demonstrates no significant difference in overall survival for patients with (red, n = 34) and without (blue, n = 114) PDGFRA amplification, P = 0.45. (B) Comparison of overall survival in larger cohort of patients stratified for IDH1 mutation (IDH1R132H) demonstrating decreased survival in IDH1 mutant GBM with PDGFRA amplification (red, n = 16) as compared with those without PDGFRA amplification (blue, n = 22) by Kaplan–Meier survival analysis, P = 0.023. Survival curves are also shown for IDH1 non‐mutant GBM with (green, n = 29) and without (black, n = 108) PDGFRA amplification. A similar analysis in adult patients with anaplastic astrocytoma (AA) demonstrated overall survival was similar regardless of PDGFRA amplification in (C) all patients [amplified, red (n = 11); not amplified, blue (n = 58) ] and in (D) patients stratified for IDH1 mutation status [IDH1 mutant and amplified, red (n = 9); IDH1 mutant and not amplified, blue (n = 45); IDH1 non‐mutant and amplified, green (n = 2); IDH1 non‐mutant and not amplified, black (n = 21) ] by Kaplan–Meier survival analysis, P = 0.41 or 0.52, respectively.
The R132H mutation in IDH1 was present in 12 of 160 (7.5%) adult de novo GBM. Based on the enrichment of PDGFRA amplification in IDH1 mutant tumors, we performed a subset analysis focused only on those tumors with mutant IDH1 (IDH1R132H). To increase the number of tumors available for analysis, we obtained an additional 27 IDH1 mutant de novo GBM (the clinical characteristics of all 39 tumors are summarized in Table 5). As expected, patients with de novo GBM with IDH1 mutation had significantly better overall survival than patients without IDH1 mutations (median survival 1927 days (n = 38) vs. 424 days (n = 137), respectively, log‐rank, P < 0.0001). Stratification of these IDH1 mutant tumors by PDGFRA amplification status revealed a striking difference in median overall survival between patients with and without amplification (Figure 3B). Overall median survival was 480 days (n = 16) for patients with IDH1 mutant de novo GBM with PDGFRA amplification vs. 2179 days (n = 22) without PDGFRA amplification (log‐rank, P = 0.023). Other clinical characteristics including age and sex were not significantly different between PDGFRA‐amplified and non‐amplified tumors.
Table 5.
Clinical characteristics of 39 adult patients with IDH1 mutant GBM †
| Patient characteristics | No amplification | PDGFRA amplification | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Number (%) | 22 (56.4%) | 17 (43.6%) | ||
| Mean age (years ± SD) | 44.7 ± 12.8 | 46.8 ± 16.8 | 0.67 | |
| Sex ratio (M : F) | 1.8 | 2 | 0.9167 (0.2121–3.963) | 1.0 |
| Median survival (days) | 2179 (n = 22) | 480 (n = 16) | 0.023 |
CI = confidence interval; GBM = glioblastoma; IDH1R132H = isocitrate dehydrogenase 1; SD = standard deviation.
†All tumors were de novo GBM and diagnosed at initial presentation as GBM.
In a multivariate analysis of all de novo GBMs, we examined PDGFRA status and IDH1 mutation status. While PDGFRA status alone was not a significant prognostic factor, the interaction of PDGFRA amplification and IDH1 mutation status (i.e. the group of tumors which are both PDGFRA‐amplified and IDH1 mutated) was found to be a significant prognostic factor (P = 0.01) (Table 6) and remained significant when age was included in the model (P = 0.049).
Table 6.
Hazard ratios from a multivariate survival analysis in adult patients with de novo GBM †
| No amplification | PDGFRA amplification | |
|---|---|---|
| IDH1 wt | 1.0 |
0.739 (0.477–1.145) P = 0.18 |
| IDH1 mutant |
0.140 (0.0639–0.3055) P = 8.2 × 10−7 |
0.416‡ (0.2131–0.8105) P = 0.01 |
GBM = glioblastoma; IDH1R132H = isocitrate dehydrogenase 1.
†GBM = complete information was available for 136 of the 178 patients, including 38 IDH1 mutant tumors.
‡Both IDH1 is mutated and PDGFRA is amplified.
Cross‐validation is a technique used for model selection as well as to asses if the model will be useful in an independent data set. Using 10‐fold cross‐validation and the integrated Brier score, a measure of the strength of the model, we compared the predictive error scores between several models: baseline (no variables in the model); main effect models with IDH1 mutation and/or PDGFRA amplification status; and, an interaction model that included the two main effects as well as an interaction term that signifies both IDH1 mutation and PDGFRA amplification. The interaction model had the lowest prediction error and was considered to be the best model, resulting in a marked reduction in error over baseline (16%) and a marginal, but consistent reduction in error over IDH1 mutation alone (2%).
In adult AAs, the frequency of PDGFRA amplification was less than in GBMs, with 19 of 103 (18.4%) tumors amplified, including 8 (7.8%) high‐level and 11 (10.7%) low‐level (Table 7). PDGFRA‐amplified tumors tended to be located in the frontal lobes, 10 of 15 (66.7%), as compared with the non‐amplified tumors, 29 of 69 (42.0%), although this was not statistically significant (P = 0.15). PDGFRA amplification was not prognostic for overall survival (Figure 3C). Interestingly, PDGFRA amplification was not increased in IDH1 mutant AAs, mutations seen in 61.2% of amplified tumors and 62.8% of non‐amplified tumors. Next, we analyzed the subset of 60 IDH1 mutant AA to determine whether PDGFRA had prognostic benefit in IDH1 mutant adult AA (Table 8). Unlike de novo GBM in which the frequency of PDGFRA amplification was highly enriched in IDH1 mutant tumors (17/39; 43.6%) vs. IDH1 non‐mutant tumors (30/151; 19.9%), in AA the frequency of PDGFRA amplification was similar in IDH1 mutant tumors (11/60; 18.3%) and in IDH1 non‐mutant tumors (7/36; 19.5%). Furthermore, in IDH1 mutant AA, there was no statistically significant difference in overall survival between PDGFRA‐amplified and non‐amplified tumors (median survival 960 days (n = 9) vs. 3300 (n = 45) days, respectively, P = 0.52). While PDGFRA‐amplified tumors were more common in the frontal lobes with 8 of 9 (88.9%) amplified tumors located in the frontal lobes as compared with 22 of 47 (46.8%) of the non‐amplified tumors, this difference was not statistically significant (P = 0.26).
Table 7.
Clinical and molecular characteristics of 103 adult anaplastic astrocytoma patients
| Patient and tumor characteristics | No amplification | PDGFRA amplification | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Number | 84 (81.6%) | 19 (18.4%) | ||
| Mean age (years ± SD) | 50.5 ± 13.2 | 50.9 ± 16.8 | 0.91 | |
| Sex ratio (M : F) | 9.2 | 5.5 | 1.673 (0.2857–9.794) | 0.62 |
| IDH1 mutant | 62.8% (n = 78) | 61.2% (n = 18) | 1.075 (0.3750–3.083) | 1.00 |
| Median survival (days) | 2070 (n = 58) | 960 (n = 11) | 0.41 | |
| Location: frontal vs. other | 42.0% (n = 69) | 66.7% (n = 15) | 0.3718 (0.1146–1.206) | 0.15 |
CI = confidence interval; IDH1R132H = isocitrate dehydrogenase 1; SD = standard deviation.
Table 8.
Clinical characteristics of 60 adult IDH1 mutant anaplastic astrocytoma
| Patient characteristics | No amplification | PDGFRA amplification | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Number | 49 (81.7%) | 11 (18.3%) | ||
| Mean age (years ± SD) | 45.1 ± 10.9 | 41.3 ± 7.30 | 0.29 | |
| Sex ratio (M : F) | 10 | 2.5 | 4.000 (0.5281–30.30) | 0.20 |
| Median survival (days) | 3300 (n = 45) | 960 (n = 9) | 0.52 | |
| Location: frontal vs. other | 46.8% (n = 47) | 88.9% (n = 9) | 0.5266 (0.1790–1.549) | 0.26 |
CI = confidence interval; IDH1R132H = isocitrate dehydrogenase 1; SD = standard deviation.
IDH1 mutant de novo GBM share many features with secondary GBM, derived from the progression of a lower‐grade astrocytoma 15. Similar to IDH1 mutant de novo GBM, PDGFRA amplification was frequent in IDH1 mutant secondary GBMs, 7 of 17 (41.2%) tumors. However, unlike in de novo, PDGFRA amplification was not associated with shorter overall survival (Table 9).
Table 9.
Clinical characteristics of 17 adult IDH1 mutant secondary GBM †
| Patient characteristics | No amplification | PDGFRA amplification | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|
| Number | 10 (58.8%) | 7 (41.2%) | ||
| Mean age (years ± SD) | 41.4 ± 10.2 | 39.5 ± 7.41 | 0.89 | |
| Sex ratio (M : F) | 3 | 6 | 0.5000 (0.0415–6.021) | 1.0 |
| Median survival (days) | 540 (n = 10) | 317.5 (n = 7) | 0.15 |
CI = confidence interval; GBM = glioblastoma; IDH1R132H = isocitrate dehydrogenase 1; SD = standard deviation.
†Secondary GBM = GBM with documented progression from a lower‐grade astrocytoma.
Discussion
HGAs are a heterogeneous group of tumors and improvements in therapy will likely require stratification of patients based upon clinical, histopathologic and molecular characteristics. Using FISH, we define a set of criteria to evaluate PDGFRA copy number alterations and determine the frequency of amplification in a large cohort of pediatric and adult HGAs. Our data suggest that PDGFRA amplification is higher than previously estimated in both pediatric (29.3%) and adult (20.7%) HGAs. To our knowledge this represents the largest reported number of pediatric and adult HGAs studied by FISH, and these data support the notion that abnormal PDGFRA signaling is important in HGA. In adults, PDGFRA amplification was associated with significantly worse overall survival in IDH1 mutant de novo GBM. Indeed, in a multivariate analysis of all adult de novo GBMs, the combination of PDGFRA amplification and IDH1 mutation status was identified as a significant prognostic factor. While additional studies in larger patient cohorts are required, our data suggest that IDH1 mutant de novo GBM may be a more heterogeneous group than previously thought.
PDGFR signaling is an important driver of glioma development and progression, and based on whole‐genome technologies, PDGFRA copy number gain/amplification is the second most common genetic alteration in RTKs in adult GBM, commonly estimated at 11% 27. This reported frequency is nearly identical to that of the high‐level amplification pattern that we noted in the current series; however, our techniques also allowed us to uncover a large number of low‐level amplifications. Using FISH on routinely processed pathology specimens, we identified a high frequency of PDGFRA amplification in adult HGA, including 22.5% in GBM and 18.4% in AA. In contrast to EGFR FISH, where widespread high‐level amplification is the rule, focal or low‐level PDGFRA amplifications were even more common. As such, this alteration may be particularly susceptible to underestimation by dose‐averaging techniques, such as PCR and array CGH. To reflect the diversity of PDGFRA signals observed by FISH, our scoring system included both low‐level and high‐level amplification. With the clinical variables available, we did not observe a significant survival difference between tumors with low‐ and high‐level amplification; thus, we considered both “positive” for amplification (data not shown). In future studies, the level of PDGFRA amplification may have unique and unexpected prognostic associations as has been seen with EGFR 11.
In adult de novo GBM with IDH1 mutations, the frequency of PDGFRA amplification was striking with nearly half of tumors positive for amplification. Interestingly, this was not true in AAs. In IDH1 mutant AA, the frequency of PDGFRA amplification was only 18.3%. While the number of IDH1 mutant GBM and AA was relatively small (39 GBM and 60 AA) this difference was significant [43.6 vs. 18.3%, P = 0.011 (odds ratio 3.442; confidence interval 1.385–8.554) ]. These data suggest that PDGFRA amplification may be an important event in the transition from AA to GBM in IDH1 mutant tumors. In support of this idea, analysis of a small number of IDH1 mutant secondary GBMs revealed a high frequency of PDGFRA amplification. Furthermore, the percent of cells with PDGFRA amplification tended to be less in IDH1 mutant de novo GBM as compared with IDH1 wild‐type GBM, as suggested by the number of cases with low‐level amplification (12/17; 71%) vs. (20/36; 56%), respectively. Gene amplification may be particularly important in the progression of IDH1 mutant astrocytoma as Lai et al 15 identified EGFR amplification in a smaller percentage of cells in IDH1 mutant vs. IDH1 wild‐type tumors. Interestingly, while survival tended to be shorter in PDGFRA‐amplified, IDH1 mutant AA and secondary GBM this did not reach statistical significance. The difference in survival between IDH1 mutant de novo GBM vs. AA and secondary GBM may reflect an insufficient sample size and high number of censored subjects for the latter or it may suggest potential biologic differences between clinically defined de novo GBM and IDH1 mutant HGAs that progress from a lower‐grade tumor.
Reports on the frequency of PDGFRA amplification in pediatric HGA vary (range 3.4–12%) with up to 50% reported in irradiation‐induced HGAs 16, 20, 23. In a large study of pediatric HGAs, PDGFR amplification as detected by array CGH was identified in 12% of HGAs overall and 17% of GBM 20. In DIPG, PDGFRA amplification may be even more common than in other HGAs, with reported estimates of 29% 22, 32. Using FISH we identified PDGFRA amplification in 29.3% of HGAs, 38.6% in GBM and 21.2% in AA. While we did not observe a significant association between brainstem location and PDGFRA amplification, we did not specifically target this group of tumors for analysis and only 12 HGAs involved the brainstem or cerebellum. PDGFRA status was not associated with overall survival in our cohort of pediatric HGAs.
This large multi‐institutional study included a broad cross‐section of cases from 10 major medical centers; however, it was a retrospective study and has inherent biases including potential case selection bias. In addition, we had access to only limited clinical and molecular data. Indeed, genetic information such as PDGFRA mutation status, shown to be common in amplified tumors 18, and co‐amplification of EGFR was not assessed 24, 25. Because of several cases with co‐amplification of PDGFRA and the centromeric region for which a ratio near 1.0 would falsely exclude PDGFRA amplification, we did not use the PDGFRA/CEP4 ratio to define gene amplification. While this allowed us to identify all cases with PDGFRA amplification we were not able to assess whether PDGFRA was selectively amplified or was co‐amplified with other potential oncogenic genes on chromosome 4, particularly KIT and KDR (VEGFR2), given that specific probes for these other genes were not applied. In addition, while our M:F ratio for adult GBM (1.6) was similar to the reported ratio of 1.58, our adult AAs had a very high M:F ratio (8.1) relative to the reported ratio of 1.39 4. As PDGFRA amplification tended to be more common in female patients, we may have underestimated the overall frequency of PDGFRA amplification in this population.
In this study we define a set of criteria to assess PDGFR amplification in routine clinical samples and provide an estimate of the frequency of PDGRA amplification in a large set of pediatric and adult HGAs. In our cohort, PDGFRA amplification did not have prognostic significance in pediatric HGA. In adults, we identified PDGFRA amplification as an independent prognostic factor in IDH1 mutant de novo GBM. These data have important potential implications regarding tumor biology and prognosis and additional studies in a larger number of IDH1 mutant de novo GBM are required.
Funding
This work was supported in part by the National Institutes of Health (K08 NS063456 to JJP; 1U01CA168878 to JJP; and P01CA095616 to KLL), the UCSF Brain Tumor SPORE (CA097257), the Ivy Foundation Early Phase Clinical Trials Consortium—Virtual Tissue Bank, and funds from the University of California San Francisco Departments of Pathology and Neurological Surgery.
Conflict of interest
The authors declare they have no conflict of interest.
Acknowledgments
The authors would like to thank Cynthia Cowdrey, Yunita Lim, and King Chiu from the UCSF Brain Tumor Research Center tissue core for their assistance with acquisition and preparation of tissue. In addition, we are grateful to the generosity of the contributing institutions.
References
- 1. Alentorn A, Marie Y, Carpentier C, Boisselier B, Giry M, Labussiere M et al (2012) Prevalence, clinico‐pathological value, and co‐occurrence of PDGFRA abnormalities in diffuse gliomas. Neuro‐Oncol 14:1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baxter EJ, Hochhaus A, Bolufer P, Reiter A, Fernandez JM, Senent L et al (2002) The t(4;22)(q12;q11) in a typical chronic myeloid leukaemia fuses BCR to PDGFRA. Hum Mol Genet 11:1391–1397. [DOI] [PubMed] [Google Scholar]
- 3. Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E (2009) Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS ONE 4:e7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CBTRUS (2011) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. Source: Central Brain Tumor Registry of the United States, Hinsdale, IL. Available at: http://www.cbtrus.org (accessed 7 March 2013).
- 5. Elkhaled A, Jalbert LE, Phillips JJ, Yoshihara HA, Parvataneni R, Srinivasan R et al (2012) Magnetic resonance of 2‐hydroxyglutarate in IDH1‐mutated low‐grade gliomas. Sci Transl Med 4:116ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graf E, Schmoor C, Sauerbrei W, Schumacher M (1999) Assessment and comparison of prognostic classification schemes for survival data. Stat Med 18:2529–2545. [DOI] [PubMed] [Google Scholar]
- 7. Guha A, Dashner K, Black PM, Wagner JA, Stiles CD (1995) Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer 60:168–173. [DOI] [PubMed] [Google Scholar]
- 8. Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474. [DOI] [PubMed] [Google Scholar]
- 9. Hermanson M, Funa K, Hartman M, Claesson‐Welsh L, Heldin CH, Westermark B, Nister M (1992) Platelet‐derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 52:3213–3219. [PubMed] [Google Scholar]
- 10. Hirsch FR, Herbst RS, Olsen C, Chansky K, Crowley J, Kelly K et al (2008) Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non‐small‐cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol 26:3351–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hobbs J, Nikiforova MN, Fardo DW, Bortoluzzi S, Cieply K, Hamilton RL, Horbinski C (2012) Paradoxical relationship between the degree of EGFR amplification and outcome in glioblastomas. Am J Surg Pathol 36:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horbinski C, Miller CR, Perry A (2011) Gone FISHing: clinical lessons learned in brain tumor molecular diagnostics over the last decade. Brain Pathol 21:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molinaro AM, Lostritto K (2010) Statistical resampling for large screening data analysis such as classical resampling Bootstrapping, Markov chain Monte Carlo, and statistical simulation and validation strategies. In: Statistical Bioinformatics: A Guide for Life and Biomedical Science Researchers, Lee JK (ed.), pp. 219–248. John Wiley & Sons, Inc: Hoboken, NJ. [Google Scholar]
- 14. Kim YH, Nonoguchi N, Paulus W, Brokinkel B, Keyvani K, Sure U et al (2012) Frequent BRAF Gain in Low‐Grade Diffuse Gliomas with 1p/19q Loss. Brain Pathol 22:834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai A, Kharbanda S, Pope WB, Tran A, Solis OE, Peale F et al (2011) Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol 29:4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicolaides TP, Li H, Solomon DA, Hariono S, Hashizume R, Barkovich K et al (2011) Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res 17:7595–7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ozawa T, Brennan CW, Wang L, Squatrito M, Sasayama T, Nakada M et al (2010) PDGFRA gene rearrangements are frequent genetic events in PDGFRA‐amplified glioblastomas. Genes Dev 24:2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paugh BS, Qu C, Jones C, Liu Z, Adamowicz‐Brice M, Zhang J et al (2010) Integrated molecular genetic profiling of pediatric high‐grade gliomas reveals key differences with the adult disease. J Clin Oncol 28:3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD et al (2006) Molecular subclasses of high‐grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173. [DOI] [PubMed] [Google Scholar]
- 22. Puget S, Philippe C, Bax DA, Job B, Varlet P, Junier MP et al (2012) Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS ONE 7:e30313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley MY, Yu M et al (2010) Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res 70:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ et al (2011) Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20:810–817. [DOI] [PubMed] [Google Scholar]
- 25. Szerlip NJ, Pedraza A, Chakravarty D, Azim M, McGuire J, Fang Y et al (2012) Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A 109:3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Team R Development Core Team (2005) R: a language and environment for statistical computing, reference index version 2.15.0. R Foundation for Statistical Computing. Vienna, Austria.
- 27. The Cancer Genome Atlas Research Network (TCGA) (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tiseo M, Bartolotti M, Gelsomino F, Bordi P (2010) Emerging role of gefitinib in the treatment of non‐small‐cell lung cancer (NSCLC). Drug Des Devel Ther 4:81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toedt G, Barbus S, Wolter M, Felsberg J, Tews B, Blond F et al (2011) Molecular signatures classify astrocytic gliomas by IDH1 mutation status. Int J Cancer 128:1095–1103. [DOI] [PubMed] [Google Scholar]
- 30. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zarghooni M, Bartels U, Lee E, Buczkowicz P, Morrison A, Huang A et al (2010) Whole‐genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet‐derived growth factor receptor alpha and poly (ADP‐ribose) polymerase as potential therapeutic targets. J Clin Oncol 28:1337–1344. [DOI] [PubMed] [Google Scholar]


