Abstract
Rates of synapse formation and elimination change over the course of postnatal development, but little is known of molecular mechanisms that mediate this developmental switch. Here we report that the dendritic RNA binding protein Fragile X Mental Retardation Protein (FMRP) bi-directionally and cell autonomously regulates excitatory synaptic function which depends on developmental age as well as function of the activity-dependent transcription factor Myocyte-Enhancer Factor 2 (MEF2). Acute postsynaptic expression of FMRP in CA1 neurons of hippocampal slice cultures (during the first postnatal week; P6–7) promotes synapse function and maturation. In contrast, acute expression of FMRP or endogenous FMRP in more mature neurons (during the second postnatal week; P13–16) suppresses synapse number. The ability of neuronal depolarization to stimulate MEF2 transcriptional activity increases over this same developmental time period. Knockout of endogenous MEF2 isoforms causes acute postsynaptic FMRP expression to promote, instead of eliminate, synapses onto two week old neurons. Conversely, expression of active MEF2 in neonatal neurons results in a precocious FMRP-dependent synapse elimination. Our findings suggest that FMRP and MEF2 function together to fine tune synapse formation and elimination rates in response to neuronal activity levels over the course of postnatal development.
Keywords: MEF2, FMRP, synapse development, synapse elimination, hippocampus, CA1
Introduction
The establishment of proper neural circuits requires the precise orchestration of synapse formation, maturation and elimination. During early postnatal development there is a robust period of excitatory synaptogenesis, when synapses and dendritic spines are dynamic; rapidly forming and eliminating (Holtmaat and Svoboda, 2009; Hua and Smith, 2004; Zuo et al., 2005a; Zuo et al., 2005b). As neurons mature, synapse formation rates decelerate, but elimination rates remain high which often results in a net loss of synaptic inputs (Chung and Barres, 2011; Holtmaat and Svoboda, 2009; Hua and Smith, 2004; Zuo et al., 2005a; Zuo et al., 2005b). By adulthood, synapse elimination rates decline to equal formation rates, and a stable synaptically-connected circuit is maintained (Holtmaat and Svoboda, 2009). Little is known of the mechanisms that control the developmental changes in cortical synapse formation and elimination rates, but this likely relies on activity-dependent mechanisms that sense when synaptic connections are mature, and then inhibit additional synapse formation, as well as maintain elimination rates (Goda and Davis, 2003).
Neuronal activity promotes synapse elimination through activation of the myocyte enhancer factor 2 (MEF2) family of transcription factors (Barbosa et al., 2008; Flavell et al., 2006; Pulipparacharuvil et al., 2008). MEF2 factors are activated by neuronal depolarization and subsequent Ca2+ influx that activates the Ca2+/Calmodulin (CaM) effectors, the protein phosphatase, calcineurin and CaM kinases (Flavell et al., 2006; McKinsey et al., 2002; Pulipparacharuvil et al., 2008; Shalizi et al., 2006). Selective activation of MEF2-dependent transcription causes rapid and robust synapse elimination, and knockdown or deletion of MEF2 isoforms results in increased synapse number in a number of brain regions (Barbosa et al., 2008; Flavell et al., 2006; Pulipparacharuvil et al., 2008; Tian et al., 2010). As a result, MEF2 regulates learning, memory and synaptic plasticity in response to experience (Akhtar et al., 2012; Barbosa et al., 2008; Chen et al., 2012; Cole et al., 2012; Li et al., 2008; Pulipparacharuvil et al., 2008).
Recently we demonstrated that MEF2-triggered synapse elimination requires the RNA binding protein and translational regulator, Fragile X Mental Retardation Protein (FMRP) (Pfeiffer et al., 2010). Our results suggested that FMRP functions downstream of MEF2 to regulate translation of MEF2-generated transcripts that lead to synapse elimination. Importantly, loss-of-function mutations in FMRP result in Fragile X Syndrome (FXS), the most common inherited cause of mental retardation and autism (Bassell and Warren, 2008). Cortical neurons of Fragile X patients, as well as the mouse model of FXS, Fmr1 knockout mice (Fmr1 KO), display an excess number of dendritic spines (Bagni and Greenough, 2005; Pfeiffer and Huber, 2009). Furthermore, spine elimination rates of neocortical Fmr1 KO neurons are insensitive to manipulations of sensory experience, unlike wildtype littermates (Pan et al., 2010). These data support a role for FMRP in experience and activity-dependent synapse elimination.
Interestingly, there is also evidence for reduced excitatory synapse formation and maturation in cortical neurons of Fmr1 KO mice suggesting that FMRP can also promote synapse function (Bureau et al., 2008; Harlow et al., 2010; Pilpel et al., 2009). However, the acute, cell autonomous role of FMRP in synapse formation or maturation is unknown. Remarkably, FMRP interacts directly with 25–30% of the mRNAs that encode the synaptic proteome (Darnell et al., 2011) suggesting that FMRP regulates multiple and diverse aspects of synapse structure and function. The factors that influence or control the varied effects of FMRP at synapses are largely unknown.
Here we present evidence for a cell autonomous, postsynaptic role of FMRP in both promotion and elimination of excitatory synapses onto hippocampal CA1 neurons. The effect of FMRP is determined by the developmental age and the level of MEF2 transcriptional activity. These data provide insight into the mechanisms that regulate the developmental changes in synapse formation and elimination and highlight the multifunctional nature of FMRP and its dependence on the transcriptional state of neurons.
Materials and Methods
Mice
Fmr1 KO congenic C57BL/6 mouse strain were originally obtained from Dr. Stephen Warren at Emory University and were backcrossed to C57BL/6N mice (Charles River Laboratories). GFP/Fmr1 mosaic mice were generated by breeding Fmr1 KO and Fmr1 wild type mice carrying a green fluorescent protein (GFP) transgene on the X chromosome (Hadjantonakis et al., 1998) (Jackson Labs). MEF2A, 2C, and 2D conditional knockout mice have been previously described (Akhtar et al., 2012; Arnold et al., 2007; Kim et al., 2008). CAG-CREERTM mice (Hayashi and McMahon, 2002) were provided by Dr. Jane Johnson (UT Southwestern). Fluorescent (td-Tomato) Cre-reporter mice were obtained from Jackson labs (Stock # 007909) (Madisen et al., 2009)
Organotypic Hippocampal Slice Cultures and Transfections
Organotypic hippocampal slice cultures were prepared from postnatal day (P) 0–1 or 6–7 mice as described (Pfeiffer and Huber, 2007). Cultures were biolistically transfected at 3 DIV (McAllister, 2004). For experiments with MEF2-VP16, neurons were cotransfected with the MEF2-transcriptional reporter, MRE-GFP and GFP expression was used as confirmation of MEF2 activated transcription in individual recorded neurons. MEF2-VP16 was expressed for 16–36 hrs. Cultures from CAG-CREERTM mice (Figure 4; Fmr1 KO/MEF2ACD-fl/CAGCre) were treated with 10μM 4-hydroxytamoxifen (4-OHT) on the day of culture and 4-OHT was reapplied every 48 hours for a total of six days prior to FMRP transfection for electrophysiology or immunoblotting and RT-PCR procedures.
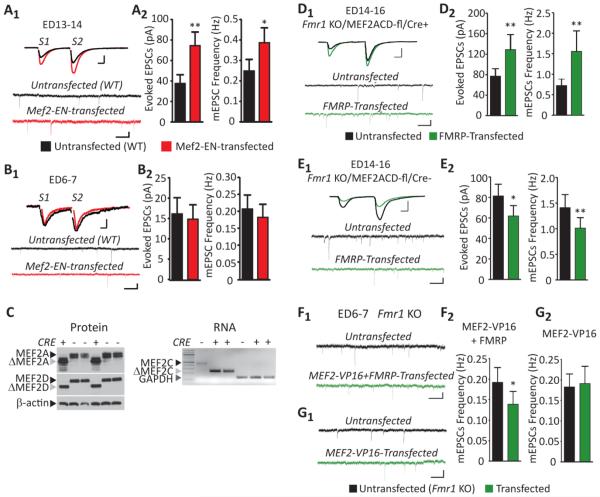
Figure 4. MEF2 activity controls the bidirectional and developmental effects of FMRP on excitatory synapse function.
(A1) Representative evoked EPSCs and mEPSCs from an untransfected and neighboring MEF2-EN-transfected CA1 neurons from mature (ED13-14) WT slice cultures. Scale bars: evoked EPSCs=25pA/10ms; mEPSCs=10pA/500ms. (A2) MEF2-EN increases the average evoked EPSC amplitude (n=17 cell pairs) and mEPSC frequency (n=23) in mature cultures. (B1) Representative evoked EPSCs and mEPSCs from an untransfected and neighboring MEF2-EN-transfected neuron from a neonatal (ED6-7) WT slice culture. Scale bars: evoked EPSCs=10pA/10ms; mEPSCs=10pA/500ms. (B2) MEF2-EN does not affect the average evoked EPSC amplitude (n = 20; p > 0.1) or mEPSC frequency (n=23; p > 0.1) in neonatal cultures. * p< 0.05; **p< 0.01. (C) Slice cultures from P6–7 Fmr1 KO/MEF2ACD-fl/CAGCre+ or Fmr1 KO/MEF2ACD-fl/CAGCre− mice were treated with 10μM 4-hydroxytamoxifen (4OHT) for six days and then processed for immunoblotting, RT-PCR. Left: Western blots of hippocampal slice culture lysates from ED14 Fmr1 KO/MEF2ACD-fl/CAGCre+ (CRE+) and Fmr1 KO/MEF2ACD-fl/CAGCre− (Cre−) reveal the truncated MEF2 protein that is produced upon Cre-mediated excision of exon 2 of the MEF2A and MEF2D genes (Akhtar et al., 2012; Arnold et al., 2007; Kim et al., 2008). β-actin is a loading control. Right: RT-PCR reveals the deletion of full length MEF2C with GAPDH as the reaction control. (D1) Representative evoked EPSCs and mEPSCs from an untransfected and neighboring FMRP-transfected neuron at ED14-16 in CRE+ slice cultures. Scale bars: evoked EPSCs=100pA/10ms; mEPSCs=10pA/500ms. (D2) FMRP transfection into mature MEF2ACD-KO cultures (Fmr1 KO/MEF2ACD-fl/CAGCre+) increases average evoked EPSC amplitude (n=17) and mEPSC frequency (n=21). (E1) Representative evoked EPSCs and mEPSCs from an untransfected and neighboring FMRP-transfected neuron at ED14-16 in CRE− slice cultures. Scale bars: evoked EPSCs=100pA/10ms; mEPSCs=10pA/500ms. (E2) FMRP transfection into mature Cre− cultures (Fmr1 KO/MEF2ACD-fl/CAGCre−) decreases average evoked EPSC amplitude and mEPSC frequency (n=25). (F,G) Representative mEPSCs from untransfected and neighboring transfected Fmr1 KO neurons with either MEF2VP16 (F1) or MEF2VP16+FMRP (G1) in neonatal (ED6-7) cultures. Scale bar=10pA/500ms. (F2) MEF2VP16 and FMRP cotransfection reduces the average mEPSC frequency in neonatal cultures (n=20). (G2) MEF2VP16 transfection has no effect on the average mEPSC frequency in neonatal cultures (n=21). * p< 0.05; **p< 0.01.
Acute slices
Acute hippocampal slices were prepared from GFP/Fmr1 mosaic females as described (Huber et al., 2000; Volk et al., 2007). Briefly, mice were anesthetized with ketamine (125mg/kg)/xylazine (75mg/kg), decapitated, and the hippocampus with overlying neocortex were dissected in chilled (4°C) sucrose dissection buffer containing (in mM): 2.6 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 5 MgCl2, 212 sucrose, and 10 dextrose aerated with 95% O2/5% CO2. Transverse hippocampal slices (400μm thickness) were obtained on a Leica VT1200S slicer. The CA3 region was cut off to avoid epileptogenic activity during recordings in picrotoxin. Slices were recovered for 30 min at 35°C and for another 30 min at room temperature in artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 3 CaCl2, 2 MgCl2, 26 NaHCO3, 1 NaH2PO4 and 11 D-Glucose aerated with 95% O2/5% CO2 to pH 7.4.
Electrophysiology
Simultaneous whole-cell recordings were made under visual guidance using IR-DIC optics and GFP-fluorescence to identify transfected or GFP-positive neurons as described (Pfeiffer and Huber, 2007). Recordings were made at 30°C in a submersion chamber perfused at 3ml/min with artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 26 NaHCO3, 1 NaH2PO4, 11 D-Glucose, 3 CaCl2, 2 MgCl2, 0.1 picrotoxin, 0.002 2-chloroadenosine; 0.1% DMSO pH 7.4, 300 mOsm and saturated with 95%O2/5%CO2. For evoked EPSC and mEPSC recordings, neurons were voltage clamped at −60mV through whole cell recording pipettes (~3–7 MΩ) filled with an intracellular solution containing (in mM): 0.2 EGTA, 130 K-Gluconate, 6 KCl, 3 NaCl, 10 HEPES, 2 QX-314, 4 ATP-Mg, 0.4 GTP-Na, 14 phosphocreatine-Tris, pH7.2 adjusted by KOH, 285 mOsm. To obtain isolated NMDAR mediated EPSCs, the ASCF was supplemented with 20μM DNQX and 20μM glycine and the neuron was voltage clamped at +40 mV. The internal pipette solution for NMDAR EPSCs contained (in mM): 2.5 BAPTA, 125 Cs-Gluconate, 6 CsCl, 3 NaCl, 10 HEPES, 2 QX-314, 4 ATP-Mg, 0.4 GTP-Na, 14 phosphocreatine-Tris, pH7.2 adjusted by CsOH, 290 mOsm. EPSCs were confirmed to be NMDAR-mediated by blockade with 100μM DL-APV treatment. To block GluN2B-mediated EPSCs, 3μM ifenprodil was washed in for 20 minutes. For mEPSC measurements, ACSF was supplemented with 1μM TTX. EPSCs were evoked by a single bipolar cluster electrode (FHC; cat #CE) placed in stratum radiatum of area CA1 (along the Schaffer collaterals) ~50μm from the recorded neurons with biphasic current pulses (1–60μA, 0.06–0.15 ms). Series and input resistance were measured in voltage clamp with a 400ms, −10 mV step from a −60 mV holding potential (filtered at 30 kHz, sampled at 50 kHz). Cells were only used for analysis if the series resistance was less than 30 MΩ and stable throughout the experiment. Input resistance ranged from 75–600 MΩ (Table 1). Synaptic currents were filtered at 2 kHz, acquired and digitized at 10 kHz on a PC using custom software (Labview; National Instruments, Austin, TX). mEPSCs were detected off-line using an automatic detection program (MiniAnalysis; Synaptosoft Inc.) with a detection threshold set at a value greater than at least 2 S.D. of the noise values, followed by a subsequent round of visual confirmation. The detection threshold remained constant for the duration of each experiment. For evoked EPSCs shown in figures, the stimulation artifact has been digitally removed for clarity. Data analysis was performed in LabView. Significant differences between neuron pairs were determined with a paired t-test or a paired non-parametric test (Wilcoxon signed-rank test) depending on if the data sets were normally distributed. Normality was determined by a D'Agostino and Pearson omnibus normality test using GraphPad Prism. * p<0.05, ** p<0.01, *** p<0.001. In all figures, averages +SEM are plotted.
The decay time constant of NMDAR-mediated EPSCs was calculated in LabView using a double exponential model and the following formula (Philpot et al., 2001):
Where τw is weighted decay time constant, If is the percentage of the fast NMDAR-mediated current, τf is the estimated fast decay time constant, Is is the percentage of the slow NMDAR-mediated current, τs is the estimated slow decay time constant.
Immunoblotting
Hippocampal slice cultures were homogenized in a sucrose lysis buffer (in mM): 320 sucrose, 5 HEPES pH 7.4, 1 EDTA, 1% SDS, 50 NaF, complete protease inhibitor cocktail tablet (Roche), phosphatase inhibitors 2 and 3 (Sigma). Protein levels were measured using BCA Protein Assay (Thermo Scientific, IL) and equal amount of proteins were loaded. Samples were boiled for 10 minutes in a SDS sample buffer, resolved by SDS-PAGE, transferred to PVDF membranes and immunoblotted. Membranes were incubated with primary antibodies, washed and exposed to horseradish peroxidase-conjugated secondary antibodies, which were visualized by enhanced chemiluminescence. Antibodies used in these studies were: 1:1,000 monoclonal anti-MEF2D (BD Biosciences, catalog# 610774), 1:500 polyclonal anti-MEF2A (Santa Cruz Biotechnology C21, catalog# sc-313), 1:20,000 polyclonal anti-beta-actin (Abcam, Cambridge, MA, catalog# ab8227), and 1:500 polyclonal anti-MEF2A S408 phospho-specific antibody (Flavell et al., 2006). Densitometry was quantified using Image J (National Institute of Health).
Constructs
pcDNA1-MEF2-VP16, MEF2-EN and MRE-GFP constructs have been described previously (Arnold et al., 2007; Flavell et al., 2006; Molkentin et al., 1996; Pfeiffer et al., 2010; Shalizi et al., 2006). The FMRP-GFP construct has been previously described (Darnell et al., 2005; Pfeiffer and Huber, 2007).
Confocal Imaging of live neurons
At 3 DIV, hippocampal slice cultures were transfected with a MEF2 transcriptional reporter, MRE-GFP, and mCherry as a transfection marker. At 7 DIV (equivalent day P7 or P14), hippocampal slice cultures were transferred to isosmotic media containing 60mM KCl. Live cells were imaged at 0, 0.5, 1, 2, 3, or 6 hours after KCl depolarization on a Zeiss inverted LSM 510 confocal microscope using a 40×/1.3 oil objective lens. Sequential excitation and acquisition of GFP and mCherry fluorescence of a 5 μm optical section through the cell soma was performed using parameters to avoid saturation of either signal. Excitation laser power and acquisition sensitivity for each channel remained constant across all imaged cells in a culture. In Image J, mCherry and MRE-GFP fluorescence intensity was measured in a 10μm circle placed in the center of each cell soma. MRE-GFP intensity was normalized to mCherry in each cell to control for differences in cell depth in the slice and transfection efficiency. A 2-way ANOVA and Bonferroni posttests were used to analyze the KCl time course data in Figure 3D.
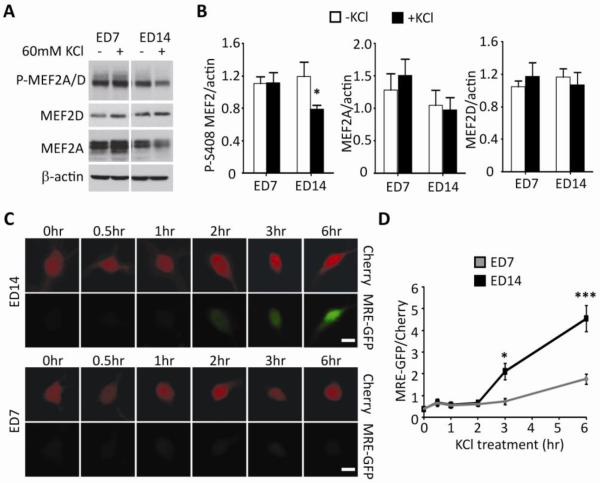
Figure 3. Activity-dependent dephosphorylation of MEF2A/D and activation of MEF2-dependent transcription increase with postnatal age in slice culture.
(A) Example western blot of phospho-MEF2 Serine408 (P-MEF2A/D), MEF2D, MEF2A and β-actin (loading control) from ED7 and ED14 slice cultures with or without KCl (10min) –induced neuronal depolarization. (B) Quantification of P-S408, MEF2A and MEF2D normalized to β-actin (n=11 cultures/condition). Quantified P-S408 MEF2A/D, MEF2D, and MEF2A normalized to β-actin (n=11/condition). KCl depolarization results in dephosphorylation of MEF2 in mature cultures. (C) Representative images of mCherry and MRE-GFP from transfected WT slice cultures at ED7 and 14 after increasing durations of KCl depolarization. Scale bar=20μm. (D) Grouped averages of MRE-GFP/mCherry intensity 0, 0.5, 1, 2, 3, 6 hours in transfected slice cultures (ED7 and 14) after KCl depolarization (n= 35–150 cells/condition). *p< 0.05; *** p< 0.001.
Dendritic Spine Imaging
Hippocampal slice cultures were prepared from P0–1 Fmr1 KO mice as described for electrophysiology and biolistically transfected (at 3 DIV) with pA1-GFP, described in (Pfeiffer et al., 2010), with or without FMRP. Three days after transfection, secondary apical dendrites (150–200 μm from the cell body) from transfected CA1 neurons were imaged live using a Zeiss LSM 510 two-photon laser scanning microscope equipped with a Chameleon-Ti: sapphire standard laser at an excitation wavelength of 920 nm using a 63× 0.9 NA water-immersion objective. Z-stacks were acquired at a z-step of 1μm at 1024×1024 pixel resolution (x=y=0.13μm, z=1μm). Images were processed using NIH ImageJ (version 1.45s). Spine density and classification of spines was determined using NeuronStudio software (Radley et al., 2008; Rodriguez et al., 2008; Rodriguez et al., 2006). Spines were identified as stubby, mushroom or thin (including filopodia) using the trainable classification scheme in NeuronStudio. 1–3 dendritic regions of interest were analyzed per cell (minimum of 3 independent slice cultures). Image acquisition and analysis were performed by an observer blind to treatment and/or genotype. The effect of FMRP on spine density was determined using an independent-t-test. The effect of FMRP on spine type was determined using a 2-way ANOVA.
RT-PCR
Slices were snap-frozen and stored at −80°C until processing. RNA was extracted from slice cultures with TRIzol reagent (Invitrogen), followed by purification with RNAeasy micro columns (Qiagen). Equal amounts of RNA were prepared for reverse transcription reactions using the Superscript II reverse transcriptase enzyme (Invitrogen). PCR reactions were run in four replicates using SYBR fast Green super mix (BioRad) on 7500 fast qPCR machine (Applied Biosystems). The primers are: GAPDH forward AGGTCGGTGTGAACGGATTTG reverse TGTAGACCATGTAGTTGAGGTCA, mef2C forward CAGGGACGAGAGAGAGAAGAAAC and reverse CAATCTTTGCCTGCTGATCATTAG.
Results
Postsynaptic FMRP bi-directionally and cell autonomously regulates excitatory synapse function that depends on postnatal age
Previously we demonstrated that acute postsynaptic transfection of FMRP into Fmr1 KO CA1 neurons in organotypic slices results in a functional and structural elimination of excitatory synapses (Pfeiffer and Huber, 2007). Here we determined if the acute, cell autonomous effects of FMRP on synapses change as a function of postnatal age. Hippocampal slice cultures were prepared from neonatal (P0–1) or one-week-old Fmr1 knockout (Fmr1 KO) pups (P6–7) and biolistically transfected with a C-terminally EGFP-tagged FMRP at three to four days in vitro (DIV). FMRP-GFP was driven by the endogenous FMR1 promoter to prevent strong overexpression of the protein (Pfeiffer and Huber, 2007; Pfeiffer et al., 2010). At 6–8 DIV, simultaneous whole cell voltage clamp recordings were obtained from pairs of transfected and neighboring untransfected Fmr1 KO neurons to assess the effects of FMRP on synapse function. Therefore, recordings were made from slice cultures at equivalent postnatal day 6–7 (ED6-7; defined here as “neonatal”) or equivalent postnatal day 13–14 (ED13-14; operationally defined here as “mature”).
Acute postsynaptic expression of FMRP in CA1 neurons of mature (ED13-14) slice cultures decreased the amplitude of evoked EPSCs, as well as mEPSC frequency (Figure 1A, B). Individual synapse strength as measured by mEPSC amplitude was not affected. Observation of a decrease in mEPSC frequency, but not amplitude, suggests that postsynaptic FMRP expression may decrease presynaptic release probability or reduce synapse number. Therefore, we measured the effect of FMRP on paired-pulse facilitation (PPF) of evoked EPSCs, a measure that is inversely correlated with presynaptic release probability of hippocampal synapses (Manabe et al., 1993) (Figure 1A, B). Acute FMRP expression in mature CA1 cultures had no effect on PPF indicating that it does not reduce presynaptic release probability. Therefore, acute FMRP-expression in mature cultures causes a decrease in functional synapse number consistent with previous results (Pfeiffer and Huber, 2007).
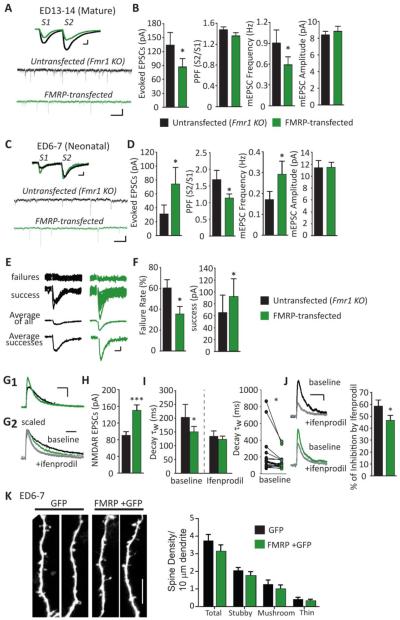
Figure 1. Acute postsynaptic transfection of FMRP bi-directionally regulates excitatory synaptic function in organotypic slice cultures prepared at different postnatal ages.
(A) Representative evoked EPSCs and mEPSCs from a simultaneous recording of an untransfected (black line) and neighboring FMRP-transfected (green line) Fmr1 KO CA1 neurons from “mature” (ED13-14) slice cultures. Scale bars: evoked EPSCs=50pA/10ms; mEPSCs=10pA/500ms. (B) Average (±SEM) of evoked EPSC amplitudes (n=28 cell pairs), paired pulse facilitation (PPF; S2/S1 evoked EPSC amplitudes; p >0.1), mEPSC frequency and amplitude (n=27) from untransfected (black filled) and FMRP-transfected (green filled) neurons at ED13-14. (C) Representative evoked EPSCs and mEPSCs from an untransfected and neighboring FMRP-transfected Fmr1 KO neuron from “neonatal” (ED6-7) slice culture. (D) Average evoked EPSC amplitude, PPF (n=26), mEPSC frequency and amplitude (n=23) from untransfected and FMRP-transfected neurons at ED6-7. (E) Representative individual traces of evoked EPSC failures and successes. Average of all EPSCs or only the successes from an untransfected and neighboring FMRP-transfected Fmr1 KO neuron. Scale bar=10pA/10ms. (F) FMRP transfection decreases the percent of failures to observe an EPSC with extracellular stimulation. When an EPSC is observed (a “success”), its amplitude is increased in FMRP transfected neurons compared to untransfected neurons (n=18). (G1) Representative pharmacologically isolated NMDAR-mediated EPSCs from an untransfected and neighboring FMRP-transfected Fmr1 KO neuron. Scale bar =50pA/100ms. (G2) Scaled NMDAR-mediated EPSCs from an untransfected and neighboring FMRP-transfected Fmr1 KO neurons before (baseline) and after ifenprodil (gray line). Scale bar=100 ms. (H) FMRP transfection increases average evoked NMDA-mediated EPSC amplitude compared to untransfected neurons (n=39). (I) Weighted time constant (τw) of the decay of NMDAR-EPSCs from untransfected and neighboring FMRP-transfected Fmr1 KO neurons before and after ifenprodil (n=19); Left: average (±SEM); Right: individual cell pairs. (J) Left: Representative NMDAR EPSCs from untransfected and neighboring FMRP-transfected Fmr1 KO neurons before and after ifenprodil. Right: Average % of inhibition of NMDAR EPSC amplitudes by ifenprodil (n=16). (K) Left: Representative segments of secondary dendrites from neonatal Fmr1 KO CA1 neurons transfected with GFP alone or FMRP + GFP. Scale bar = 10 μm. Right: Group data demonstrating that dendritic spine density of GFP+FMRP transfected Fmr1 CA1 neurons (n = 15 cells/ 2–3 dendrites per cell) is not different from neurons transfected with GFP only (n = 11) measured from all spines (total; p > 0.1) or spines of different types (stubby, mushroom or thin; p = 0.07; 2-way ANOVA). * p< 0.05; ***p< 0.001.
In contrast to mature cultures, FMRP expression in neonatal neurons (ED6-7) enhanced synapse function as measured by an increase in evoked EPSC amplitude and mEPSC frequency, but did not affect mEPSC amplitude (Figure 1C, D). This result reveals a developmental shift in the effects of postsynaptic FMRP expression on excitatory synaptic function. We next attempted to determine the synaptic locus for the effects of acute FMRP expression on neonatal synapses. In neonatal slice cultures we observed that the probability of observing an EPSC with extracellular stimulation was often low in comparison to more mature cultures. Specifically, we observed a ~60% failure rate to evoke an EPSC with extracellular stimulation in untransfected, neonatal Fmr1 KO neurons (Figure 1E, F). The high failure rate of evoked EPSCs may reflect the low rate of synaptic connectivity (De Simoni et al., 2003) or low presynaptic release probability of neonatal hippocampal neurons (Mozhayeva et al., 2002). FMRP decreased the failure rate of evoked EPSCs to ~30% and also decreased PPF (Figure 1C, D) suggesting that postsynaptic FMRP increases presynaptic release probability. However, when an EPSC occurred in an FMRP transfected neuron (e.g. a “success”), its amplitude was greater (Figure 1E, F). This result suggests that FMRP also increases either the strength of an individual synapse (e.g. quantal amplitude) or synapse number. Because FMRP does not affect mEPSC amplitudes (Figure 1D) this argues against an effect on quantal amplitude and supports a role for FMRP in increasing synapse number.
FMRP may also decrease failure rate by “unsilencing” synapses (Liao et al., 1995). Silent synapses refer to those synapses with NMDA receptors (NMDAR) but not functional AMPA receptors (AMPAR). If acute FMRP expression in neonatal neurons “unsilenced” existing synapses, we would expect to observe a greater enhancement of AMPAR-mediated EPSCs as compared to pharmacologically-isolated NMDAR-mediated EPSCs. However, acute FMRP expression in neonatal neurons also enhanced NMDAR-mediated EPSCs (~60%; Figure 1G1, H) similar to its effects on AMPAR EPSCs (~50%; Figure 1D), suggesting that FMRP does not unsilence synapses. Taken together our data suggest that FMRP enhances synapse function in neonatal neurons by stimulating the formation of new synapses and/or increasing presynaptic release probability.
We next determined if acute, cell autonomous expression of FMRP affected synapse maturation, as measured by the kinetics and subunit composition of NMDAR-mediated EPSCs. In hippocampal CA1, NMDAR subunit composition switches from GluN2B- to GluN2A-containing NMDARs during the first and second postnatal week (Cull-Candy et al., 2001; Gray et al., 2011). GluN2A-mediated EPSCs on mature neurons have a faster rate of decay and are less-sensitive to the GluN2B selective antagonist, ifenprodil (Cull-Candy et al., 2001; Gray et al., 2011). We previously demonstrated that acute FMRP expression in mature neurons reduced the amplitude of NMDAR-EPSCs, but did not affect the decay kinetics (Pfeiffer et al., 2010). In contrast, acute FMRP expression in neonatal neurons decreased the decay time constant (τw) of NMDAR-EPSCs as well as the sensitivity to ifenprodil (Figure 1G2, I, J). This result suggests that FMRP expression in neonatal neurons promotes postsynaptic maturation as measured by the expression of synaptic GluN2A-containing NMDARs.
To determine if the enhancement of synaptic transmission observed with FMRP expression in neonatal neurons correlates with an increased number of structural synapses, we measured the effects of acute FMRP expression on a structural correlate of excitatory synapses, dendritic spines. To visualize dendritic spines, neonatal slice cultures from Fmr1 KO mice were biolistically transfected with GFP, either alone or with FMRP. Three days after transfection, dendritic spines on secondary dendrites were visualized on live neurons using 2-photon, laser-scanning microscopy as described (Pfeiffer et al., 2010). Acute FMRP expression did not affect the total dendritic spine density, nor the density of specific spine types (mushroom, stubby and thin) (Radley et al., 2008; Rodriguez et al., 2008; Rodriguez et al., 2006) (Fig 1K) indicating the enhancement of mEPSC frequency and evoked EPSCs by FMRP is not due to an increase in dendritic spines or a change in spine type.
The effects of endogenous postsynaptic FMRP on synapse function are developmentally regulated
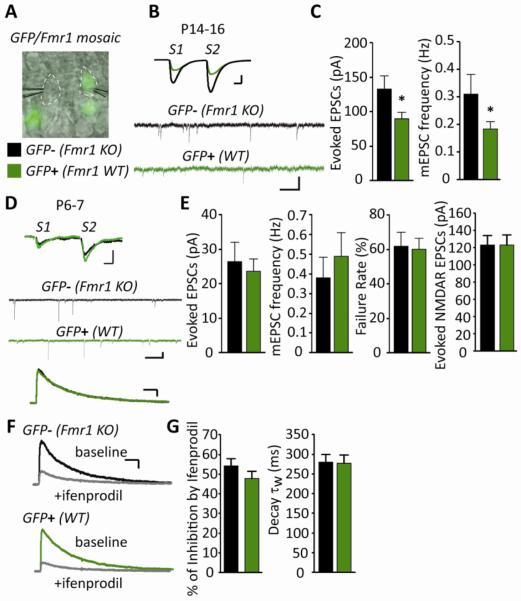
To determine if endogenous FMRP differentially regulates synapse function across development in vivo as observed with acute expression of exogenous FMRP, we utilized GFP/Fmr1KO mosaic mice that were generated by crossing Fmr1 KO mice with mice that express GFP on the X chromosome (Hadjantonakis et al., 1998; Hanson and Madison, 2007). Due to X-inactivation, cells of heterozygous females are mosaic. In other words, GFP-positive cells are wildtype for Fmr1 and neighbor GFP-negative Fmr1 KO cells (Niere et al., 2012). Acute hippocampal slices were prepared from postnatal day (P) 6–7 and P14–16 GFP/Fmr1 mosaic mice; ages which are equivalent to the postnatal ages of slice cultures used for acute FMRP expression experiments. Simultaneous recordings of evoked EPSCs and mEPSCs from pairs of wild-type (WT; GFP-positive) and Fmr1 KO (GFP-negative) CA1 pyramidal neurons demonstrated that the effects of endogenous FMRP on synaptic function were developmentally regulated (Figure 2). At P14–16, wildtype neurons displayed a 30% decrease in evoked EPSC amplitude and mEPSC frequency (Figure 2B, C; Table S1) in comparison to neighboring Fmr1 KO neurons and no change in mEPSC amplitude or PPF was detected. These results support a role for endogenous FMRP in suppression of synapse number in vivo. However, at P6–7, synaptic function, measured by the amplitude of evoked AMPAR or NMDAR-EPSCs, PPF, mEPSC amplitude or frequency, was not different between neighboring WT and Fmr1 KO CA1 neurons (Figure 2D–G; Table S1). Therefore, endogenous FMRP recapitulated the synapse elimination effects we observed with acute FMRP expression in mature cultures, but not the synapse promoting or maturation effects we observed in neonatal neurons.
Figure 2. The effects of endogenous postsynaptic FMRP on excitatory synaptic function are developmentally regulated.
(A) Representative DIC and epifluorescence image during simultaneous whole cell recordings from GFP− (Fmr1 KO) and GFP+ (WT) CA1 neurons in acute hippocampal slices from a GFP/Fmr1 mosaic mouse. White dotted lines outline recorded neurons (B) Representative evoked EPSCs and mEPSCs from simultaneous recordings of GFP− (Fmr1 KO) and GFP+ (WT) neurons in acute slices from a P14–16 GFP/Fmr1 mosaic mouse. Scale bars: evoked EPSCs=50pA/10ms; mEPSCs=10pA/500ms. (C) Average EPSC amplitude (n=16 cell pairs) and mEPSC frequency (n=31) are reduced in GFP+ (WT) neurons in comparison to neighboring GFP− (Fmr1 KO) neurons. (D) Representative evoked EPSCs, mEPSCs and evoked, pharmacologically isolated NMDAR-EPSCs from GFP− (Fmr1 KO) and GFP+ (WT) neurons in acute slices from a P6–7 GFP/Fmr1 mosaic mouse. Scale bars: evoked EPSCs=10pA/10ms; mEPSCs=10pA/500ms; NMDAR EPSCs=50pA/100ms. (E) Average evoked EPSC amplitude (p > 0.1), failure rate (n=17; p > 0.1)), mEPSC frequency (n=20; p > 0.1) and evoked NMDAR-mediated EPSC amplitude (n=58; p > 0.1) are not different between GFP− (Fmr1 KO) and GFP+ (WT) neurons at P6–7. (F) Representative evoked NMDAR-mediated EPSCs before and after 3μM ifenprodil (scale bar = 50pA/100ms). (G) Average % inhibition by ifenprodil (n=44; p > 0.1) and weighted time constant (τw) of the decay of NMDAR-EPSCs (n=49; p > 0.1) from GFP− (Fmr1 KO) and GFP+ (WT) neurons at P6–8 are not different. * p< 0.05.
MEF2 levels and transcriptional activity are developmentally regulated
Because FMRP and MEF2 function together to eliminate synapses in mature cultures (Pfeiffer et al., 2010), we hypothesized that developmental changes in MEF2 activity may underlie the bidirectional effects of FMRP on synaptic function. To test this hypothesis, we first examined whether MEF2 levels or transcriptional activity differed between neonatal (ED7) and mature (ED14) hippocampal slice cultures. The major MEF2 isoforms expressed in hippocampal CA1 are MEF2A and MEF2D (Flavell et al., 2006; Lyons et al., 1995). Dephosphorylation of MEF2A and MEF2D at Ser408 by the Ca2+-dependent protein phosphatase, calcineurin, occurs in response to KCl-induced neuronal depolarization and increases MEF2 transcriptional activity (Flavell et al., 2006). Therefore, we measured basal and activity-induced changes in phosphorylation of MEF2A/D at Ser408 as a measure of MEF2 activation in neonatal and mature cultures. Basal levels of total or phosphorylated-S408 MEF2A/D did not differ between neonatal and mature cultures (Figure 3A, B). However, depolarization (60 mM KCl; 10 min) caused dephosphorylation of MEF2A/D in mature cultures, but not in neonatal cultures (Figure 3B). These results suggest that as the hippocampal cultures mature neuronal activity is more able to stimulate MEF2 transcriptional activity.
To measure MEF2 transcriptional activity in the cultures at the two different ages, we used a fluorescent MEF2 transcriptional reporter, MRE-GFP (Flavell et al., 2006; Pfeiffer et al., 2010). Using the MRE-GFP, we measured MEF2 transcriptional activity in individual, live CA1 neurons in slice culture, the same preparation used for electrophysiology. MRE-GFP was transfected into mature (ED14) and neonatal (ED7) wildtype neurons together with mCherry as a transfection marker and normalization factor. Neurons were depolarized for different durations (0.5, 1, 2, 3, 6 hours of KCl treatment; Figure 3C, D). Basal MRE-GFP intensity (t=0) was similar between neonatal and mature neurons. However, 3 hours after depolarization MRE-GFP expression was increased in mature (p<0.05), but not neonatal cultures. Furthermore, there was greater MRE-GFP expression induced at 6 hours in mature cultures, in comparison to neonatal (p<0.001), indicative of greater MEF2 transcriptional activation in response to neuronal depolarization (Figure 3C, D).
As cultures mature, the ability of neuronal depolarization to activate MEF2 increases. Therefore, MEF2 may function to suppress synapse number in mature, but not neonatal neurons. To test this possibility, we acutely transfected a dominant negative form of MEF2, MEF2-Engrailed (MEF2-EN) (Pfeiffer et al., 2010) into neonatal and mature CA1 neurons to suppress endogenous MEF2 activity and examined excitatory synaptic function. As we previously reported, expression of MEF2-EN in mature neurons results in a robust enhancement of synapse function as measured by evoked EPSC amplitude and mEPSC frequency (Pfeiffer et al., 2010) (Figure 4A). MEF2-EN did not affect mEPSC amplitude or PPF (Table S1). In contrast, expression of MEF2-EN in neonatal cultures had no effect on synaptic function (Figure 4B; Table S1). These results are consistent with previous work indicating that inhibition of MEF2 increases synapse number (Barbosa et al., 2008; Flavell et al., 2006; Pfeiffer et al., 2010) and here we show this preferentially occurs in mature cultures.
MEF2 activity controls the developmental switch of FMRP on synaptic function
Our previous work suggested that FMRP functions downstream of MEF2 to mediate synapse elimination in mature neurons (Pfeiffer et al., 2010). Therefore, we hypothesized that MEF2 activity in mature neurons is required for FMRP-induced synapse elimination. Furthermore, deletion of MEF2 in mature cultures may also reveal the synapse promoting effects of FMRP usually observed in neonatal cultures. To test this hypothesis, we deleted endogenous MEF2 isoforms (MEF2A/C/D) from mature cultures and examined the effects of acute FMRP expression on synaptic function. To delete endogenous MEF2 isoforms in slice cultures, Fmr1 KO mice were crossed with mice harboring floxed alleles of mef2A, mef2C, mef2D (Fmr1 KO/MEF2ACD-fl) (Akhtar et al., 2012; Arnold et al., 2007; Kim et al., 2008), a ubiquitously expressed tamoxifen-inducible Cre–driver mouse line (Fmr1 KO/MEF2ACD-fl/CAGCre+) (Hayashi and McMahon, 2002), and a fluorescent Cre-reporter mouse (floxed td-tomato; (Madisen et al., 2009). The loxP sites of MEF2ACD-fl flank the second exon containing the MADS/MEF2 domains that mediates DNA binding. Therefore, CRE-mediated recombination in MEF2ACD-fl generates a truncated MEF2 protein that lacks the DNA binding domain and transcriptional activity (Akhtar et al., 2012; Arnold et al., 2007; Kim et al., 2008). A 6-day application of tamoxifen to slice cultures of Fmr1 KO/MEF2ACD-fl/CAGCre+ mice deleted all MEF2 isoforms as indicated by the presence of truncated MEF2A and MEF2D protein products and mef2c RNA (Figure 4C). After MEF2 deletion, FMRP was transfected into mature cultures of Fmr1 KO/MEF2ACD-fl/CAGCre+ mice. Consistent with our prediction, FMRP transfection into mature cultures of MEF2-deleted mice enhanced evoked EPSC amplitude and mEPSC frequency (Figure 4D). However, FMRP did not alter PPF in this experiment (Table S1). As a control, FMRP transfection of mature cultures prepared from tamoxifen-treated, Cre-negative littermates (Fmr1 KO/MEF2ACD-fl/CAGCre−) induced a decrease in evoked EPSCs and mEPSC frequency (Figure 4E). Therefore, MEF2 deletion from mature cultures inhibits FMRP-induced synapse elimination and/or reveals an enhancement of synaptic function that is normally observed in neonatal cultures that are wildtype for MEF2.
As another test of our hypothesis we determined if expression of a constitutively active MEF2 (MEF2VP16) in neonatal Fmr1 KO cultures would cause a precocious synapse elimination by FMRP transfection. Acute expression of MEF2VP16 alone in neonatal Fmr1 KO cultures had no effect on synaptic function as previously observed in mature Fmr1 KO cultures (Pfeiffer et al., 2010). In contrast, transfection of MEF2VP16 and FMRP in neonatal Fmr1 KO cultures reduced mEPSC frequency consistent with a functional synapse elimination (Figure 4F,G; Table S1). Together with the effects of MEF2 deletion, these results suggest that the level of MEF2 transcriptional activity determines the developmental effect of postsynaptic FMRP on synaptic function.
Discussion
FMRP interacts with mRNAs that encode 25–30% of the synaptic proteome (Darnell et al., 2011). Consequently, there are multiple examples of altered synaptic structure, function and plasticity in Fmr1 KO mice. Little is known of the determinants of the multiple and diverse functions of FMRP at synapses. Here we demonstrate cell autonomous and bidirectional effects of postsynaptic FMRP on excitatory synaptic function that vary as a function of postnatal age. The developmental regulation of FMRP function is dependent on MEF2 transcriptional activity because we can accelerate or delay the developmental effects of FMRP on synaptic function by overexpression or deletion of MEF2, respectively. Importantly, these results suggest that the identity and levels of specific transcripts in a neuron determine the effect of FMRP on synaptic function. Our work suggests a model, depicted in Figure 5, where FMRP and MEF2 function together as an activity-dependent sensor of synapse development and maturation that can refine synaptic connectivity over the course of brain development. Specifically, in neonatal neuronal cultures with few synapses, there is a low level of MEF2-mediated transcription. Therefore, FMRP may interact with a set of transcripts that function to promote synapse formation, stabilization and/or maturation (Figure 5). As neurons mature and more excitatory synapses are formed, neurons are more electrically active which stimulates Ca2+ influx and activates MEF2-dependent transcription. The increase in MEF2-generated transcripts in mature neurons may then interact with FMRP and function to destabilize existing synapses and/or inhibit additional synapse formation (Figure 5). This model would predict that FMRP interacts with distinct mRNA targets that depend on the electrical activity, developmental age, and the active transcription factors in neurons. Deletion of endogenous MEF2 isoforms reveals the synapse promoting effects of FMRP in mature neurons as measured by increased evoked EPSCs and mEPSC frequency. Whether these synapse promoting effects of FMRP are simply due to a lack of MEF2 transcripts in the cell or if other specific transcription factors are required is a topic for future investigation. An interesting possibility is that FMRP and/or other dendritic RNA-regulatory proteins are generally required for transcriptional regulation of synapses (Greer and Greenberg, 2008).
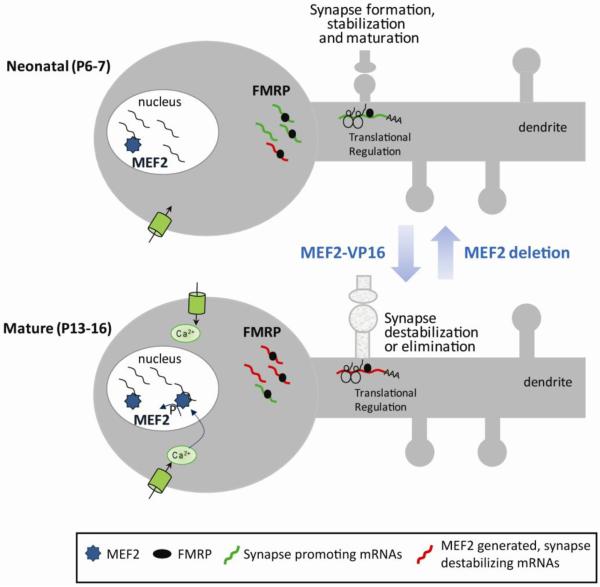
Figure 5. Working model: The bidirectional and developmental effects of FMRP on excitatory synaptic function depend on the availability of MEF2 transcripts.
In neonatal neurons (postnatal day 6–7) FMRP may interact and translationally regulate mRNAs (depicted as green) that promote synapse formation, stabilization and/or maturation. As neurons mature and more excitatory synapses are formed, neurons are more electrically active which stimulates Ca2+ influx, dephosphorylates MEF2 and activates MEF2-dependent transcription. The increase in MEF2-generated mRNAs (red) in mature neurons may then interact with FMRP and function to destabilize existing synapses or inhibit additional synapse formation. Deletion of MEF2 isoforms reveals the immature, synapse promoting effects of FMRP presumably by reducing the availability of the “synapse destabilizing” transcripts. Expression of constitutively active MEF2 (MEF2VP16) in neonatal neurons increases the level of “synapse destabilizing” transcripts and results in precocious synapse elimination upon acute FMRP expression.
A major molecular function for FMRP is to suppress translation of its mRNA targets. Neural activity relieves translational repression of these target mRNAs, through posttranslational regulation of FMRP and FMRP-interacting proteins (Darnell et al., 2011; Nalavadi et al., 2012; Napoli et al., 2008; Niere et al., 2012). Therefore, FMRP is also necessary for translational activation of many of its target mRNAs. FMRP has also been implicated in dendritic mRNA transport of some target mRNAs (Dictenberg et al., 2008). Intact RNA-binding domains of FMRP are necessary to elicit synapse elimination (Pfeiffer and Huber, 2007; Pfeiffer et al., 2010) suggesting that translation and/or transport of FMRP target mRNAs is required. Deletion of MEF2 isoforms inhibits synapse elimination with acute FMRP expression and/or reveals FMRP-dependent facilitation of synaptic function. Therefore, we hypothesize that FMRP translationally regulates MEF2-generated transcripts to suppress synapse number. Candidate transcripts include Arc, SynGAP (Flavell et al., 2006), Pcdh10 (Tsai et al., 2012) and others (Darnell et al., 2011) known to weaken synapse strength and/or reduce spine number.
Postsynaptic FMRP expression in neonatal cultures increased synapse function as measured by evoked EPSCs (both AMPA and NMDA receptor-mediated) and mEPSC frequency. FMRP also caused a decrease in paired-pulse facilitation and EPSC failure rate. All of these effects are consistent with postsynaptic FMRP causing an increase in presynaptic release probability. However, the observed increase in the amplitude of EPSC successes without an effect on mEPSC amplitude, or dendritic spine density suggests that FMRP may also increase the number of functional synapses not associated with spines. FMRP may stimulate presynaptic maturation of existing synapses and/or the formation and stabilization of new synapses with a high release probability. Known target mRNAs of FMRP whose protein products are known to stimulate synapse formation, maturation or stabilization include the Neuroligins, PSD-95, SHANKs, NCAM, CamKII (α and β), and NMDAR subunits (Darnell et al., 2011). Notably, postsynaptic expression of PSD-95 and/or Neuroligin 1 (NL1) stimulate synapse formation, presynaptic release probability and synapse maturation (Futai et al., 2007; Peixoto et al., 2012; Wittenmayer et al., 2009).
FMRP expression in neonatal neurons also stimulated postsynaptic maturation, as measured by the quickening of the NMDAR EPSC and decreased sensitivity to the GluN2B antagonist ifenprodil which suggests that FMRP facilitates the developmental switch from GluN2B to GluN2A subunits at synapses (Cull-Candy et al., 2001; Gray et al., 2011). FMRP interacts directly with mRNAs for the NMDAR subunits themselves (GluN1, GluN2A and GluN2B), as well as components of the NMDAR complex (Darnell et al., 2011) and GluRN2B protein levels are elevated in young Fmr1 KO hippocampi (Schutt et al., 2009). Therefore, FMRP-mediated suppression of GluN2B translation may be a mechanism by which FMRP enhances neonatal synapse function. Interestingly, deletion of GluN2B enhances evoked EPSCs and mEPSC frequency, similar to what we observe with acute FMRP expression in neonatal neurons (Gray et al., 2011). Deletion of MEF2 in mature neurons reinstated the synapse promoting effects of FMRP as measured by evoked EPSCs and mEPSC frequency, but not decreased PPF. This result suggests that under these conditions FMRP stimulates functional synapse number, but not through a presynaptic release probability mechanism. The different maturational state of presynaptic terminals impinging on FMRP transfected neurons in neonatal vs. mature cultures may be differentially susceptible to the effects of postsynaptic FMRP. Additional experiments are necessary to explore this hypothesis.
Using the GFP/Fmr1 mosaic we confirmed a cell autonomous, postsynaptic role for endogenous FMRP in suppression of synapse number in vivo. However, we only observed the synapse promoting and maturation effects of FMRP with acute FMRP expression. Compensatory mechanisms in the Fmr1 mosaic may establish proper synapse number in neurons with constitutive deletion of FMRP. Furthermore, biolistic transfection of FMRP results in overexpression of FMRP in CA1 neurons by about 1.8 fold of endogenous levels, despite using the endogenous Fmr1 promoter to drive FMRP (Darnell et al., 2005; Pfeiffer and Huber, 2007). This suggests that acute and/or overexpression of FMRP are required to observe its cell autonomous, synapse promoting effects. There may also be However, consistent with a role for FMRP in synapse stabilization and maturation, previous work observes weak and immature excitatory synapses in the hippocampus and neocortex of Fmr1 KO mice, but only within an early postnatal time window (Bureau et al., 2008; Harlow et al., 2010; Pilpel et al., 2009).
The ability of neuronal depolarization to dephosphorylate MEF2 and activate MEF2 transcription increased as the cultures matured. Furthermore, inhibition of MEF2 using MEF2-EN enhanced synaptic function in mature, but not neonatal, cultures. These results support the idea that neuronal activity driven MEF2 suppresses synapse number beginning at the 2nd postnatal week. Increased excitatory synapse density, maturation, cell excitability or Ca2+ influx may lead to enhanced MEF2 activity in mature cultures. In addition, developmental changes in expression of other cofactors or mechanisms that regulate MEF2 activation may also contribute. Importantly, the MEF2-EN-induced increase in synapse number requires FMRP because MEF2-EN transfection in mature Fmr1 KO cultures has no effect on synapse function (Pfeiffer et al., 2010). Therefore FMRP is required to observe effects of MEF2 activation or inhibition on synapse function. This fact may explain why we do not observe differences in mEPSC frequency between cultures prepared from Fmr1 KO/MEF2ACD-fl/CAGCre− with Fmr1 KO/MEF2ACD-fl/CAGCre+ mice (Figure 4D,E).
The mechanisms we have revealed in cultured neurons may have important implications for experience-dependent postnatal refinement of cortical circuits in vivo. Sensory deprivation reduces spine elimination rates in WT cortical neurons, but spines in Fmr1 KO neurons are insensitive to sensory experience (Pan et al., 2010). We hypothesize that MEF2 and FMRP function to couple experience-driven activity in cortical neurons with spine elimination and thus are necessary for the proper developmental refinement of circuits by experience. In FXS patients, symptoms such as hyperactivity, language and developmental delay become evident at 2–4 years of age (Tranfaglia, 2011) which corresponds to the period of synaptogenesis and elimination in the human cortical neurons (Huttenlocher and Dabholkar, 1997). This suggests that these synaptic regulatory mechanisms of FMRP that we have discovered likely contribute to the early behavioral deficits associated with the disease. Understanding their mechanisms will help to provide novel therapeutic strategies for FXS and related disorders.
Supplementary Material
Acknowledgements
This research was supported by FRAXA Postdoctoral Fellowship (T.Z.), and grants from the NIH HD052731, Autism Speaks (K.M.H) and Simons Foundations (K.M.H. and C.W.C.). We would like to thank Lorea Ormazabal, Nicole Cabalo and Brad Pfeiffer for technical assistance; members of the Huber lab for helpful discussions and suggestions on the manuscript; and J. Johnson for providing the CAG-CREERTM transgenic mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtar MW, Kim MS, Adachi M, Morris MJ, Qi X, Richardson JA, Bassel-Duby R, Olson EN, Kavalali ET, Monteggia LM. In vivo analysis of MEF2 transcription factors in synapse regulation and neuronal survival. PLoS One. 2012;7:e34863. doi: 10.1371/journal.pone.0034863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12:377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci U S A. 2008;105:9391–9396. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SX, Cherry A, Tari PK, Podgorski K, Kwong YK, Haas K. The Transcription Factor MEF2 Directs Developmental Visually Driven Functional and Structural Metaplasticity. Cell. 2012;151:41–55. doi: 10.1016/j.cell.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Chung WS, Barres BA. The role of glial cells in synapse elimination. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CJ, Mercaldo V, Restivo L, Yiu AP, Sekeres MJ, Han JH, Vetere G, Pekar T, Ross PJ, Neve RL, Frankland PW, Josselyn SA. MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat Neurosci. 2012;15:1255–1264. doi: 10.1038/nn.3189. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Mostovetsky O, Darnell RB. FMRP RNA targets: identification and validation. Genes Brain Behav. 2005;4:341–349. doi: 10.1111/j.1601-183X.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J Neurosci. 2007;27:4014–4018. doi: 10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kim Y, Phan D, van Rooij E, Wang DZ, McAnally J, Qi X, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest. 2008;118:124–132. doi: 10.1172/JCI33255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, Soussou W, Nie Z, Kang YJ, Nakanishi N, Okamoto S, Roberts AJ, Schwarz JJ, Lipton SA. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U S A. 2008;105:9397–9402. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Lyons GE, Micales BK, Schwarz J, Martin JF, Olson EN. Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J Neurosci. 1995;15:5727–5738. doi: 10.1523/JNEUROSCI.15-08-05727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2009;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Biolistic transfection of cultured organotypic brain slices. Methods Mol Biol. 2004;245:197–206. doi: 10.1385/1-59259-649-5:197. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27:40–47. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Black BL, Martin JF, Olson EN. Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Mol Cell Biol. 1996;16:2627–2636. doi: 10.1128/mcb.16.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalavadi VC, Muddashetty RS, Gross C, Bassell GJ. Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation. J Neurosci. 2012;32:2582–2587. doi: 10.1523/JNEUROSCI.5057-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, Witke W, Costa-Mattioli M, Sonenberg N, Achsel T, Bagni C. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Niere F, Wilkerson JR, Huber KM. Evidence for a fragile x mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered arc translation and long-term depression. J Neurosci. 2012;32:5924–5936. doi: 10.1523/JNEUROSCI.4650-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, Philpot BD, Ehlers MD. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron. 2012;76:396–409. doi: 10.1016/j.neuron.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The State of Synapses in Fragile X Syndrome. Neuroscientist. 2009 doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Zang T, Wilkerson JR, Taniguchi M, Maksimova MA, Smith LN, Cowan CW, Huber KM. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Pilpel Y, Kolleker A, Berberich S, Ginger M, Frick A, Mientjes E, Oostra BA, Seeburg PH. Synaptic ionotropic glutamate receptors and plasticity are developmentally altered in the CA1 field of Fmr1 knockout mice. J Physiol. 2009;587:787–804. doi: 10.1113/jphysiol.2008.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Hof PR, Wearne SL. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat Protoc. 2006;1:2152–2161. doi: 10.1038/nprot.2006.313. [DOI] [PubMed] [Google Scholar]
- Schutt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284:25479–25487. doi: 10.1074/jbc.M109.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Tian X, Kai L, Hockberger PE, Wokosin DL, Surmeier DJ. MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons. Mol Cell Neurosci. 2010;44:94–108. doi: 10.1016/j.mcn.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranfaglia MR. The psychiatric presentation of fragile x: evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndrome. Dev Neurosci. 2011;33:337–348. doi: 10.1159/000329421. [DOI] [PubMed] [Google Scholar]
- Tsai NP, Wilkerson JR, Guo W, Maksimova MA, DeMartino GN, Cowan CW, Huber KM. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell. 2012;151:1581–1594. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenmayer N, Korber C, Liu H, Kremer T, Varoqueaux F, Chapman ER, Brose N, Kuner T, Dresbach T. Postsynaptic Neuroligin1 regulates presynaptic maturation. Proc Natl Acad Sci U S A. 2009;106:13564–13569. doi: 10.1073/pnas.0905819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005a;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005b;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.