Abstract
Purpose
Higher physical activity (PA) has been associated with greater attenuation of body-fat gain and preservation of lean mass across the lifespan. These analyses aimed to determine relationships of change in PA to changes in fat and lean body mass in a longitudinal prospective study of postmenopausal women.
Methods
Among 11,491 women enrolled at three Women’s Health Initiative (WHI) clinical centers were selected to undergo dual-energy x-ray absorptiometry (DXA), 8,352 had baseline body composition measurements, with at least one repeated measure at yr 1, 3, and 6. PA data were obtained by self-report at baseline, 3 and 6 yr of follow-up. Time-varying PA impact on change in lean and fat mass during the six-yr study period for age groups (50–59y, 60–69y, 70–79y) was estimated using mixed effects linear regression.
Results
Baseline PA and body composition differed significantly among the three age groups. The association of change in fat mass from baseline and time-varying PA differed across the three age groups (p=0.0006). In women aged 50–59, gain in fat mass from baseline was attenuated with higher levels of physical activity. Women aged 70–79 lost fat mass at all PA levels. In contrast, change in lean mass from baseline and time-varying PA did not differ by age group (p=0.1935).
Conclusions
The association between PA and change in fat mass varies by age group, with younger, but not older, women benefitting from higher levels of aerobic PA. Higher levels of aerobic activity are not associated with changes in lean mass, which tends to decrease in older women regardless of activity level. Greater attention to resistance training exercises may be needed to prevent lean mass loss as women age.
Keywords: lean mass changes, exercise, aging, women, sarcopenia
INTRODUCTION
Sarcopenia, the age-associated loss of skeletal muscle mass, and increasing body fat mass are both hallmarks of the aging process. Sarcopenia has been cited as a major factor in strength decline (8), as well as functional impairment, disability, and loss of independence (26) with aging. In addition to loss of skeletal muscle mass, women experience bone mineral density (BMD) decreases (9) concomitant with increased central body fatness, which may promote a loss of fat-free mass during the menopausal transition. This adverse change in the ratio of fat-to-lean mass may be related to a decline in energy expenditure, loss of muscular strength and a decline in physical activity (5, 28, 30). Preventing gain of fat mass and loss of skeletal muscle mass and function is preferable to trying to reverse these body composition changes in old age.
Current evidence suggests that maintaining physical activity over time is key to preventing these adverse age-related body composition changes associated with aging (14,20,25). Hankinson and colleagues (13) examined the relationship of habitual PA levels and changes in body mass index (BMI)) over a 20-year period and observed that those individuals, particularly women, who reported high levels of PA through young adulthood experienced less weight gain and central adiposity through the transition to middle age. Andreoli and colleagues (2) examined the differences in BMD, body composition, and bone mineral content in women who had been elite athletes during their youth as compared to sedentary controls and observed that long term PA significantly improved BMD and muscle mass, with a reduction in the adverse aging affects on body composition.
The Women’s Health Study reported that PA was associated with less weight gain only in women with BMI lower than 25 kg/m2 (17). We recently reported a significant interaction of age and initial (baseline) PA in relation to weight changes over an 8-yr period in the large Women’s Health Initiative (WHI) clinical trial (CT) cohort (27). Among women aged 50–59 years at baseline, there was significantly less weight gain in those reporting moderate (>500–1200 MET-min-wk−1) and high (>1200 MET-min-wk−1) levels of PA compared to sedentary women (≤100 MET-min-wk−1); whereas, among women aged 70–79 years, higher PA (>1200 MET-min-wk1) was associated with significantly less weight loss, compared to those who reported sedentary, low or moderate activity (27).
Only total weight changes were investigated in these earlier reports; however, healthy weight management should emphasize the prevention of fat mass gain and lean mass loss, both of which are believed to be favorably influenced by physical activity (11,31). Furthermore, prior analyses have focused on baseline physical activity, without taking into account changes in activity level during follow-up. The availability of dual-energy x-ray absorptiometery (DXA) data from a subgroup (the DXA cohort) of the large WHI cohort provided the opportunity to extend our investigation of the relationship of PA to weight changes to focus on changes in body composition over a six-year follow up period, in relationship to changes in physical activity (PA) levels.
METHODS
The WHI DXA cohort was drawn from three WHI clinical centers (Pittsburgh, PA; Birmingham, AL; and Tuscon-Phoenix, AZ), among 40 US clinical centers which enrolled the large multiethnic cohort of 68,132 postmenopausal women into the WHI Clinical Trials (CT) of Diet Modification (DM), Hormone Therapy (HT) and/or calcium plus vitamin D supplementation (CaD), as well as 93,676 women into the WHI Observational Study (OS) cohort, from October 1993 to December 1998. Details of recruitment, baseline data collection, and baseline characteristics of the CT and OS cohort have been published previously (15). All procedures and protocols were approved by the institutional review boards at each participating institution, and all participants provided written informed consent.
Body composition by DXA scans was assessed at baseline and after 1,3, and 6 years of follow-up in CT and OS participants who were randomly selected for DXA measurements at each of the three centers (total N = 11,941; OS N = 6,365 and CT N=4,655). Diversity based on race/ethnicity was maximized at the WHI DXA sites. Participants who completed the baseline and at least one follow-up DXA scan post randomization, with an accompanying physical activity questionnaire, were included in this analysis. The present analyses further excluded participants who had missing baseline information for variables used in statistical models, including PA, physical measures, dietary and alcohol intake, smoking status, menopausal hormone therapy (HT) use, education, and sleep information. These exclusions reduced the sample to a final analytic cohort of 8,352 participants.
Body Composition Measurements
Whole-body DXA (QDR2000, 2000+, or 4500W; Hologic Inc, Bedford MA) scans were used to determine both regional and total body compositions. Measurements included bone-mineral density (BMD), lean body mass (lean soft tissue mass) fat mass, and percentage of fat mass. As previously described (7), standard WHI protocols were used for the positioning and analysis of DXA scans by radiology technicians, trained and certified by Hologic and the WHI Bone Density Coordinating Center at the University of California, San Francisco.
Assessment of Physical Activity
Recreational PA was assessed by questions on the frequency and duration of recreational activities, and metabolic equivalent task (MET) scores (defined as the ratio of work metabolic rate to a standard resting metabolic rate, with one metabolic equivalent task roughly equivalent to the resting metabolism while sitting quietly) were computed as the product of days per week, minutes per day, and metabolic equivalent task value for each activity (1).
Information on walking and recreational PA was used to generate a summary variable in MET-minutes/week. Participants were asked how often they currently walked outside the home for more than 10 minutes without stopping and the usual duration and speed of their walks. Categories of frequency were rarely/never, 1 to 3 times per month, 1 time per week, 2 to 3 times per week, 4 to 6 times per week, and 7 or more times per week. Duration categories were less than 20 minutes, 20 to 39 minutes, 40 to 59 minutes, and 1 hour or more. Four speed categories were used: less than 2 mph (casual strolling or walking, 2.0 MET), 2–3 mph (average or normal walking, 3.0 MET)), 3–4 mph (fairly fast walking 4.0 MET)) or more than 4 mph (very fast walking, 4.5 MET). The MET values were further calculated using the corrected MET equation (Harris-Benedict equation) to adjust the standard MET level for age, sex, height, and body weight as described in the Compendium of Physical Activities (2011).
Based on the questions asked, women were classified into four groups of PA levels at baseline, year 3 and year 6: sedentary, i.e. reporting ≤100 MET · min wk−1; low PA level, >100–500 MET · min wk−1; moderate PA, >500 to 1200 MET · min wk−1; and high PA, >1200 MET · min wk−1. Achieving a minimum of 500 MET · min wk−1 meets current national guidelines to engage in at least 150 minutes per week of moderate-intensity PA, i.e. the minimum health-related dose of activity recommended by the 2008 US Physical Activity Guidelines (32).
Assessment of other Covariates
Covariates including age, energy intake and other dietary variables, energy expenditure, ethnicity, smoking and alcohol habits, sleep duration, medications with a known impact on body composition (oral steroids, thyroid medications, psychotropics/antidepressants) and prior HT were all assessed from baseline questionnaires. Waist-hip ratio (WHR), and BMI were assessed at each clinic visit. Caloric intake was assessed using a validated food-frequency questionnaire, based on instruments previously used in large-scale dietary intervention trials (6, 23).
STATISTICAL ANALYSES
The primary outcomes were change in lean body mass and fat mass from baseline. The primary variable of interest was self-reported physical activity at baseline, year 3, and year 6. Physical activity was incorporated in a time-varying fashion; for the difference in outcome at the year 1 clinical visit, baseline physical activity was used. Similarly, at the year 3 and year 6 visits, physical activity reported on year 3 and year 6 questionnaires, respectively, were used. Mixed effects linear regression techniques were used to describe the association between the primary outcomes (change in lean mass, fat mass, and BMI from baseline) and time-varying physical activity over the 6-year study period. In particular, the main question of interest was whether the association between PA and change in body composition varied by age group. Thus, our main parameter of interest was an interaction between time varying in PA and age group. Our modeling approach accounted for the correlation of responses within a subject over time by including a subject-specific random intercept in the model. Estimates of the association were adjusted for the following potential confounders: years post-randomization, baseline measurement of the outcome, age group at study entry, ethnicity, education, smoking, prior and current HT usage, medications associated with body composition change (oral steroids, thyroid, psychotropic/antidepressants), hours of sleep, total energy intake, protein intake, alcohol intake, fruit and vegetable servings per day, treatment assignment in the WHI DM trial and scanner used for body composition measures. The hypothesis corresponding to presence of an interaction effect between time-varying MET and age group on each primary outcome was tested with a two-sided Wald test at the 0.05 level of significance. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary NC) and Stata 12 (StataCorp LP, College Station TX).
RESULTS
Baseline Characteristics
Table 1 presents baseline socio-demographic and lifestyle characteristics of the analytic cohort by self-reported PA group, with 24.9% of participants categorized as sedentary; 29.5% as low PA level; 26.2% as moderate and 19.5% as high. Less than half (45.6%) reported baseline PA levels that meet current recommended PA levels. Compared to the moderate and high PA groups, a greater proportion of women in the sedentary and low categories were obese, current smokers, and were distributed in the highest quartiles of waist-hip ratio.
Table 1.
Baseline characteristics by baseline MET category
| Physical Activity Category at Baseline | p-value | ||||
|---|---|---|---|---|---|
|
| |||||
| 0–<100 | 100–<500 | 500–<1200 | 1200+ | ||
| Total (N= 8,352) | 2080 | 2460 | 2186 | 1626 | |
| % Total | 24.90 | 29.45 | 26.17 | 19.47 | |
|
| |||||
| Demographics | |||||
|
| |||||
| Age | <.0001 | ||||
|
| |||||
| <50–59 | 778 | 815 | 655 | 467 | |
| 37.40 | 33.13 | 29.96 | 28.72 | ||
|
| |||||
| 60–69 | 873 | 1087 | 1013 | 744 | |
| 41.97 | 44.19 | 46.34 | 45.76 | ||
|
| |||||
| 70–79+ | 429 | 558 | 518 | 415 | |
| 20.63 | 22.68 | 23.70 | 25.52 | ||
|
| |||||
| Race/Ethnicity | <.0001 | ||||
|
| |||||
| American Indian or Alaskan Native | 28 | 23 | 16 | 16 | |
| 1.34 | 0.93 | 0.73 | 0.98 | ||
|
| |||||
| Asian or Pacific Islander | 7 | 9 | 8 | 5 | |
| 0.34 | 0.37 | 0.37 | 0.31 | ||
|
| |||||
| Black or African-American | 433 | 353 | 217 | 119 | |
| 20.80 | 14.34 | 9.92 | 7.31 | ||
|
| |||||
| Hispanic/Latino | 124 | 149 | 120 | 72 | |
| 5.96 | 6.05 | 5.48 | 4.42 | ||
|
| |||||
| White (not of Hispanic origin) | 1480 | 1915 | 1822 | 1408 | |
| 71.09 | 77.81 | 83.27 | 86.43 | ||
|
| |||||
| Other | 10 | 12 | 5 | 9 | |
| 0.48 | 0.49 | 0.23 | 0.55 | ||
|
| |||||
| Education | <.0001 | ||||
|
| |||||
| High school | 793 | 828 | 586 | 368 | |
| 38.13 | 33.66 | 26.81 | 22.63 | ||
|
| |||||
| Some college | 785 | 903 | 851 | 603 | |
| 37.74 | 36.71 | 38.93 | 37.08 | ||
|
| |||||
| College | 502 | 729 | 749 | 655 | |
| 24.13 | 29.63 | 34.26 | 40.28 | ||
|
| |||||
| Lifestyle | |||||
|
| |||||
| Smoking | <.0001 | ||||
|
| |||||
| Never Smoked | 1176 | 1410 | 1198 | 849 | |
| 56.54 | 57.32 | 54.80 | 52.21 | ||
|
| |||||
| Past Smoker | 685 | 854 | 860 | 694 | |
| 32.93 | 34.72 | 39.34 | 42.68 | ||
|
| |||||
| Current Smoker | 219 | 196 | 128 | 83 | |
| 10.53 | 7.97 | 5.86 | 5.10 | ||
|
| |||||
| Hormone therapy | <.0001 | ||||
|
| |||||
| None | 1022 | 1157 | 1001 | 705 | |
| 49.13 | 47.03 | 45.79 | 43.36 | ||
|
| |||||
| < 5 Years | 478 | 515 | 457 | 325 | |
| 22.98 | 20.93 | 20.91 | 19.99 | ||
|
| |||||
| 5 to < 10 Years | 216 | 271 | 257 | 203 | |
| 10.38 | 11.02 | 11.76 | 12.48 | ||
|
| |||||
| 10 to < 15 Years | 364 | 517 | 471 | 393 | |
| 17.50 | 21.02 | 21.55 | 24.17 | ||
|
| |||||
| Sleep(h/night) | <.0001 | ||||
|
| |||||
| <=5 hours | 257 | 249 | 158 | 126 | |
| 12.36 | 10.12 | 7.23 | 7.75 | ||
|
| |||||
| 6–7 hours | 1340 | 1615 | 1458 | 1050 | |
| 64.42 | 65.65 | 66.70 | 64.58 | ||
|
| |||||
| 8–9 hours | 464 | 578 | 558 | 437 | |
| 22.31 | 23.50 | 25.53 | 26.88 | ||
|
| |||||
| >=10 hours | 19 | 18 | 12 | 13 | |
| 0.91 | 0.73 | 0.55 | 0.80 | ||
|
| |||||
| Diet | |||||
|
| |||||
| Energy intake (kCals) | |||||
|
| |||||
| <1249 | 654 | 808 | 716 | 494 | 0.0310 |
| 31.44 | 32.85 | 32.75 | 30.38 | ||
|
| |||||
| 1249–1625 | 495 | 573 | 563 | 428 | |
| 23.80 | 23.29 | 25.75 | 26.32 | ||
|
| |||||
| 1626–2090 | 450 | 549 | 471 | 388 | |
| 21.63 | 22.32 | 21.55 | 23.86 | ||
|
| |||||
| >2090 | 481 | 530 | 436 | 316 | |
| 23.13 | 21.54 | 19.95 | 19.43 | ||
|
| |||||
| Alcohol | 2.76 | 3.17 | 4.32 | 5.60 | <.0001 |
| 8.44 | 7.75 | 8.88 | 10.91 | ||
|
| |||||
| Fruit servings per day | 1.44 | 1.71 | 1.91 | 2.16 | <.0001 |
| 1.15 | 1.18 | 1.20 | 1.28 | ||
|
| |||||
| Vegetable servings per day | 1.67 | 1.98 | 2.14 | 2.47 | <.0001 |
| 1.07 | 1.19 | 1.23 | 1.38 | ||
|
| |||||
| Grains servings per day | 4.59 | 4.64 | 4.60 | 4.78 | 0.1546 |
| 2.78 | 2.74 | 2.56 | 2.62 | ||
|
| |||||
| Protein intake in grams | 66.67 | 67.86 | 67.93 | 68.96 | 0.2256 |
| 35.31 | 35.10 | 32.33 | 30.63 | ||
|
| |||||
| Trial Arm | |||||
|
| |||||
| DM Trial | <.0001 | ||||
|
| |||||
| Not randomized to DM | 1352 | 1651 | 1565 | 1285 | |
| 65.00 | 67.11 | 71.59 | 79.03 | ||
|
| |||||
| Intervention | 289 | 332 | 238 | 142 | |
| 13.89 | 13.50 | 10.89 | 8.73 | ||
|
| |||||
| Control | 439 | 477 | 383 | 199 | |
| 21.11 | 19.39 | 17.52 | 12.24 | ||
At baseline, MET hours per week was significantly different among the three age groups (p=0.0004), with greater MET hours per week observed in the 70–79y. Fat mass, lean mass, and BMI were also significantly different across the age groups at baseline (p<.0001 for fat mass, lean mass, BMI) with all measures lower for older age groups (Table 2a).
Table 2a.
DXA Cohort current physical activity by BMD visit year
| Physical activity category
| ||||||
|---|---|---|---|---|---|---|
| BMD visit year | MET value from: | 0–<100 | 100–<500 | 500–<1200 | 1200+ | Total |
| 1 | Baseline (%) | 919 | 1020 | 812 | 518 | 3269 |
| 28.11 | 31.20 | 24.84 | 15.85 | |||
|
| ||||||
| 3 | Year 3 (%) | 1921 | 2279 | 1964 | 1488 | 7652 |
| 25.10 | 29.78 | 25.67 | 19.45 | |||
|
| ||||||
| 6 | Year 6 (%) | 1749 | 1842 | 1527 | 1259 | 6377 |
| 27.43 | 28.89 | 23.95 | 19.74 | |||
Change in Physical Activity
At year 3, 8,127 women reported their current PA (Table 2b). Physical activity decreased by 0.06 MET hours per week from baseline levels on average (SD=12.0). At year 6, 7,457 women reported current PA with an average decrease of 0.53 MET hours per week from baseline levels (SD=13.0). Difference in physical activity from baseline was significant at year 6 (p=0.0004) but not at year 3 (p=0.6385).
Table 2b.
DXA Cohort Characteristics by Age Group
| Mean (SD) | Age 50–59 | Age 60–69 | Age 70–79 | P-value (ANOVA) |
|---|---|---|---|---|
| MET hours per week at baseline | 10.78 (13.76) | 11.84 (13.56) | 12.31 (14.20) | 0.0004 |
| Fat mass in kg at baseline | 33.32 (12.05) | 32.42 (11.04) | 30.22 (10.00) | <.0001 |
| Lean mass in kg at baseline | 38.99 (5.55) | 37.60 (5.12) | 36.03 (4.70) | <.0001 |
| BMI in kg/m2 at baseline | 28.58 (6.23) | 28.06 (5.61) | 27.23 (5.22) | <.0001 |
Relationships of Time-Varying Physical Activity on Body Composition
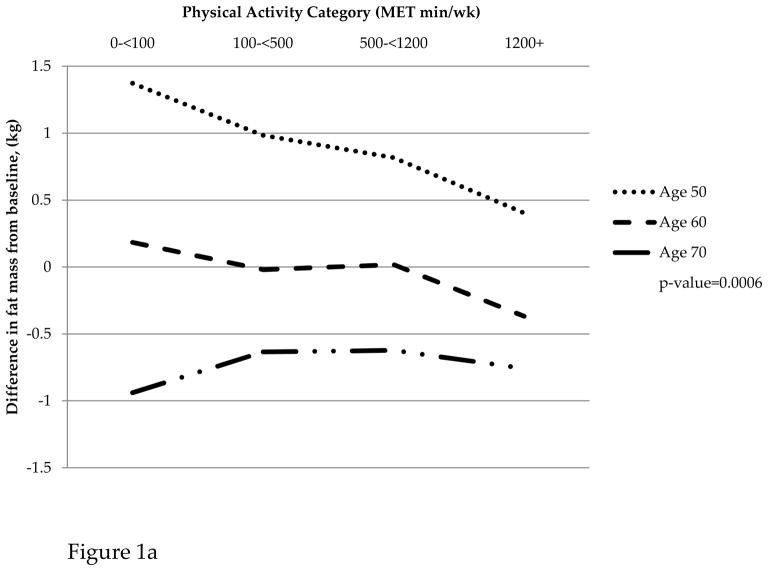
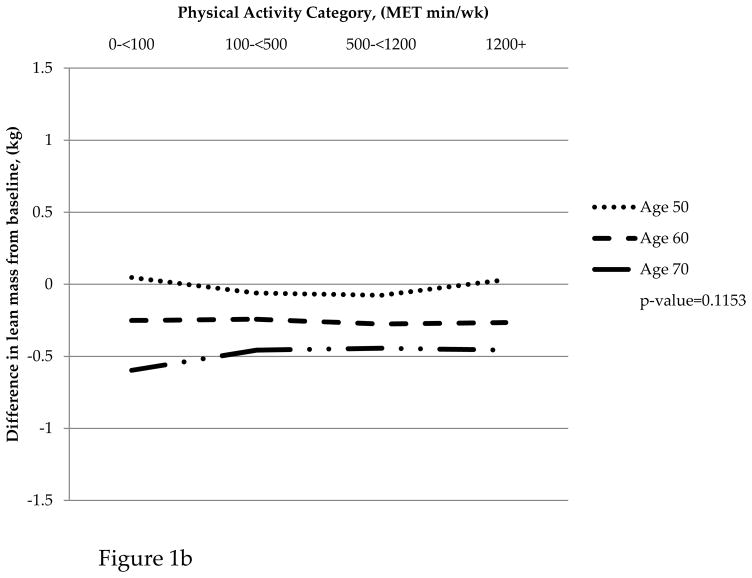
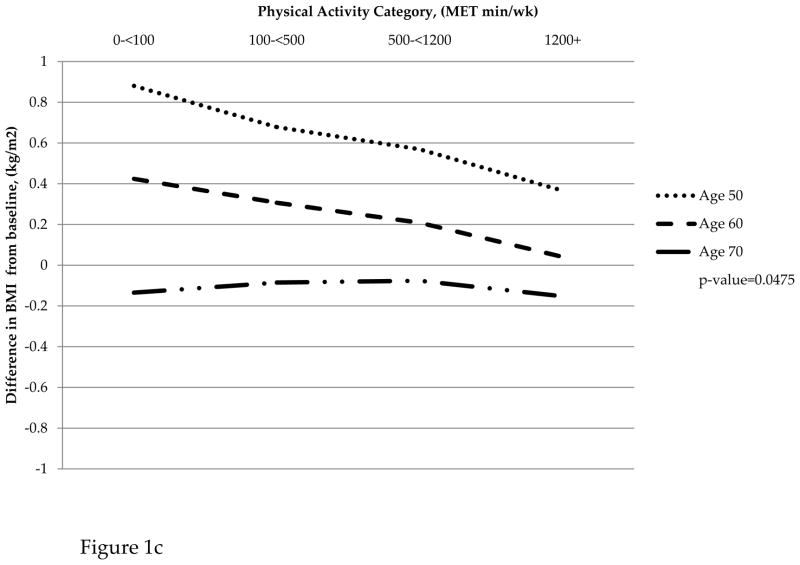
Table 3 presents results from fitting mixed effects linear regression models that adjusted for baseline MET category and other confounders mentioned above to describe associations between time-varying PA and changes in body composition variables (lean mass, fat mass, and BMI) from baseline. For change in fat mass and BMI, the associations with time-varying PA varied by age group (p=0.0006 and p=0.0395, respectively). In contrast, the association between time-varying PA and change in lean mass did not vary significantly by age group (p=0.1935). Further, PA was not significantly associated with change in lean mass from baseline (p=0.8147).
Table 3.
Results from linear mixed models of change in fat mass, lean mass, and BMI from baseline on average over the six-year study period1
| Outcome | Age Group | Physical Activity
|
p-value of interaction of PA and age group | Global p-value for PA | |||
|---|---|---|---|---|---|---|---|
| Sedentary <100 | Low 100–<500 | Moderate 500–<1200 | High 1200+ | ||||
| Change in fat mass from baseline | 50–59 | 1.11 | 0.70 | 0.55 | 0.23 | 0.0006 | |
| 60–69 | 0.00 | −0.21 | −0.21 | −0.56 | |||
| 70–79 | −1.14 | −0.78 | −0.85 | −0.91 | |||
|
| |||||||
| Change in lean mass from baseline | 50–59 | 0.09 | −0.03 | −0.01 | 0.07 | 0.1935 | |
| 60–69 | −0.18 | −0.17 | −0.20 | −0.17 | |||
| 70–79 | −0.51 | −0.40 | −0.37 | −0.38 | |||
|
| |||||||
| Change in lean mass from baseline | All age groups | −0.18 | −0.20 | −0.20 | −0.16 | 0.8147 | |
|
| |||||||
| Change in BMI from baseline | 50–59 | 0.60 | 0.38 | 0.30 | 0.10 | 0.0395 | |
| 60–69 | 0.18 | 0.06 | −0.06 | −0.20 | |||
| 70–79 | −0.40 | −0.34 | −0.34 | −0.39 | |||
Adjusted for baseline MET category, visit year, baseline BMI, age group at screening, ethnicity, education, smoking, hormone therapy status, hours of sleep, energy in kcal, Dietary Modification study arm, Hormone Therapy Study Arm, alcohol servings, fruit servings, vegetable servings, grain servings, and protein intake.
For women aged 50–59, those in the sedentary MET category gained 1.11kg of fat mass while those in the most active MET category gained 0.23kg on average over the nine years of follow up. Women aged 60–69 in the sedentary category did not gain or lose fat mass, but those in the most active category lost 0.56kg. Women in the oldest age group (70–79) all lost fat mass on average with the sedentary and most active category having a greater loss than the low and moderate activity categories. A similar pattern was observed for change in BMI from baseline on average over the 6-year study period. Women in the 50–59 age group who reported high PA gained 0.10kg/m2; sedentary women in this age group gained 0.60kg/m2. A similar trend was observed for women aged 60–69; however, women aged 70–79 all reported a loss in BMI that was similar across categories of PA. Women in all age groups lost similar amounts of lean mass across categories of PA (Figure 1).
Figure 1.
(a) Change in fat mass by physical activity category and age group. (b) Change in lean mass by physical activity category and age group. (c) Change in BMI by physical activity category and age group
DISCUSSION
The current report demonstrates changes in body composition in postmenopausal women over a 6-year period in association with time-varying physical activity, with higher PA levels associated with an attenuation in fat mass and BMI gain for women aged 50–59 and 60–69. Women in the oldest age group, however, lost fat mass and BMI at all levels of physical activity. Lean mass loss was not significantly associated with physical activity and invariant across the age groups.
While it is generally stated that higher levels of physical activity attenuate fat mass gain and lean mass loss, most large studies rely on baseline measurements of PA and the projected trajectory of PA levels (3,10,12,15,22,29). The current study incorporates physical activity at baseline, three and six years after follow up; PA has decreased significantly by year 6 from baseline.
There is considerable discussion of the role of PA in preventing weight gain, in particular the attenuation of fat mass gain, across the lifespan in the current literature on obesity (13,15,17,19,21,22,27,28,30). Although the maintenance of high levels of PA is believed to lessen weight gain, there are caveats to these data. First, it seems to be assumed that baseline activity levels persist in older adults over time (barring disease, chronic pain, or other illness), whereas the current study demonstrates that over a 6-year period, postmenopausal women changed their habitual PA levels significantly. Further, our results suggest that higher levels of physical activity reduce fat mass and BMI gain in women aged 50–59 and 60–69 but not in the oldest age group, 70–79. This in turn may suggest that efforts to encourage increased physical activity in postmenopausal women may be more effective at earlier ages.
Our data suggest that there should be greater emphasis on the maintenance of lean mass in post-menopausal women. Firstly, the prevention of sarcopenia may attenuate risk of falls, loss of independence, and loss of physical function (8,9,12,14,16,26,33). Secondly, lean mass is more metabolically active and may therefore, reduce the risk of cardiovascular and metabolic diseases (12, 16,24,33). The current study demonstrates postmenopausal women in all age groups experience loss of lean mass as they age, invariant of physical activity levels. It is important to note that the physical activity reported in this study was primarily aerobic in nature. Especially in regards to lean mass, the potential role of muscle-strengthening exercises deserves consideration. Peterson et al. (24) conducted a meta-analysis of resistance training studies amongst young and old adults. The evidence strongly indicated that resistance training elicits an approximate 1kg increase in LBM among older adults. Although modest compared to the expected adaptation with healthy young adults, this increase is in contrast to the 0.18 kg annual decline that may occur through sedentary lifestyles, beyond fifty years of age. Moreover, volume of training and age of participation are important determinants of effectiveness, suggesting that higher dosages result in greater adaptive-response, and that aging individuals should consider starting a regimen of resistance exercise as early as possible, to optimize results. The lack of association between physical activity and lean mass in our cohort may be due to the majority of PA being aerobic in nature.
Lifetime PA plays a major role in the overall aging process. Booth, Laye, and Roberts (4) describe the consequences of primary vs. secondary aging. Secondary aging is defined as physiological changes that are not inevitable, yet significantly alter quality of life and life expectancy. There is ample evidence that physical inactivity and, to a lesser degree, decreased PA, over the lifespan, plays a major role in “unsuccessful” aging (aging accompanied by decreased life expectancy, increased cardiometabolic risk factors, decreased physical function with concomitant decreased skeletal muscle strength and function). Thus the message of maintaining high and/or increasing overall aerobic and resistance training PA levels becomes fundamental in the notion of successful aging.
Strengths of this present study include the prospective design, the large size and diversity of the WHI DXA cohort, the detailed assessment of PA at multiple points during follow-up as well as sedentary behavior, long-term follow-up, and high retention rates (90.8% at 6 years of follow up).
Several limitations of the present analyses deserve mention. Despite the fact that we controlled for a large number of potential confounding variables in our multivariable analyses, residual confounding by lifestyle-related factors cannot be excluded, and although the WHI PA questionnaire was shown to have good reliability and validity (6), self-reported PA is a limitation. The magnitude and consistency of the relations between PA and body composition across the variety of analytical approaches used here suggest that confounding by diet is not a likely explanation of the findings. An important limitation of these analyses is that adjusting for intensity and mode of exercise to demarcate resistance training from yoga-type and lower intensity aerobic exercise was not feasible due to the nature of the data collection.
In conclusion, physical activity levels throughout the postmenopausal years are associated with changes in BMI and fat mass and this association differs by age group. Physical activity was not associated with change in lean mass for all age groups. Moreover, our results reinforce the role of physical activity in minimizing the adverse effects of aging on body composition changes, which have been associated with improved health outcomes and successful aging.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at https://cleo.whi.org/researchers/SitePAges/Write%20a%20Paper.aspx
Footnotes
There are no conflicts of interest declared for any of the authors.
The results of the present study do not constitute endorsement by ACSM.
References
- 1.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Androeoli A, Celi M, Volpe SL, Sorge R, Taranttion U. Long-term effect of exercise on bone mineral density and body composition in post-menopausal ex-elite athletes:a retrospective study. Euro J Clin Nutri. 011 Jun;65(6) doi: 10.1038/ejcn.2011.104. [DOI] [PubMed] [Google Scholar]
- 3.Bea JW, Zhao Q, Cauley JA, LaCroix AZ, et al. Effect of hormone therapy on lean body mass, falls, and fractures: 6-year results from the Women’s Health Initiative hormone trials. Menopause. 2010;18:1. doi: 10.1097/gme.0b013e3181e3aab1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol. 2011;111:1497–1504. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- 5.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: The Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 6.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition- a substudy of the Estrogen Plus Progestin Trial of the Women’s Health Initiative. Am J Clin Nutr. 2005;82:651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 8.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metabl. 2008;93:861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleg JL, Morrell CH, Bos AG, Brant LJ, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112 (5):674–82. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher D, Ruts E, Visser M, Heshka S, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Won Park S, Harris TB, Kritchevsky SB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging, and Body Composition Study. J Gerontol. 2006;61A(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 13.Hankinson AL, Daviglus ML, Bouchard C, Carnethon M, et al. Maintaining a high physical activity level over 20 years and weight gain. JAMA. 2010;304(23):2603–2610. doi: 10.1001/jama.2010.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1452–1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hays J, Hunt J, Hubbell A, Anderson G, Limacher M, Allen C, Rossouw J. The WHI Recruitment Methods and Results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 16.Lange T, Streeper T, Cawton P, Baldwin K, Taaffee DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporosis Int. 2010;21(4):543–59. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee I-M, Djousse L, Sesso HD, Wang L, Buring JE. Physical Activity and Weight Gain Prevention. JAMA. 2010;303(12):1173–1179. doi: 10.1001/jama.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Greenland P, LaCroix A, Stefanick ML, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 20.Marks B. Physiologic responses to exercise in older women. Top Geriatr Rehabil. 2002;18:9–20. [Google Scholar]
- 21.McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY Women’s Health Initiative Investigators. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14(9):1662–77. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 22.Newman AB, Lee JS, Visser M, Goodpaster GH, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–8. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- 23.Patterson RE, Kristal AR, Coates RJ, et al. Low-fat diet practices of older women: prevalence and implications for dietary assessment. J Am Diet Assoc. 1996;96:670–9. doi: 10.1016/s0002-8223(96)00186-1. [DOI] [PubMed] [Google Scholar]
- 24.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: A meta-analysis. Med Sci Sports Exerc. 2011;43(2):249–258. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips SM. Resistance exercise: good for more than just grandma and grandpa’s muscles. Appl Physiol Nutr Metab. 2007;32:1198–1205. doi: 10.1139/H07-129. [DOI] [PubMed] [Google Scholar]
- 26.Rantanen T. Muscle strength, disability, and mortality. Scan J Med Sci Sports. 2003;13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 27.Sims ST, LaMonte MJ, Michael YL, Larson JC, Martin LW, JohnsonK C, Sarto GE, Stefanick ML. Physical activity and Body Mass: Changes in younger vs older postmenopausal women. Med Sci Sport Exer. 2012;1:24–32. doi: 10.1249/MSS.0b013e318227f906. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen MB. Changes in body composition at menopause--age, lifestyle or hormone deficiency?-140. J Br Menopause Soc. 2002;8(4):137. doi: 10.1258/136218002100321974. [DOI] [PubMed] [Google Scholar]
- 29.Sowers MF, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP. Menopause, physical activity and body composition/fat distribution in midlife women. Med Sci Sports Exerc. 2005;37(7):1195–1202. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- 31.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998 Feb;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services. Physical activity guidelines for Americans. Hyattsville, MD: 2008. [Accessed on 1 April 2012]. Accessible at http://www.health.gov/PAGuidelines/guidelines/default.aspx. [Google Scholar]
- 33.Visvanathan R, Chapman I. Review: Preventing sarcopenia in older people. Maturitas. 2010;66:383–388. doi: 10.1016/j.maturitas.2010.03.020. [DOI] [PubMed] [Google Scholar]