Abstract
The rate of co-dependency for alcohol and nicotine is extremely high. Numerous studies have indicated that there is a common genetic association for alcoholism and nicotine dependency. The current experiments examined whether selective breeding for high alcohol preference in rats may be associated with increased sensitivity of the posterior ventral tegmental area (pVTA) to the reinforcing properties of nicotine. In addition, nicotine can directly bind to the serotonin-3 (5-HT3) receptor, which has been shown to mediate the reinforcing properties of other drugs of abuse within the pVTA Wistar rats were assigned to groups that were allowed to self-infuse 0, 10, 50, 100, 200, 400 or 800 μM nicotine in 2-lever (active and inactive) operant chambers. P rats were allowed to self-infuse 0, 1, 10, 50 or 100 μM nicotine. Co-infusion of 5-HT3 receptor antagonists with nicotine into the pVTA was also determined. P rats self-infused nicotine at lower concentrations than required to support self-administration in Wistar rats. In addition, P rats received more self-infusions of 50 and 100 μM nicotine than Wistar rats. Including a 5HT3 receptor antagonist (LY-278,584, or zacopride) with nicotine reduced responding on the active lever. Overall, the data support an association between selective breeding for high alcohol preference and increased sensitivity of the pVTA to the reinforcing properties of nicotine. In addition, the data suggest that activation of 5HT3 receptors may be required to maintain the local reinforcing actions of nicotine within the pVTA.
Keywords: Alcohol-preferring rats, Intracranial Self-Administration, Nicotine self-infusions, Serotonin-3 receptors, Ventral Tegmental Area
INTRODUCTION
Epidemiological and clinical studies estimate that 80-95% of alcohol-dependent individuals are regular smokers (Hurt et al., 1994; Pomerleau, Aubin, and Pomerleau, 1997; Romberger and Grant, 2004). Alcohol dependent individuals also have higher rates of nicotine dependence (Hughes, Rosa, and Callas, 2000; Room, 2004). Alcohol intake is also significantly higher in nicotine users than alcohol users alone (York and Hirsch, 1995; Williamson et al., 1997). Common genetic factors may make certain individuals vulnerable to both alcohol and smoking addiction (Carmelli, Swan, and Robinette, 1993; Enoch and Goldman, 2001; Swan, Carmelli, and Cardon, 1997). Twin studies show that there may be a genetic linkage between alcohol dependence and nicotine dependence (True et al., 1999; Volk et al., 2007; Nurnberger et al., 2004).
The selectively bred alcohol-preferring (P) line of rat has been well characterized both behaviorally and neurobiologically and satisfies criteria proposed as essential for an animal model of alcoholism (reviewed in McBride and Li 1998; Murphy et al. 2002). Behavioral pharmacological studies demonstrated that ethanol (EtOH) discriminative stimulus generalizes to nicotine in P rats compared to NP rats (Gordon, Meehan, and Schechter, 1993; McMillan, Li, and Shide, 1999) and that P rats are more likely to substitute nicotine for EtOH (McMillan et al., 1999). Le at al. (2006) findings showed that EtOH-naïve P rats will intravenously (i.v.) self-administer more nicotine and express greater nicotine-seeking behavior than NP rats. The Le et al. (2006) findings with P rats provide support for the hypothesis that nicotine and alcohol addiction may share common genetic vulnerabilities. Mice selected for differential sensitivity to the locomotor stimulatory effects of EtOH (FAST and SLOW) are also differentially affected by nicotine. FAST mice are more responsive to nicotine than SLOW mice (Bergstrom et al., 2003). Furthermore, reverse selection of FAST and SLOW mice (breeding FAST mice with mice that were not responsive to EtOH, and SLOW to EtOH-responsive mice) reduced the enhanced locomotor stimulatory profile in FAST mice, while producing an enhancement in SLOW mice (Bergstrom et al., 2003).
Nicotinic receptors are present in the ventral tegmental area (VTA) and the reinforcing effects of nicotine are mediated mainly via stimulation of nicotinic receptors within the VTA (Corrigall, Coen, and Adamson, 1994; Nisell, Nomikos, and Svensson, 1994). The reinforcing effects of EtOH are thought to be partially mediated by central nicotinic receptors (Blomqvist et al., 1996; Ericson et al., 2003; Soderpalm et al., 2000) and EtOH can elevate dopamine levels via indirect activation of nicotinic receptors (Ericson et al., 2003). In the P rat, systemic administration of nicotine can increase both EtOH-seeking and EtOH relapse drinking in a time dependent manner (Hauser et al., 2012).
Nicotine can increase the firing rate of dopaminergic (DA) neurons (Grenhoff, Aston-Jones, and Svensson, 1986) and enhance somatodendritic DA release in the VTA (Rahman, Zhang, and Corrigall 2003; 2004). In addition, nicotine can increase the release of DA in the nucleus accumbens (Yoshida et al., 1993; Nisell et al., 1994; Ferrari et al., 2002; Tizabi et al., 2002).
The VTA appears to be involved in mediating i.v. nicotine self-administration (Corrigall et al., 1994). The posterior (but not the anterior) VTA is a neuroanatomical site supporting the reinforcing actions of both EtOH (Rodd et al., 2004; Rodd-Henricks et al., 2000) and nicotine (Ikemoto, Qin, and Liu, 2006). There is evidence that genetic factors influence the reinforcing actions of EtOH within the VTA, with an association between selective breeding for alcohol preference and enhanced sensitivity of the posterior VTA to the reinforcing effects of EtOH (Rodd et al., 2004).
The reinforcing effects of nicotine within the posterior VTA were blocked by the co-infusion of mecamylamine, a nicotinic receptor antagonist (Ikemoto et al., 2006). Previous studies indicated that the self-infusions of EtOH (Rodd-Henricks et al., 2003) and cocaine (Rodd et al., 2005a) into the posterior VTA were inhibited with the co-infusion of serotonin-3 (5-HT3) receptor antagonists. In addition, a 5-HT3 receptor agonist was self-infused by P and Wistar rats into the posterior VTA, supporting the idea that local activation of 5-HT3 receptors can produce rewarding effects.
Nicotine is not specific for cholinergic receptors. In fact, nicotine binds at a lower affinity to the 5-HT3 receptors than any cholinergic nicotinic receptors (c.f., Jackson and Yakel, 1995; Breitinger, Geetha, and Hess, 2001; Gurley and Lanthorn, 1998). Originally, the 5-HT3 receptor was classified as a nicotinic acetylcholine receptor (NAchR; Jackson and Yakel, 1995). The increase in the affinity of CNS acetylcholine receptors to nicotine compared to acetylcholine receptors in the periphery (muscle) is predicated upon a cation-π interaction (Xiu et al., 2008). The 5-HT3 receptor also has a cation-π interaction that enhances its affinity for nicotine (Beene et al., 2002). In fact, the NAchRs are sensitive to a reduction in affinity for nicotine following point mutations, while the cation-π interaction in the 5-HT3 receptor is resistant to the loss of affinity to nicotine following point mutations (Beene et al., 2002).
Agonist and antagonist for the NAchRs are not specific. The NAchR agonist (epibatidine) and antagonist (mecamylamine) have at least a 4-fold greater affinity for the 5-HT3 receptor than the NAchR (Drisdel et al., 2008). In contrast, 5-HT3 receptor antagonists (LY-278,584 and Zacopride) do not have an appreciable affinity for nicotinic receptors (Macor et al., 2001; Kidd et al., 1993). Specificity of antagonists for NAchRs and 5-HT3 receptors are further complicated by the observation that these two receptors can co-assemble. The α4 NAchR subunit can co-assemble with the 5-HT3 receptor to produce a heteromeric 5-HT3 receptor channel with enhanced Ca permeability and a decrease in sensitivity to the ability of antagonists to block activation of the novel receptor (van Hooft et al., 1998).
Because of the similarities in structure between nicotinic and 5-HT3 receptors (Mascia, Trudell, and Harris, 2000; Peters et al., 2006), and the potential interactions of these receptors and of nicotine at the 5-HT3 receptor (Bianchi et al., 1995; Gurley and Lanthorn 1998; Nayak et al., 2000; Dougherty and Nichols 2009), it is possible that the reinforcing effects of nicotine within the posterior VTA may be mediated in part through activation of 5-HT3 receptors.
The objectives of the present study were to test the hypotheses that (a) the posterior VTA of the P rat is more sensitive than the posterior VTA of Wistar rats to the reinforcing effects of nicotine, and (b) the reinforcing actions of nicotine within the posterior VTA are mediated in part by activation of 5-HT3 receptors.
MATERIALS AND METHODS
Animals
Female alcohol-naïve Wistar rats (Harlan, Indianapolis, IN) and P rats (52nd and 53rd generations) weighing 250-320 g at time of surgery were used. Female rats were used in the present study because (a) they were used in the initial and previous studies (Gatto et al. 1994; Rodd-Henricks et al. 2000, 2003), and (b) they maintain their body weights and head size better than male rats for more accurate stereotaxic placements. The estrous cycle was not monitored in the present study. However, counterbalanced experiments were conducted on different days so that any effect of a given phase of the estrous cycle was distributed across experimental conditions. Animals were double-housed upon arrival and were maintained on a 12-hr reverse light-dark cycle (lights off at 0900 hr). Food and water were freely available except in the test chamber. All research protocols were approved by the Institutional Animal Care and Use Committee and are in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse, NIH, and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Data for rats that did not complete all experimental test sessions were eliminated from the analyses. The number of animals indicated for each experiment represents approximately 85% of the total number that underwent surgery; 15% of the animals were not included for analyses mainly due to the loss of the guide cannula before completion of all experimental sessions. The data for these animals were not used because their injection sites could not be verified.
Drug and Vehicle
The artificial cerebrospinal fluid (aCSF) vehicle consisted of (in mM): 120.0 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 Mg SO4, 25.0 NaHCO3, 2.5 CaCl2, and 10.0 d-glucose. Nicotine tartrate (Sigma-Aldrich, St. Louis, MO USA), LY-278,584 (Eli Lilly Company, Indianapolis, IN, USA) and zacopride (Tocris Bioscience, Ellisville, MO) were dissolved in aCSF. When necessary, 0.1 M HCl or 0.1 M NaOH was added to the solutions to adjust the pH to 7.4 + 0.1.
Apparatus
Standard 2-lever operant chambers (previously described: Rodd-Henricks et al., 2003; Rodd et al., 2004) were situated in sound-attenuating cubicles ( Coulbourn Instruments, Allentown, PA) which were illuminated by a dim house-light during testing. Two identical levers were mounted on a single wall of the test chamber, 15 cm above a grid floor, and were separated by 12 cm. Levers were raised to this level to avoid accidental brushing against the lever and to reduce responses as a result of locomotor activation. Directly above each lever was a row of three different colored cue lights. The light (red) to the far right over the active bar was illuminated during resting conditions. A desktop computer equipped with an operant control system (L2T2 system, Coulbourn Instruments) recorded the data and controlled the delivery of infusate in relation to lever response.
An electrolytic microinfusion transducer (EMIT) system, as previously described (Rodd-Henricks et al. 2003; Rodd et al. 2004) was used to control microinfusions of nicotine or vehicle. Depression of the active lever delivered the infusion current for 5 sec, which led to the rapid generation of H2 gas (raising the pressure inside the airtight cylinder), and, in turn, forcing 100 nl of the infusate through the injection cannula. During the 5-sec infusion and additional 5-sec timeout period, the house light and right cue light (red) were extinguished and the left cue light (green) over the active lever flashed on and off at 0.5 sec intervals.
Animal Preparation
While under isoflurane anesthesia, a unilateral 22-gauge guide cannula (Plastics One) was stereotaxically implanted in the right hemisphere of each subject and, aimed 1.0 mm above the target region. Coordinates for placements into the pVTA were 5.4 to 6.0 mm posterior to bregma, 2.1 mm lateral to the midline, and 8.5 mm ventral from the surface of the skull at a 10° angle to the vertical. In between experimental sessions, a 28- gauge stylet was placed into the guide cannula and extended 0.5 mm beyond the tip of the guide. Following surgery, all rats were individually housed and allowed to recover 7-10 days. Animals were handled for at least 5 min daily following the fourth recovery day. Subjects were not acclimated to the test chamber prior to the commencement of data collection, nor were they trained on any other operant paradigm.
General Test Conditions
For testing, subjects were brought to the testing room, the stylet was removed, and the injection cannula screwed into place. Rats were placed individually in the test chamber. To avoid trapping air at the tip of the injection cannula, the infusion current was delivered for 5 sec during insertion of the injector that resulted in a non-contingent administration of nicotine or aCSF at the beginning of the session. Injection cannulae extended 1.0 mm beyond the tip of the guide. The test chamber was equipped with two levers. Depression of the ‘active lever’ (FR1 schedule of reinforcement) caused the delivery of a 100-nl bolus of infusate over a 5-sec period followed by a 5-sec time-out period. During both the 5-sec infusion period and 5-sec time-out period, responses on the active lever did not produce further infusions. The assignment of active and inactive lever with respect to the left or right position was counterbalanced among subjects. However, the active and inactive levers remained the same for each rat throughout the experiment. No shaping technique was used to facilitate the acquisition of lever responses. The number of infusions and responses on the active lever was recorded. Responses on the ‘inactive lever’ were recorded, but did not result in infusions. The duration of each test session was 4 hr and sessions occurred every other day.
Nicotine Self-Administration: Dose Response P vs. Wistar rats
Wistar (n = 6-10/group) and P (n = 5-7/group) rats were randomly assigned to one group to receive aCSF or a given concentration of nicotine. Wistar rats were allowed to self-infuse 0 - 800 μM nicotine; P rats were given 0 – 100 μM nicotine. Wistar rats were allowed to self-infuse higher nicotine doses than P rats to determine the maximal concentration of nicotine for that strain. For P rats, the maximal concentration appeared to be around 50 – 100 μM nicotine. The original infusate solution was available for self-administration during the first four sessions (acquisition). During the fifth and sixth sessions (extinction), all animals received infusions of aCSF. On the seventh session, rats were allowed to respond for their originally assigned infusate.
Wistar rats were used instead of NP rats because previous ICSA studies indicated that NP rats would not self-infuse EtOH over a range of 25 to 200 mg/100 ml concentrations (Gatto et al. 1994). However, Wistar rats, using the ICSA technique will self-infuse EtOH (Engleman et al. 2009; Rodd et al. 2004, 2005a; Rodd-Henricks et al. 2000), cocaine (Katner et al. 2011), nicotine (Ikemoto et al. 2006) and other drugs (Rodd-Henricks et al. 2003; Rodd et al. 2008) with no prior operant training, and within the limited number of injection sessions required for ISCA procedure. In addition, P and NP rats were originally derived from a colony of Wistar rats (Lumeng et al. 1977).
Co-infusion of 5-HT3 receptor antagonists with Nicotine
LY-278,584 and zacopride are two potent 5-HT3 antagonists that were selected because both can block EtOH ICSA in pVTA at doses from 25–100 μM or 10–100 μM, respectively (Rodd-Henricks et al. 2003). Wistar rats were randomly assigned to groups that self-administered 200 μM nicotine for the initial 4 sessions, 200 μM nicotine containing 100 or 200 μM LY-278,584, or 10 or 100 μM zacopride (n = 6 - 8/dose) during session 5 and 6, and 200 μM nicotine alone during session 7.
Histology
At the termination of the experiment, 1% bromophenol blue (0.5 ul) was injected into the infusion site. Subsequently, the animals were given a fatal dose of Nembutal (100 mg/kg) and then decapitated. Brains were removed and immediately frozen at −70° C. Frozen brains were equilibrated at −15° C in a cryostat microtome and then sliced into 40 um sections. Sections were then stained with cresyl violet and examined under a light microscope for verification of the injector site using the rat brain atlas of Paxinos and Watson (1998).
Statistical Analysis
Data analysis consisted of a group x day mixed ANOVA, with a repeated measure of session performed on the number of infusions. Additionally, for each individual group, lever discrimination was determined by type (active or inactive) x day mixed ANOVA with a repeated measure of ‘session.’
RESULTS
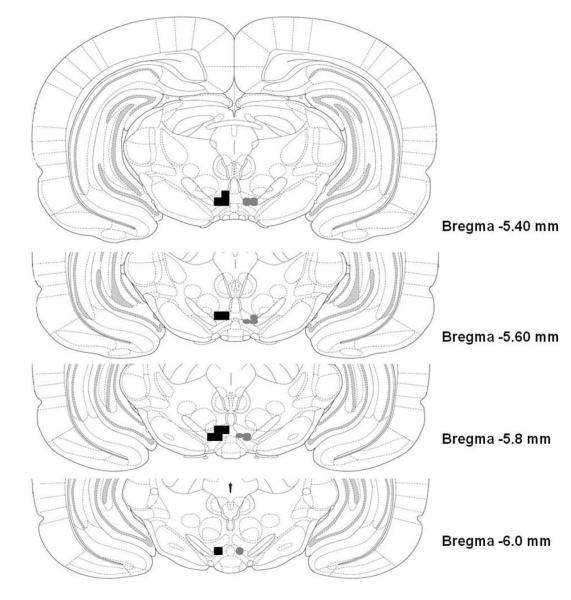
The pVTA was defined as the VTA region at the level of the interpeduncular nucleus, coronal sections at −5.4 to −6.04 bregma (Fig. 1). Rats with injector tip placements outside the VTA (i.e., substantia nigra, red nucleus, and caudal linear nucleus) displayed an overall low level of infusions and active lever responding throughout all sessions (average infusions and active lever responses for initial 4 sessions – 5.2 + 1.3 and 12.3 + 2.2, respectively). For all sessions, the number of infusions of nicotine outside the VTA was not significantly different than the aCSF group with injection sites in the VTA (p values > 0.53, data not shown). Similarly, examination of the active lever responses revealed that rats administering nicotine into areas outside the VTA displayed equivalent amounts of low levels of responding on both the active and inactive levers (p values > 0.73, data not shown).
Fig. 1.
Illustrated is a representation of the placements of injection sites within the posterior VTA (defined as –5.4 to –6.0 mm Bregma) of Wistar rats (left, squares) and P rats (right, circles) self-administering aCSF or various concentrations of nicotine.
Nicotine Self-infusions: Dose Response P vs. Wistar rats
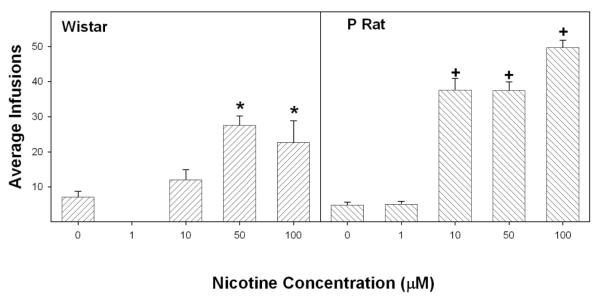
A mixed factor ANOVA performed on the number of self-infusions for each session with between factors of nicotine concentrations (0, 10, 50 and 100 μM nicotine) and rat line (P vs Wistar) revealed a significant session X nicotine concentration X rat line interaction (F18, 147 = 3.4; p < 0.001), a significant effect of nicotine concentration (F3, 52 = 19.2; p < 0.001) and rat line (F1,52 = 31.8; p < 0.001). Simplifying the analysis, by examining effects of the between subject factors rat line and nicotine concentration on the average number of self-infusions between session 1-4 (Fig. 2), similarly revealed a significant rat line x nicotine concentration interaction (F3,52 = 6.7; p < 0.001). Examining the self-infusion of nicotine within each line indicated that P rats self-administered nicotine at lower concentrations and received more self-infusions than Wistar rats (Fig. 2).
Fig. 2.
The average number of infusions (± SEM) across the initial 4 sessions (acquisition) by Wistar and P rats as a function of infusate concentration (0 to 100 μM) with cannula placements in the posterior VTA. Asterisks indicate infusions significantly higher than aCSF and 10μM nicotine infusions; plus symbols indicate significantly higher values compared to aCSF, 1 μM nicotine and to the number of infusions by Wistar rats (p < 0.05; Tukey’s b post-hoc).
For the P rat (including the 1 μM nicotine group), the analysis indicated a significant effect of nicotine concentration (F4, 32 = 86.98; p < 0.001). Post-hoccomparisons (Tukey’s b) indicated that P rats given 10 and 50 μM nicotine received more self-infusions than P rats given aCSF or 1 μM nicotine (Fig. 2; right panel). In addition, post-hoc comparisons indicated that P rats given 100 μM nicotine received more self-infusions compared to all other nicotine concentrations. For Wistar rats, there was also a significant effect of nicotine concentration (F6, 24 = 4.7; p = 0.009) on the average number of infusions. Post-hoc comparisons (Tukey’s b) indicated that the number of infusions obtained by Wistar rats given 50 and 100 μM nicotine were significantly higher than infusions of aCSF, and that Wistar rats given 50 μM nicotine received significantly more infusions than Wistar rats given 10 μM nicotine (Fig. 2; left panel). There was no difference in the number of aCSF infusions between P and Wistar rats (F1,12 = 0.98: p = 0.34). In contrast, P rats given 10, 50, and 100 μM nicotine received significantly more self-infusions than Wistar rats at each of the concentrations (F values > 7.1; p values < 0.019).
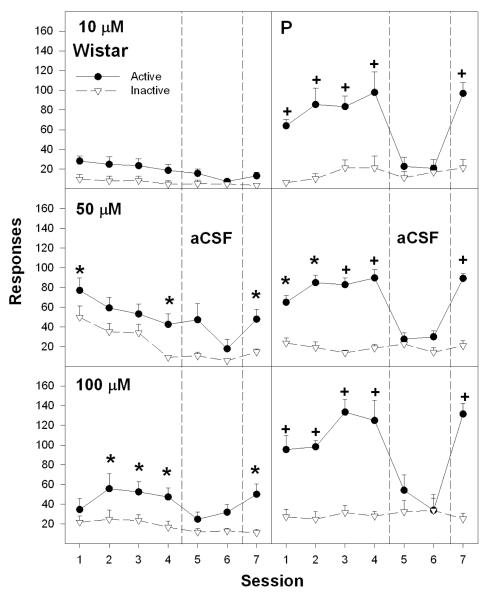
Further analyses allowed for the determination of lever discrimination during acquisition, extinction and reinstatement. The overall analysis revealed a significant session X lever X nicotine concentration interaction term (F24, 120 = 3.2; p < 0.001). For P rats given aCSF or 1 μM nicotine into the pVTA, there were no significant differences in responses on the active and inactive levers across the 7 sessions (all p values > 0.172; data not shown). In contrast, P rats self-infusing 10, 50, and 100 μM nicotine into the pVTA responded significantly more on the active than inactive lever (all p values < 0.003) during sessions 1-4 and session 7 (Fig. 3, right panel), but not during aCSF substitution (session 5 and 6; Fig. 3, right panel).
Fig. 3.
The number of active and inactive lever presses (means ± SEM) for Wistar and P rats self-administering 10, 50 or 100 μM nicotine into the posterior VTA during sessions 1-4, aCSF for sessions 5 and 6, and the original infusate during session 7. Asterisks represent significant (p<0.05; Tukey’s) differences from responding observed for rats self-infusing aCSF, and responding on the active lever significantly differed (p < 0.05) from responding on the inactive lever (determined by one-way ANOVAs performed on individual sessions contrasting active and inactive lever presses). Plus symbols indicated differences from responding observed for rats self-infusing aCSF, responses on the active lever significantly differed (p < 0.05) from responding on the inactive lever and higher responding on active lever by P rats compared to Wistar rats.
To determine if active lever responses were extinguished during aCSF substitution (sessions 5 and 6), a repeated measure ANOVA was performed for P rats given 10, 50 and 100 μM nicotine. For active lever responding, there were significant differences between sessions 4-6 (p values < 0.001; Fig. 3, right panel). In each separate group of rats, t-tests indicated a significant reduction in active lever responses between sessions 4 and 5, and between sessions 4 and 6 (p values < 0.002; Fig. 3, right panel).
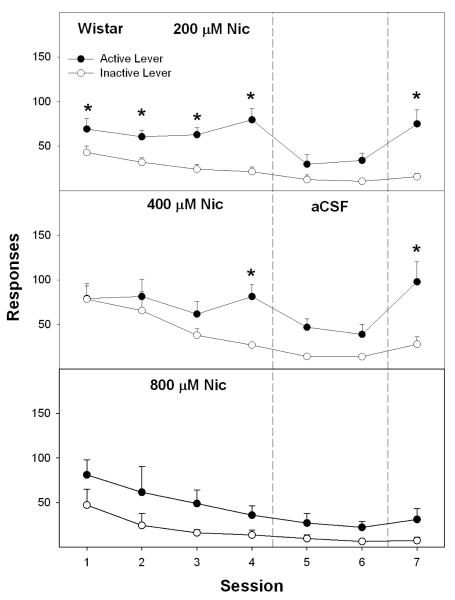
The analysis for lever discrimination for Wistar rats revealed a significant session X lever X nicotine concentration interaction term (F36, 282 = 1.5; p < 0.001). For Wistar rats given aCSF or 10 μM nicotine into the pVTA, there were no significant differences in responses on the active vs. inactive lever across the 7 sessions (all p values > 0.085). In contrast, Wistar rats self-infusing 50, 100 or 200 μM nicotine into the pVTA discriminated between active and inactive levers (all p values < 0.04; Fig. 3, left panel and Fig. 4), which was altered across sessions (session X lever interaction terms – all p values < 0.02). At 50 and 100 μM nicotine, lever discrimination occurred in some of the sessions between 1 and 4, but was always observed during session 7 (Fig. 3, left panel). For 200 μM nicotine, lever discrimination was observed during sessions 1-4 and session 7 (Fig. 4). At 400 μM nicotine lever discrimination was only observed during sessions 4 and 7 (p values < 0.05); lever discrimination was not observed during any session with 800 μM nicotine (p values > 0.05) (Fig. 4).
Fig. 4.
The number of active and inactive lever presses (means ± SEM) for Wistar rats self-infusing 200, 400 or 800 μM nicotine into the posterior VTA during sessions 1-4, aCSF for sessions 5 and 6, and the original infusate during session 7. Asterisks represent significant (p<0.05; Tukey’s) difference from responding observed for rats self-infusing aCSF, and responses on the active lever significantly differed (p < 0.05) from responding on the inactive lever.
To determine if active lever responding and the number of self-infusions were extinguished during aCSF substitution (sessions 5 and 6), a repeated measure ANOVA was performed for Wistar rats self-infusing 50 and 100 μM nicotine into the pVTA (Fig. 3, left panel). For active lever responding and number of self-infusions, there were significant across sessions 4 through 6 (p values < 0.037). For Wistar rats self-administering 100 (Fig. 3, left panel) and 200 μM nicotine (Fig. 4), t-tests performed indicated a significant reduction in both infusions and active lever responses between sessions 4 and 5, and between sessions 4 and 6 (p values < 0.042). A reduction in responding in Wistar rats self-administering 50 μM nicotine was only observed during session 6 (Fig. 3, left panel).
Co-infusion of 5HT3 antagonists with Nicotine
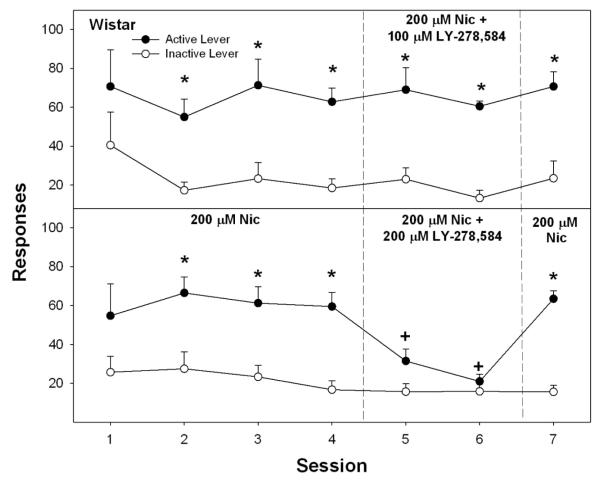
The effects of co-infusion of LY-278,584 with nicotine were determined by performing a repeated measure ANOVA on number of responses on the active lever, as a function of session and dose of LY-278,584. The overall analysis indicated a significant session x dose interaction term (F6,7 = 6.54; p = 0.013; Fig. 5). Prior to co-infusion, Wistar rats readily responded on the active lever for 200 μM nicotine, as evident by lever discrimination during sessions 2-4 (p values < 0.041). The addition of 100 μM LY-278,584 did not alter responses on the active lever for 200 μM nicotine (F6,2 = 0.48; p = 0.82; Fig. 5, upper panel). In contrast, co-infusion of 200 μM LY-278,584 did reduce responding on the active lever for 200 μM nicotine (F6,2 = 18.45; p = 0.047; Fig. 5, lower panel). T-tests indicated a significant reduction in active lever responses between sessions 4 vs 5, and between sessions 4 vs 6 (p values < 0.03). Responding on the active lever returned during session 7 when only 200 μM nicotine was given (p = 0.038).
Fig. 5.
Effects of 100 and 200 μM LY-278,584 on responding for the self-infusion of 200 μM nicotine into the posterior VTA of Wistar rats. For the first 4 sessions, 200 μM nicotine alone was given. In sessions 5 and 6, LY-278,584 was co-infused with 200 μM nicotine. In session 7, only 200 μM nicotine was given. Data are the means ± S.E.M. Asterisks indicate that responses on the active lever were significantly higher than responses on the inactive lever for that session (p < 0.05). Plus symbols indicate that LY-278,584 significantly reduced responding for 200μM nicotine during sessions 5 and 6 compared to session 4 (p < 0.05).
With co-administration of zacopride, the overall analysis indicated a significant effect of session (F6,6 = 6.65; p = 0.018; Fig. 6). Prior to addition of the antagonist, Wistar rats readily self-infused 200 μM nicotine, as evidenced by higher responding on the active than inactive lever during sessions 2-4 (p values < 0.026). Co-infusion of 10 μM zacopride reduced 200 μM nicotine self-infusions only during the 2nd co-administration session (sessions 6; p < 0.01; Fig. 6, upper panel). Co-infusion of 100 μM zacopride reduced 200 μM nicotine self-administration during both sessions (5 and 6; p values < 0.001; Fig. 6, lower panel). Responses on the active lever returned during session 7 when only 200 μM nicotine was given (p values < 0.013).
Fig. 6.
Effects of 10 and 100 μM zacopride on responding for the self-infusion of 200 μM nicotine into the posterior VTA of Wistar rats. For the first 4 sessions, 200 μM nicotine alone was given. In sessions 5 and 6, zacopride was co-infused with 200 μM nicotine. In session 7, only 200 μM nicotine was given. Data are the means ± S.E.M. Asterisks indicate that responses on the active lever were significantly higher than responses on the inactive lever for that session (p < 0.05). Plus symbols indicate that zacopride significantly reduced responding for 200μM nicotine during sessions 5 and/or 6 compared to session 4 (p < 0.05).
DISCUSSION
The major findings of this study are that the pVTA of P rats is more sensitive than the pVTA of Wistar rats to the reinforcing effects of nicotine, and that activation of local 5-HT3 receptors may be needed to maintain these effects. The current results support the hypothesis that there is an association between selective breeding for alcohol preference and enhanced sensitivity to the reinforcing effects of nicotine. This was indicated by the findings that P rats will self-infuse lower concentrations of nicotine (Fig. 2) and will readily discriminate the active from the inactive lever at 10 μM nicotine (Fig. 3, right panel), whereas Wistar rats self-administer this concentration of nicotine at the same level as aCSF and do not demonstrate lever discrimination at this dose. Moreover, P rats received more self-infusions of nicotine than did Wistar rats at the 10, 50 and 100 μM concentrations of nicotine (Fig. 2). The combination of increased responsiveness to the effects of nicotine and higher number of self-infusions suggest that nicotine may be a stronger reinforcer in the pVTA of P rats than Wistar rats. Rodd et al. (2004) reported similar differences in sensitivity of the pVTA between P and Wistar rats for EtOH. Collectively, these findings support the idea that there may be a genetic linkage between selective breeding for high alcohol preference and increased sensitivity of the pVTA to the reinforcing actions of drugs of abuse.
The dose effects of nicotine self-infusions into the pVTA of Wistar rats exhibited an inverted ‘U-shaped’ response, with active lever responding and discrimination at 50 to 200 μM nicotine but not at the 10 or above 400 μM (Figs. 3 and 4). A similar inverted ‘U-shaped’ dose-response plot was demonstrated for EtOH self-infusions into the pVTA of Wistar rats (Rodd-Henricks et al. 2000). The lack of response at the high concentrations could indicate non-specific effects of nicotine that reduced DA neuronal activity and inhibited self-infusions. The lack of a dose-response effect between 50 and 200 μM nicotine into the pVTA suggests that these are maximal concentrations and that lower doses should have also be tested with the Wistar rats.
The present results are compatible with published data indicating that nicotine can be self-infused into the pVTA of Wistar rats (Ikemoto et al., 2006). This latter study indicated that 25 mM nicotine was reliable self-infused, which is approximately 500-fold higher than the present results (Fig. 2). The concentrations of nicotine used in the current experiments fall within the physiological realm of human smokers (15-150 ng/ml; Benowitz and Jacob, 1984) and of P rats orally consuming nicotine (Hauser et al., in press). The reasons for the difference between Ikemoto et al. (2006) and the current experiment are difficult to understand, but may be due to a combination of factors, e.g., source of Wistar rats (Indianapolis Harlan vs. Virginia Harlan), the micro-infusion procedure (EMIT unit in present study vs. micro-infusion pump), placements within the pVTA, and general operant procedure. Recent data collected for other experiments have indicated a similar dose-response curve between male and female Wistar rats for nicotine ICSA into the pVTA (Rodd et al., submitted), therefore gender differences do not appear to be involved in the discrepancy. It is not possible with the available information to determine which factor or factors could contribute to the differences observed between the current study and the study of Ikemoto et al. (2006).
The present study indicated that co-administration of 5-HT3 antagonists reduced responding on the active lever, suggesting that activation of local 5-HT3 receptors are involved in mediating the reinforcing effects of nicotine within the pVTA (Figs. 5 and 6). Previous studies indicated that 5-HT3 receptor antagonists reduced the local self-infusions of EtOH (Rodd-Henricks et al., 2003) and cocaine (Rodd et al., 2005a) in the pVTA, suggesting that 5-HT3 receptors may be important regulators of the brain reward system’s response to drugs of abuse. However, there are some differences in the effectiveness of LY-278,584 and zacopride to reduce EtOH self-infusions into the pVTA of Wistar rats (Rodd-Henricks et al. 2003) and their effectiveness in reducing nicotine self-infusions into the pVTA of Wistar rats in the present study (Figs. 5 and 6). Lower doses of LY-278,584 (25 μM) inhibited EtOH self-infusions whereas a 100 μM concentration was not effective in reducing nicotine self-infusions (Fig. 5). On the other hand, in the case of zacopride, the 10 μM concentration was almost equally effective in reducing the self-infusion of EtOH (Rodd-Henricks et al. 2003) and nicotine (Fig. 6). The differences in the effectiveness of the 2 antagonists to reduce EtOH vs. nicotine self-infusions may be a result of the differences in their relative receptor specificities (Klein et al. 1994), and that different receptor mechanisms underlie the rewarding actions of EtOH and nicotine within the pVTA.
In another study, it was demonstrated that activation of 5-HT3 receptors, with a 5-HT3 agonist, in the pVTA produced reinforcing effects (Rodd et al., 2007). Furthermore, the pVTA of the P rat was more sensitive than the pVTA of Wistar rats to the reinforcing effects of the 5-HT3 agonist (Rodd et al., 2007). These latter results suggest that the difference in sensitivity of the pVTA to the reinforcing effects of nicotine between P and Wistar rats may be due to differences in 5-HT3 receptors between the two rat strains. In support of this idea, a microdialysis study (Liu et al., 2006) indicated that the pVTA of the P rat was more sensitive than the pVTA of the Wistar rat to the stimulating effects of a 5-HT3 agonist on the somatodendritic release of dopamine, suggesting differences in the number of 5-HT3 receptors and/or the functional properties of the 5-HT3 receptors between P and Wistar rats. It is likely that both 5-HT3 antagonists are acting at 5-HT3 receptors to reduce the excitatory tone of VTA DA neurons (Liu et al., 2006), and thereby prevent the self-infusion of nicotine.
The reduction in responding on the active lever when the 5-HT3 antagonists were co-infused with nicotine is not likely due to a motor impairing effect. Administration of 5-HT3 receptor antagonists, at concentrations used in the present, into the pVTA did not result in a reduction of locomotor activity (Rodd-Henricks et al., 2003) or alter oral operant self-administration for saccharin (Rodd et al., 2010). Moreover, a previous study indicated that co-infusion of a 5-HT3 antagonist (ICS 205,930), at concentrations as high as 400 μM, did not alter responses on the active lever for the self-infusion of acetaldehyde into the pVTA (Rodd et al., 2005b).
Mechanisms underlying the reinforcing effects of nicotine within the pVTA may involve the interaction of nicotine at α4β2, α6β2 and α7 receptors on DA cell bodies, and/or on excitatory nerve terminals acting on DA neurons. The expression of nicotinic receptors is greater in the pVTA compared to anterior VTA or the tail of VTA (Zhao-Shea et al. 2011). The α4 and α6 nicotinic receptors have been shown to be necessary for nicotine-induced DA neuron activity in pVTA (Zhao-Shea et al. 2011; Gotti et al., 2010). It has also been reported that α4 nicotinic receptors and 5-HT3 receptors co-exist on striatal nerve terminals (Dougherty and Nichols, 2009; Nayak et al., 2000), although it is not known if a similar co-existence also occurs within the VTA. Prolonged DA neurotransmission may be due to α7 nicotinic receptors on the presynaptic glutamate terminals, which do not desensitize to nicotine as rapidly as α4 and α6 receptors, but continue to enhance glutamatergic excitation in the presence of nicotine (Pidoplichko et al., 2004). Therefore presence of the α7 nicotinic receptor on excitatory glutamatergic terminals (Albuquerque et al., 2009; Gotti and Clementi 2004; Nayak et al., 2000; Wonnacott 1997), and activation of these receptors by nicotine could result in stimulation of DA neurons and promotion of rewarding behavior in pVTA. 5-HT3 receptors are also involved in mediating the release of glutamate (Dong et al., 2009). It is possible that the activation of both nicotinic and 5-HT3 receptors may be needed to increase the release of glutamate in the pVTA and that the inhibition of one or both receptors by the 5-HT3 receptor antagonists could prevent glutamate release and the resulting increased activation of VTA DA neurons.
In conclusion, the results of the present study suggest an association between selective breeding for high alcohol preference and enhanced sensitivity of the pVTA to the reinforcing effects of nicotine. Moreover, the current results provide additional support that there is a strong genetic influence on the pVTA response to the rewarding actions of drugs of abuse. Serotonin via activation of 5-HT3 receptors may be needed to maintain the excitatory tone of VTA DA neurons to support the reinforcing actions of nicotine within the pVTA. The biological basis for the altered sensitivity to the reinforcing actions of drugs of abuse within the pVTA as the result of selection for high alcohol preference may be predicated on differences in 5-HT3 receptors (e.g., cation-π interaction).
ACKNOWLEDGEMENTS
The skillful technical assistance of Tylene Pommer is gratefully acknowledged. Supported in part by NIAAA grants AA07611, AA07462 and AA019366. None of the authors has a conflict of interest associated with this research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Grant support: AA07462, AA07611, AA019366
Footnotes
AUTHORS’ CONTRIBUTION SRH, ALB, GAD, JET, and ZAR participated in research design. SRH, ALB, GAD and ZMD conducted experiments. SRH, WJM, WAT, ZAR and RLB performed data analysis and interpretation of findings. SRH drafted the manuscript. SRH, ALB, GAD, JET, ZMD, WAT, RLB, WJM, and ZAR provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
REFERENCES
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beene DL, Brandt GS, Zhong W, Zacharias NM, Lester HA, Dougherty DA. Cation-π interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: The anomalous binding properties of nicotine. Biochem. 2002;41:10262–10269. doi: 10.1021/bi020266d. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ. Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res. 2003;27:1535–1547. doi: 10.1097/01.ALC.0000091226.18969.B9. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Ferraro L, Tanganelli S, Morari M, Spalluto G, Simonato M, Beani L. 5-Hydroxytryptamine-mediated effects of nicotine on endogenous GABA efflux from guinea-pig slices. Br J Pharmacol. 1995;116:2724–2728. doi: 10.1111/j.1476-5381.1995.tb17233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: Effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Breitinger H, G A, Geetha N, Hess GP. Inhibition of the serotonin 5-HT3 receptor by nicotine, cocaine and fluoxetine investigated by rapid chemical kinetic techniques. Biochem. 2001;40:8419–8429. doi: 10.1021/bi0106890. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D. The relationship between quitting smoking and changes in drinking in World War II veteran twins. J Substance Abuse. 1993;5:103–116. doi: 10.1016/0899-3289(93)90055-g. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Dong L, Zhu Y, Dong Y, Yang J, Zhao Y, Qi Y, Wu P, Zhu Y, Zheng P. Neuroactive steroid dehydroepiandrosterone sulfate inhibits 5 hydroxytryptamine (5-HT)-evoked glutamate release via activation of sigma-1 receptors and then inhibition of 5-HT3 receptors in rat prelimbic cortex. J Pharmacol Exp Ther. 2009;330:494–501. doi: 10.1124/jpet.109.154294. [DOI] [PubMed] [Google Scholar]
- Dougherty JJ, Nichols RA. Cross-regulation between colocalized nicotinic acetylcholine and 5-HT3 serotonin receptors on presynaptic nerve terminals. Acta Pharmacol Sin. 2009;30:788–794. doi: 10.1038/aps.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Sharp D, Henderson T, Hales TG, Green WN. High affinity binding of epibatidine to serotonin type 3 receptors. J Biol Chem. 2008;283:9659–9665. doi: 10.1074/jbc.M703672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–71. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psych Reports. 2001;3:114–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le NN, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gordon TL, Meehan SM, Schechter MD. P and NP rats respond differently to the discriminative stimulus effects of nicotine. Pharmacol Biochem Behav. 1993;45:305–308. doi: 10.1016/0091-3057(93)90243-m. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: From structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–25. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Gurley DA, Lanthorn TH. Nicotinic agonists competitively antagonize serotonin at mouse 5-HT3 receptors expressed in Xenopus oocytes. Neurosci Lett. 1998;247:107–110. doi: 10.1016/s0304-3940(98)00306-1. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, McBride WJ, Rodd ZA. Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcohol Clin Exp Res. 2012;36:43–54. doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA, Jr, Ding ZM, Toalston JE, Scott BJ, Bell RL, McBride WJ, Rodd ZA. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (P) rats. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2012.01800.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Rosa GL, Callas PW. Nicotine is more reinforcing in smokers with a past history of alcoholism than in smokers without this history. Alcohol Clin Exp Res. 2000;24:1633–1638. [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr., Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin Exp Res. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu Z-H. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Yakel JL. The 5-HT3 receptor channel. Ann Rev Physiol. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- Katner SN, Oster SM, Ding ZM, Deehan GA, Jr, Toalston JE, Hauser SR, McBride WJ, Rodd ZA. Alcohol-preferring (P) rats are more sensitive than Wistar rats to the reinforcing effects of cocaine self-administered directly into the nucleus accumbens shell. Pharmacol Biochem Behav. 2011;99:688–95. doi: 10.1016/j.pbb.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd FJ, Levy JC, Nielsen M, Hamon M, Gozlan H. Characterisation of the non-5-HT3 high-affinity ‘R” binding site for (R)-zacopride in brain and other tissues. Eur J Pharmacol. 1993;247:45–56. doi: 10.1016/0922-4106(93)90136-w. [DOI] [PubMed] [Google Scholar]
- Klein RL, Sanna E, McQuilkin SJ, Whiting PJ, Harris RA. Effects of 5-HT3 receptor antagonists on binding and function of mouse and human GABA-A receptors. Eur J Pharmacol. 1994;268:237–246. doi: 10.1016/0922-4106(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naïve offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Thielen RJ, Rodd ZA, McBride WJ. Activation of serotonin-3 receptors increases dopamine release within the ventral tegmental area of Wistar and alcohol-preferring (P) rats. Alcohol. 2006;40:167–176. doi: 10.1016/j.alcohol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng L, Hawkins TD, Li T-K. New strains of rats with alcohol preference and non-preference. In: Thurman RG, Williamson JR, Drott H, Chance B, editors. Alcohol and aldehyde metabolizing systems. III. Academic Press; New York: 1977. pp. 537–544. [Google Scholar]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001;11:319–21. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA. 2000;97:9305–9310. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Li M, Shide DJ. Differences between alcohol-preferring and alcohol-nonpreferring rats in ethanol generalization. Pharmacol Biochem Behav. 1999;64:415–419. doi: 10.1016/s0091-3057(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- National Research Council . Institute of Laboratory Animal Resources and Guide for the care and use of laboratory animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Nayak SV, Ronde P, Spier AD, Lummis SCR, Nichols RA. Nicotinic receptors co-localize with 5-HT3 serotonin receptors on striatal nerve terminals. Neuropharmacology. 2000;39:2681–2690. doi: 10.1016/s0028-3908(00)00109-x. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Edition Academic Press; New York: 1998. [Google Scholar]
- Peters JA, Carland JE, Cooper MA, Livesey MR, Deeb TZ, Hales TG, Lambert JJ. Novel structural determinants of single-channel conductance in nicotinic acetylcholine and 5-hydroxytryptamine type-3 receptors. Biochem Soc Trans. 2006;34:882–886. doi: 10.1042/BST0340882. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–9. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Aubin HJ, Pomerleau OF. Self-reported alcohol use patterns in a sample of male and female heavy smokers. J Addict Dis. 1997;16:19–24. doi: 10.1300/J069v16n03_02. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of acute and chronic nicotine on somatodendritic DA release of the rat ventral tegmental area: in vivo microdialysis study. Neurosci Lett. 2003;348:61–64. doi: 10.1016/s0304-3940(03)00723-7. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Local perfusion of nicotine differentially modulates somatodendritic dopamine release in the rat ventral tegmental area after nicotine pre-exposure. Neurochem Res. 2004;29:1687–1693. doi: 10.1023/b:nere.0000035803.64724.17. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li T-K, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res. 2004;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Ther. 2005a;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li T-K, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005b;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Gryszowka VE, Toalston JE, Oster SM, Ji D, Bell RL, McBride WJ. The reinforcing actions of a serotonin-3 receptor agonist within the ventral tegmental area: evidence for subregional and genetic differences and involvement of dopamine neurons. J Pharmacol Exp Ther. 2007;321:1003–1012. doi: 10.1124/jpet.106.112607. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Oster SM, Ding ZM, Toalston JE, Deehan G, Bell RL, Li TK, McBride WJ. The reinforcing properties of salsolinol in the ventral tegmental area: evidence for regional heterogeneity and the involvement of serotonin and dopamine. Alcohol Clin Exp Res. 2008;32:230–9. doi: 10.1111/j.1530-0277.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, Murphy JM. Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol. 2010;44:245–255. doi: 10.1016/j.alcohol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Room R. Smoking and drinking as complementary behaviours. Biomed Pharmacother. 2004;58:111–115. doi: 10.1016/j.biopha.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. Heavy consumption of cigarettes, alcohol and coffee in male twins. J Stud Alcohol. 1997;58:182–190. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into the ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Spier AD, Yakel JL, Lummis SCR, Vijverberg HPM. Promiscuous coassembly of serotonin 5-HT3 and nicotinic α4 receptor subunits into Ca2-permable ion channels. Proc Natl Acad Sci. 1998;95:11456–11461. doi: 10.1073/pnas.95.19.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Scherrer JF, Bucholz KK, Todorov A, Heath AC, Jacob T, True WR. Evidence for specificity of transmission of alcohol and nicotine dependence in an offspring of twins design. Drug Alcohol Depend. 2007;87:225–232. doi: 10.1016/j.drugalcdep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Xiu X, Puskan NL, Shanata JAP, Lester HA, Dougherty DA. Nicotine binding to brain receptors requires a strong cation-π interaction. Nature. 2008;458:534–48. doi: 10.1038/nature07768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL, Hirsch JA. Drinking patterns and health status in smoking and nonsmoking alcoholics. Alcohol Clin Exp Res. 1995;19:666–673. doi: 10.1111/j.1530-0277.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Tanaka T, Mizoguchi K, Emoto H, Ishii H, Tanaka M. Facilitatory modulation of mesolimbic dopamine neuronal activity by a mu-opioid agonist and nicotine as examined with in vivo microdialysis. Brain Res. 1993;624:277–280. doi: 10.1016/0006-8993(93)90087-4. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–32. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]