Abstract
Pheochromocytomas and paragangliomas (PGLs) are neuroendocrine tumors of sympathetic and parasympathetic paraganglia. The present study investigated the relationships between genotype-specific differences in mitochondrial function and catecholamine content in PGL tumors. For this purpose, respiratory chain enzyme assays and 1H-NMR spectroscopy were performed on homogenates of 35 sporadic PGLs and 59 PGLs from patients with hereditary mutations in SDHB, SDHD, SDHAF-2, VHL, RET, NF1 and MAX. In SDHx related PGLs, a significant decrease in complex II activity (p<0.0001) and a significant increase in complex I, III and IV enzyme activities were observed when compared to sporadic, RET and NF1 tumors. Also, a significant increase in citrate synthase (p<0.0001) enzyme activity was observed in SDHx related PGLs when compared to sporadic, VHL, RET and NF1 related ones. An increase in succinate accumulation (p<0.001) and decrease in ATP/ADP/AMP accumulation (p<0.001) was observed when compared to sporadic PGLs and PGLs of other genotypes. Positive correlations (p<0.01) were observed between respiratory chain complex II activity and total catecholamine content and ATP/ADP/AMP and total catecholamine contents in tumor tissues. The present study for the first time establishes relationship between determinants of energy metabolism like activity of respiratory chain enzyme complex II, ATP/ADP/AMP content and catecholamine content in PGL tumors. Also, the present study for the first time successfully uses NMR spectroscopy to detect catecholamines in PGL tumors and provides in vivo evidence for the accumulation of succinate in PGL tumors with a SDHx mutation.
Keywords: respiratory chain enzyme activities, pheochromocytoma, paraganglioma, catecholamines, epinephrine, norepinephrine, energy metabolism, NMR spectroscopy
Introduction
Pheochromocytomas and paragangliomas (PGLs) are neuroendocrine tumors of sympathetic and parasympathetic paraganglia. PGLs of sympathetic origin (adrenal medulla and extra adrenal sympathetic tissue of abdomen, pelvis and chest) usually produce catecholamines whereas the tumors of parasympathetic origin (head and neck PGLs) usually do not produce significant amounts of catecholamines (1). At least 30%–35% of the PGLs are caused by germline mutations of ten identified tumor susceptibility genes (2). These include VHL (von Hippel-Lindau), RET (rearranged during transfection), NF1 (neurofibromatosis type 1), SDHA/B/C/D (succinate dehydrogenase subunits A, B, C and D), SDHAF2 (succinate dehydrogenase assembly factor 2) and the more recently reported TMEM127 (transmembrane protein 127) and MAX (myc-associated factor X) (2). In 17% of sporadic tumors, somatic mutations in RET, VHL, MAX and more recently, HIF-2α and NF1 have been reported (3–5).
Based on transcriptional profiling studies, PGLs can be classified into two clusters: cluster 1 and cluster 2 (6, 7). Cluster 1 tumors (VHL, SDHA/B/C/D/AF2) are characterized by increased expression of genes involved in (pseudo)hypoxia, cell proliferation, angiogenesis, electron transport chain and the Krebs cycle and abnormal function of oxidoreductases. Cluster 2 tumors (RET, NF1) show an increased expression of genes involved in protein synthesis, kinase signaling, endocytosis and maintenance of a differentiated chomaffin cell catecholamine biosynthetic and secretory phenotype. Sporadic PGLs are distributed between the two major clusters based on their gene expression pattern and catecholamine phenotype (6).
SDH is an important component of the mitochondrial electron transport chain. In tumors with SDHx mutations, the ability of cells for oxidative phosphorylation is compromised (7–10). Also, it has been demonstrated in vitro that accumulation of succinate in cells silenced for SDH causes inhibition of prolyl hydroxylase activity resulting in stabilization of hypoxia-inducible factors (HIF) -1α and -2α (11, 12). HIF-1α and -2α then translocate to the nucleus where, together with aryl hydrocarbon receptor nuclear translocator (ARNT), they form an active HIF complex that induces the expression of genes with hypoxia response elements that support tumor progression via different signaling pathways. Thus, in cluster 1 tumors, the pseudo-hypoxic drive is hypothesized to mediate an increase in aerobic glycolysis, also known as Warburg effect. This is supported by increased HIF-α protein level combined with lower SDH activity and increased glycolysis as indicated by lactate dehydrogenase activity (7).
The differences between cluster 1 and 2 tumors are also characterized by differences in catecholamine biosynthetic and secretory profiles (13). PGLs with mutations in RET and NF1 produce both epinephrine and norepinephrine and have low rate constants for catecholamine secretion, whereas SDHx and VHL–related tumors mainly produce norepinephrine and have high rate constants for catecholamine secretion. Tumor catecholamine content is lower in cluster 1 tumors, compared to cluster 2 tumors. Also, it is well known that sequestration of catecholamines into chromaffin granules through vesicular monoamine transporters (VMAT) and re-uptake of catecholamines via norepinephrine transporter (NET) are active energy dependent processes. Differences in catecholamine phenotypes may thus in part be explained by mutation-dependent changes in energy metabolism.
In the present study, we therefore investigated relationships between genotype-specific differences in mitochondrial function and catecholamine content in PGL tumors. A large number of PGL tissues of various genotypes was included. Besides functional assays for respiratory complex I–IV and citrate synthase (CS), 1H nuclear magnetic resonance (NMR) spectroscopy was performed to provide an overview of the intracellular metabolome with specific focus on catecholamines, AMP/ADP/ATP and intermediates of glycolysis and Krebs cycle.
Materials and methods
Patients
Patients with histologically proven PGLs evaluated at the department of General Internal Medicine, division of Endocrinology of the Radboud University Nijmegen Medical Centre (RUNMC), Nijmegen, the Netherlands and at Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Health (NIH), Bethesda, MD, USA were considered for the study. Tumor tissue samples of consecutive patients from RUNMC who underwent surgical resection between 1988 and 2012 were included in the study. NIH patients underwent surgery between 2003 and 2010. Frozen primary tumor tissues from 64 patients at RUNMC and 26 patients at NICHD were included. The presence of germline mutations and large deletions in SDHB/C/D, RET, VHL and -since 2011- in SDHA, SDHAF2, TMEM127 and MAX, was investigated using standard procedures. Data were collected under conditions of regular clinical care, with ethical committee approval obtained for the use of those data for scientific purposes at RUNMC. The study was approved by the Institutional Review Board of NICHD, and all patients gave written informed consent before testing. The details of the patients’ clinical characteristics and genotype are listed in Table 1.
Table 1.
Patient Characteristics
| Genotype | N (patients) | Age y (mean±SD) | Gender (M/F) | N (tumors) | Tumor Location (A/E/HN) | Tumor volume (cm3) |
|---|---|---|---|---|---|---|
| Sporadic | 35 | 47.5 ± 14.8 | 18/17 | 35 | 32/3/0 | 218.6 ± 452.5 |
| SDHB | 13 | 32 ± 11.9 | 10/3 | 15 | 1/14/0 | 273.1 ± 316.2 |
| SDHD | 8 | 44 ± 10.4 | 6/2 | 8 | 1/1/6 | 27.9 ± 47 |
| SDHAF2 | 1 | 24 | 1/0 | 1 | 0/0/1 | 18.9 |
| VHL | 9 | 30.7 ± 13 | 6/3 | 9 | 7/2/0 | 61.5 ± 84.9 |
| MEN-2 | 13 | 37.9 ± 12.8 | 6/7 | 15 | 15/0/0 | 93.4 ± 171.6 |
| NF1 | 8 | 43.2 ± 17.4 | 5/3 | 8 | 8/0/0 | 107.6 ± 806 |
| MAX | 3 | 48 ± 14 | 2/1 | 3 | 3/0/0 | 63.2 ± 71.9 |
N: No. of patients, y: years, M: Male, F: Female, A: Adrenal, E: Extraadrenal, HN: head and neck
Tumor tissue processing
Tumor tissues resected from the patients described above were procured as early as possible, the dimensions of the tumor were recorded and a small piece of the tumor tissue was weighed and snap frozen in liquid Nitrogen and used for experimental purposes. For histological confirmation additional slices were stained with hematoxylin and eosin and re-evaluated by an independent pathologist (BK).
Respiratory chain enzyme assays
Frozen tumor specimens (~40 mg) were homogenized on melting ice in sucrose-EDTA-phosphate buffer (0.25 M sucrose, 2 mM EDTA, 10 mM K2PO4, pH 7.4, 8% w/v) using a hand held glass/glass homogenizer. Homogenates were subsequently centrifuged at 600×g at 4°C for 10 min and supernatants used for the determination of the activities of respiratory chain enzyme complexes I, II, III and IV and the mitochondrial matrix enzyme, CS. These assays measured the formation of a spectrophotometrically detectable end-product at regular time intervals and were performed on Konelab 20XT clinical chemistry analyzer (Thermo Scientific, Finland) as described elsewhere (14). The protein concentrations in the supernatants were also measured in parallel using pyrogallol red-molybdate complex method as described earlier (15). The enzyme activities were normalized to mg protein. Five samples were excluded from analysis (Sporadic- 2, SDHB, VHL and NF1- 1 each) as the tumor tissue homogenate contained blood which could affect determinations and interpretations of respiratory chain enzyme activities and protein concentrations.
1H NMR Spectroscopy
1H NMR spectroscopy was performed in frozen tumor tissues to determine concentrations of intermediates of energy metabolism (Krebs cycle and glycolysis), catecholamines and their metabolites. One sporadic, 2 SDHB, 1 VHL and 2 MAX tumors were excluded from the experiment as the amount of starting material was low. The tumor tissues were homogenized on ice in 10% w/v of distilled water using a hand held Teflon/glass homogenizer. The samples were then centrifuged at 16,000×g for 10 min at 4°C and the supernatants were subjected to ultrafiltration with Vivaspin Turbo 15, 10 kDa filters (Sartorius, Germany). The ultrafiltrates were diluted with water to 700 μl, pH was adjusted to 2.5 and 20 μl of 20.2 mM sodium 3-trimethylsilyl-2,2,3,3-tetradeuteropropionate (TSP) in D2O was added to the samples. The samples were then placed in 5 mm NMR tubes and 1H NMR spectra were obtained using a Bruker 500 MHz spectrometer (pulse angle 90°, 7 μs pulses with a delay time of 4 s, number of scans 256). The water resonance was suppressed by gated irradiation centered on water frequency.
Concentrations of succinate in the tumor tissues were estimated by integrating the area under the peak at 2.66 ppm. Differences in peak heights were clearly observed for tumors with high, absent and low levels of succinate (Supplementary Figure 1). Tumor tissue ATP/ADP/AMP content were estimated by integrating the area under peaks in the region 6.18–6.21 ppm. Since each of the three compounds differ by the presence of one phosphate group, spectral peaks for these metabolites cannot be distinguished using 1H NMR spectroscopy. Epinephrine content was estimated by integrating area under the peaks for the triplet at 2.73 ppm and the total catecholamine content was estimated by integrating the area under the peaks for multiplets in the region 6.86–6.98 ppm (Supplementary Figure 1). Further, norepinephrine content was estimated by calculating the difference between total catecholamine and epinephrine contents. Other amines, which could contribute to peaks in the region 6.86–6.98 ppm are dopamine and 3-methoxytyramine. However, the concentration of these compounds as measured by HPLC is in the nanomolar range (16), much lower than the detection limit of 1H NMR spectroscopy, which is in micromolar range. The catecholamine content of the tumor tissue as estimated by 1H NMR spectroscopy was validated using HPLC as described previously (17), for a small subset of 22 samples and a significant correlation and linear relationship was observed between the two methods (Supplementary Figure 2).
Statistical Methods
Statistical analyses were performed using SPSS (SPSS Inc., v.18) and GraphPad Prism 6 software (GraphPad, La Jolla, CA). The data was analyzed using independent samples. Kruskal-Wallis test and Dunn’s post-test was used to compare the different genotypes. Correlation between respiratory chain enzyme activities and tumor tissue catecholamine content was examined using Spearman’s correlation test. Statistical significance was accepted at P value <0.05. Further, estimation of catecholamine content by 1H NMR spectroscopy and HPLC and comparison of respiratory chain enzyme activities with total catecholamine content was carried out using Passing-Bablok regression analysis (18).
Results
Respiratory chain enzyme activities
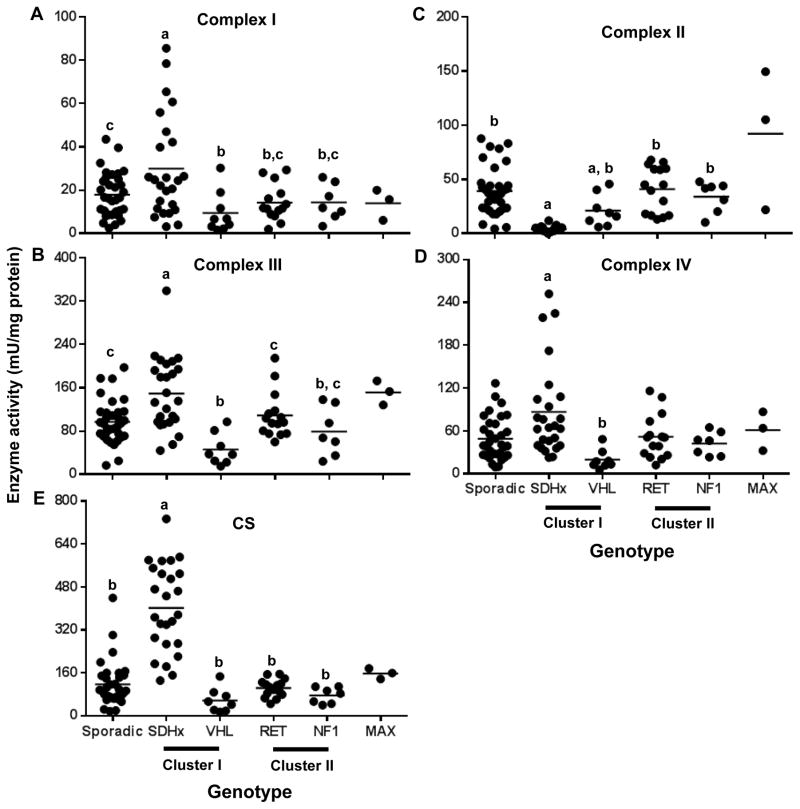
The activity of the respiratory chain complex II was deficient in all SDHx tumors as expected (Fig 1B; p<0.0001). The activity of the complexes I, III and IV was significantly higher in SDHx tumors than in VHL, RET, NF1 and sporadic tumors (Fig 1A, C, D, p<0.05). This was even more clearly so for citrate synthase activity (Fig 1E; p<0.0001). The VHL tumors showed a lower activity for Complex I when compared to sporadic tumors (Fig 1A, Table 2, p<0.05) and complex III when compared to sporadic (Fig 1C, Table 2, p<0.01) and cluster II tumors (Fig 1C, Table 2, p<0.05). In contrast, the MAX tumor group had a tendency of higher activity for the complexes II, III and IV when compared to the other genotypes (Fig 1B–D).
Figure 1.
Respiratory chain enzyme activity in PGL tumor tissues of different genotypes. A–E. Dot plots depicting the respiratory chain enzyme activities of respiratory chain enzyme complexes I, II, III and IV and CS across different genotypes in mU normalized to mg protein concentrations. Horizontal line represents the mean. Data sets having different alphabets above them are significantly different (p<0.05).
Table 2.
Comparison of respiratory chain complex activities between different genotypes
| Genotype | SDHx vs | VHL vs | ||
|---|---|---|---|---|
| Sporadic | MEN-2 and NF1 | Sporadic | MEN-2 and NF1 | |
| Complex I | 0.3164 | 0.0278 | 0.0478 | 0.4221 |
| Complex II | < 0.0001 | < 0.0001 | 0.1150 | 0.1703 |
| Complex III | 0.0050 | 0.0165 | 0.0082 | 0.0137 |
| Complex IV | 0.0397 | 0.0439 | 0.0600 | 0.0733 |
Enlisted in the table are P values (adjusted) for the various comparisons. Highlighted in bold letters are comparisons which attained statistical significance.
Energy metabolism intermediates
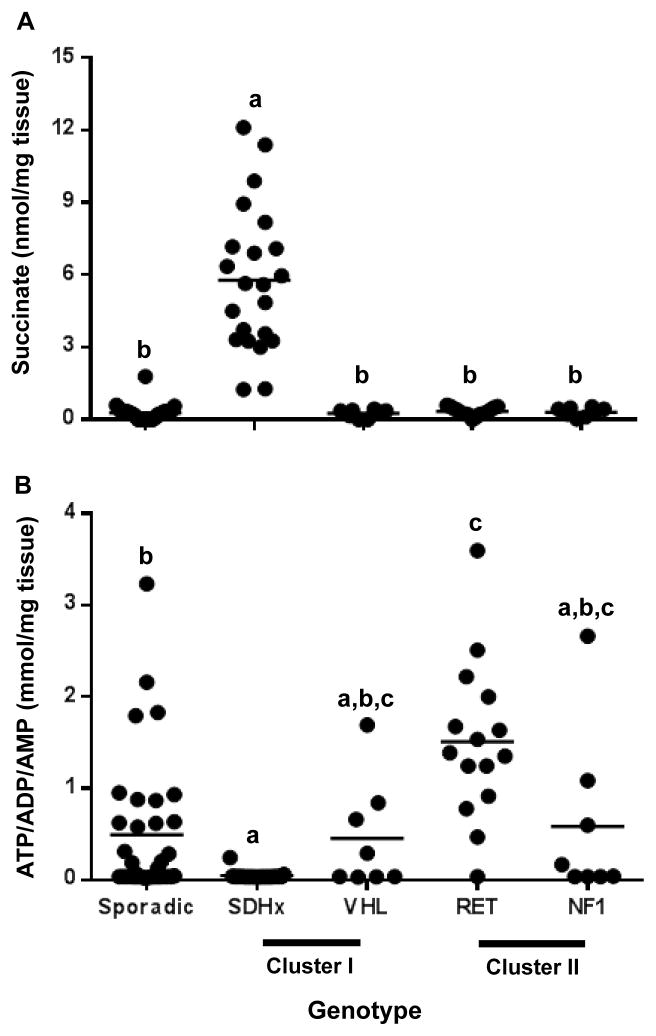
The NMR spectra of the tumor homogenates showed very high succinate (p<0.001) levels in all SDHx cases as expected (Fig 2A). Succinate was not NMR detectable or very low in all other tumor samples except for 1 tumor in the sporadic group. Citrate was present in high concentration in four sporadic tumors. Low concentrations of pyruvate, without genotype specific differences, were observed in all PGL tissues (data not shown).
Figure 2.
Accumulation of intermediates of energy metabolism in PGL tumor tissues of different genotypes as determined by 1H-NMR spectroscopy. A. Dot plot depicting the tumor tissue succinate concentrations expressed as nmol per mg tumor tissue across different genotypes. B. Dot plot depicting the tumor tissue ATP/ADP/AMP concentrations expressed as mmol per mg tumor tissue across different genotypes. Horizontal line represents the mean. Data sets having different alphabets above them are significantly different (p<0.05). Succinate and ATP/ADP/AMP were below detection limit in 13 tumor samples and have been represented as half the lowest detectable value.
The differences in high energy phosphate content between the tumors were striking. Proton NMR spectroscopy cannot discriminate between ATP, ADP and AMP and therefore figure 2B shows the sum of the three high energy phosphates. The concentration of ATP/ADP/AMP was consistently very low (p<0.0001) in all SDHx tumors. A very low content also occurred in the other tumor groups but the ATP/ADP/AMP concentration was rather variable in these groups. RET tumors had high ATP/ADP/AMP when compared to sporadic (p<0.01) and SDHx tumors (p<0.0001).
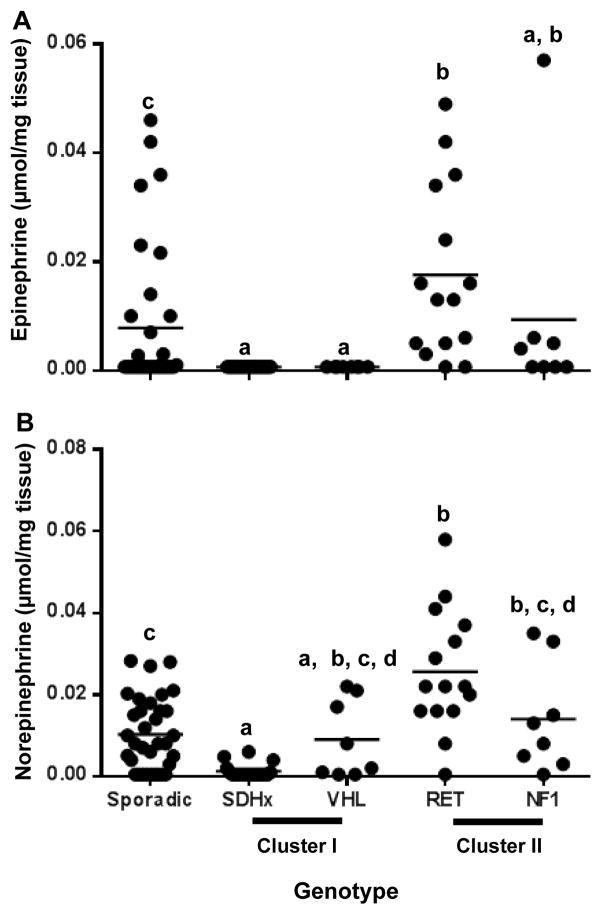
Detection of tumor tissue catecholamines using 1H-NMR spectroscopy
Epinephrine was proton NMR-undetectable in all SDHx and VHL tumors. The RET tumors showed high epinephrine concentrations when compared to sporadic (p<0.05), SDHx (p<0.0001) and VHL (p<0.001) tumors (Fig 3A). In SDHx tumors norepinephrine was also very low when compared to sporadic (p<0.01), RET (p<0.0001) and NF1 tumors (p< 0.05) while 50% of the VHL tumors produced significant amounts of norepinephrine (Fig 3B). Total catecholamine peaks were below detection limit in 33 tumor samples. Seven of these samples were subjected to HPLC which detected the presence of catecholamines in these tissues.
Figure 3.
Tumor tissue catecholamine content as determined by 1H-NMR spectroscopy. A. Dot plot representing the tumor tissue epinephrine concentrations expressed as μmol per mg tumor tissue across different genotypes. B. Dot plot representing the tumor tissue norepinephrine concentrations expressed as μmol per mg tumor tissue across different genotypes. Data sets having different alphabets above them are significantly different (p<0.05). Total catecholamine peaks were below detection limit in 33 tumor samples and have been represented as half the lowest detectable value. E- epinephrine, NE - norepinephrine
Correlation of energy metabolism with tumor tissue catecholamine content
Positive correlations (p<0.001) were observed between activities of respiratory chain complex II and concentrations of epinephrine, norepinephrine and total catecholamine content in tumor tissues. ATP/ADP/AMP content of PGL tumors also showed a positive correlation with tumor tissue epinephrine, norepinephrine and total catecholamine levels (Table 3). Sympathetic PGLs were excluded from this analysis as they do not produce catecholamines.
Table 3.
Correlation of respiratory chain enzyme activity with tumor tissue catecholamine content
| Catecholamines | CI (mU/mg protein) | CII (mU/mg protein) | CIII (mU/mg protein) | CIV (mU/mg protein) | ATP/ADP/AMP (mmol/mg tissue) | ||
|---|---|---|---|---|---|---|---|
| Spearman’s rho | E (nmol/mg tissue) | Correlation Coefficient | 0.226 | 0.423 | 0.182 | 0.006 | 0.366 |
| Sig. (2-tailed) | 0.046 | 0.0001 | 0.097 | 0.955 | 0.0001 | ||
| N | 78 | 78 | 78 | 78 | 82 | ||
| NE (nmol/mg tissue) | Correlation Coefficient | 0.160 | 0.479 | 0.078 | 0.066 | 0.390 | |
| Sig. (2-tailed) | 0.163 | 0.0001 | 0.495 | 0.569 | 0.0001 | ||
| N | 78 | 78 | 78 | 78 | 82 | ||
| Total catecholamines (nmol/mg tissue) | Correlation Coefficient | 0.208 | 0.517 | 0.148 | 0.033 | 0.458 | |
| Sig. (2-tailed) | 0.067 | 0.0001 | 0.196 | 0.774 | 0.0001 | ||
| N | 78 | 78 | 78 | 78 | 82 | ||
E: epinephrine, CI, II, III and IV: respiratory chain enzyme complexes I, II, III and IV. Highlighted in bold letters are comparisons which attained statistical significance.
Further, Passing-Bablok regression statistics for comparison between complex II activity, tumor ATP/ADP/AMP content and total catecholamine content demonstrated a linear relationship (Figure 4).
Figure 4.
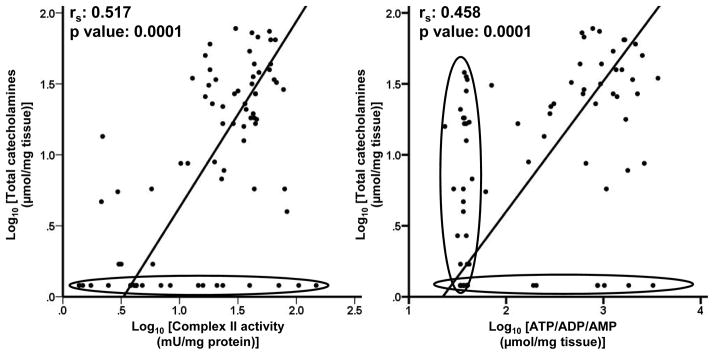
Relationship between tumor tissue catecholamine content and activity of respiratory chain enzyme complex II and tumor ATP/ADP/AMP content. Passing-Bablok regression plots for activities respiratory chain enzyme complex II (mU per mg protein) versus total catecholamine content (nmol per mg tissue) in PGL tumor tissues represented in Log10 scale. Points for samples below detection limit of 1H NMR spectroscopy are encircled in black.
Discussion
The present study establishes differences in mitochondrial energy pathways in metabolic processes in PGLs and provides novel insight into how deregulation of energy metabolism might impact catecholamine phenotypic features of cluster 1 and cluster 2 tumors. Further the study also provides in vivo evidence for the accumulation of succinate in SDH-related tumors and successfully uses 1H-NMR spectroscopy for detection of catecholamines in PGL tumor tissues.
In accordance with previous reports (7–10) we observed that the activity of SDH or respiratory chain enzyme complex II is low in SDH-related tumors. Interestingly, reduction of complex II activity in SDH-related tumors was associated with increased activities of other respiratory chain complexes I, III and IV and CS. The increased CS activity also indicates an increase in the mitochondrial content. This observation is in agreement with report by Douwes et al., (19) in which elevated numbers of tightly packed mitochondria were observed in SDHD linked head and neck PGLs. All these factors suggest a compensatory response to the lack of SDH activity and associated activity of complex II. However as currently shown, the apparently increased activity of complex I, III, IV and CS in the tumors does not lead to full restoration of ATP/ADP/AMP. This contrasts with the report of Favier et al., (7) in which no differences for complex III activity were observed among NF1/RET, SDH and VHL-related PGLs and that of Rapizzi et al., (10) where no differences in CS activity were observed across different genotypes. Our findings of higher complex IV activity in SDH-related tumors than NF1 and RET related ones contradicts with the findings by Favier et al., (7) who observed a decreased Complex IV activity in SDHx and VHL- related tumors. However, our observations are in agreement with the increased expression of complex IV protein reported in four out of five SDH-related tumors by Rapizzi et al., (10). Nevertheless, this study also indicated a high degree of variability in complex IV activities in these tumors.
Previous studies by Lopez-Jimenez et al., (20) and Favier et al. (7) have demonstrated that there is a preferential activation of HIF-1α target genes such as glycolytic pathway enzymes in VHL tumors while activation of HIF-2α is observed in both VHL and SDHx tumors. It is also thought that activation of hypoxic response may be directly involved in decreased mitochondrial respiration. Interestingly, in the present study we observed an increase in complex I, III and IV and CS in SDHx tumors while there was a trend of overall down regulation of respiratory chain activities in VHL tumors. Also, we observed that in contrast to VHL tumors, accumulation of ATP/ADP/AMP in SDHx tumors was undetectable (except in one sample). This indirectly supports the observations by Favier et al., (7) that there is an activation of glycolysis as evidenced by increased lactate dehydrogenase activity in VHL tumors and not SDHx tumors. However, increased expressions of GLUT-1, GLUT-3 and Hexokinase II mRNAs observed in SDH tumors (7) can explain the high sensitivity of [18F]-FDG PET to SDH tumors (21–23).
In in vitro experiments with cells silenced for SDH, it was observed that the accumulation of succinate leads to stabilization of HIF-1α (11). Later, Pollard et al., (24) described succinate accumulation in tumor tissues with germline SDH mutation, however, it was described in a single patient with pathogenic SDH mutation. In the present study, we for the first time provide strong in vivo evidence to support the hypothesis that there is an accumulation of succinate in SDH-related PGL tumor tissues. This supports the concept that stabilization of HIF-α in SDH-related tumors reflects the inhibitory effects of succinate on prolyl hydroxylase. High succinate accumulation observed in tumors with SDHx mutations also makes this a reliable parameter to indicate mutations in SDH subunits or assembly factors.
Genotype specific differences in catecholamine production by PGLs are well known. In the present study, our observations that tumors with mutations in VHL and SDHx mainly produce noradrenaline and those with mutations in RET and NF1 produce both adrenaline and noradrenaline support the previous findings by Eisenhofer et al.,(13). This difference in the catecholamine phenotype has been attributed to the lack of the enzyme phenylethanolamine N-methyltransferase (PNMT) in VHL and SDHx tumors (25). In line with the previous studies (13), we also observed that the tumor tissue total catecholamine content was lower in SDHx tumors when compared to RET and sporadic PGLs. These differences could be possibly attributed to the increased tyrosine hydroxylase activity in RET tumors (25) however, tyrosine hydroxylase activity or expression has not been investigated in SDHx tumors in comparison with other genotypes albeit a lack of the expression of this enzyme has been observed in biochemically silent SDHB tumors (26).
In the present study we established that tumor tissue concentrations of ATP/ADP/AMP, as determined by low peak heights at relevant resonance positions in 1H-NMR spectra, are lower in SDH - related tumors than in other tumors. Further, our findings of positive relationships between respiratory enzyme complex II function, tumor ATP/ADP/AMP content and tumor catecholamine contents suggest the possibility that differences in energy metabolism might also contribute to the lower tumor tissue catecholamine contents in cluster 1 than in cluster 2 tumors. To this end it is well known that the sequestration of catecholamines into secretory vesicles and re-uptake of catecholamines into chromaffin cells are active energy dependent processes. Sequestration of catecholamines is facilitated by vesicular monoamine transporters (VMATs). The H+ gradient necessary to maintain the activity of VMATs is generated by ATP dependent vesicular membrane proton pump (27). Chromaffin granules also contain strikingly high concentrations of ATP due to the activity of vesicular nucleotide transporter (28). This contributes to the stability and ability of chromaffin granules to maintain stores of catecholamines (29, 30). Further, norepinephrine transporter (NET) responsible for the sodium-chloride (Na+/Cl−)-dependent reuptake of extracellular norepinephrine and dopamine is also indirectly dependent on cellular store of ATP. NET functions by coupling the transport of norepinephrine and dopamine with the influx of sodium and chloride (Na+/Cl−). The ion gradients of Na+ and Cl− generated by the Na+/K+-ATPase make this reuptake energetically favorable (31, 32). Clearly therefore, energy metabolism has an important role in maintaining the stability of chromaffin granules and thus catecholamine storage. It thereby seems possible that genotype specific differences in the energy metabolism, along with associated differences in expression of various genes encoding components of secretory pathway and exocytotic machinery (33), could in conjuction contribute to genotype specific differences in tumor catecholamine phenotypic features.
In the present study we included 35 sporadic tumors 60% of which were not tested for SDHA and SDHAF-2. Two of the 3 sporadic tissues which had low respiratory chain complex II activities comparable to SDHx tumors also belong to the group which were not tested for SDHA and SDHAF-2. Thus, mutations in SDHA and SDHAF-2 cannot be ruled out in these tumors. Also, the low activity may indicate that these tumors may have an as yet unidentified intronic or promotor mutations in SDH subunit genes or unidentified mutations in assembly factors genes.
We used 1H NMR spectroscopy to determine the tumor tissue metabolite concentrations as it provides a holistic view on the tumor metabolome. This technique can very well identify various metabolites and quantify differences in the metabolite concentrations among different samples, but it is limited by its sensitivity. It can quantify metabolites only in micromolar range because of which, many intermediates of energy and catecholamine metabolism could not be determined in the present study. This is clearly visible in the Passing-Bablok regression analysis of total catecholamines vs Complex II activity and ATP/ADP/AMP levels where reduced sensitivity of NMR spectroscopy separates out a group of samples which if analysed with a more sensitive method could have reflected the linear relationship better. Nevertheless, the study was successful in identifying the relationship between catecholamine content of PGLs and energy metabolism.
Catecholamine contents are particularly low in tumors due to SDHB mutations and it has been suggested that this along with diversion of energy from maintaining catecholamine phenotypic features to growth might contribute to the larger sizes and more aggressive features of these tumors (16). The present study, establishing relationships between tumor energetics and catecholamine phenotypic features, provides new insight into how such diversions of energy might occur with implications for novel therapeutic strategies targeting energy pathways.
Supplementary Material
Acknowledgments
Grants: The work leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 259735 (ENSAT CANCER).
Ms. Anne M. Leenders and Mr. Nicolai J. Grebenchtchikov for their help with respiratory chain enzyme assays and statistics, respectively.
Footnotes
Reprint requests: Same as above
Disclosures: Authors have no disclosures to make
References
- 1.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 2.Welander J, Soderkvist P, Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocrine-related cancer. 18(6):R253–76. doi: 10.1530/ERC-11-0170. [DOI] [PubMed] [Google Scholar]
- 3.Burnichon N, Buffet A, Parfait B, et al. Somatic NF1 inactivation is a frequent event in sporadic pheochromocytoma. Human molecular genetics. doi: 10.1093/hmg/dds374. [DOI] [PubMed] [Google Scholar]
- 4.Burnichon N, Vescovo L, Amar L, et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Human molecular genetics. 20(20):3974–85. doi: 10.1093/hmg/ddr324. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. The New England journal of medicine. 367(10):922–30. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahia PL. Transcription association of VHL and SDH mutations link hypoxia and oxidoreductase signals in pheochromocytomas. Annals of the New York Academy of Sciences. 2006;1073:208–20. doi: 10.1196/annals.1353.023. [DOI] [PubMed] [Google Scholar]
- 7.Favier J, Briere JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PloS one. 2009;4(9):e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimenez-Roqueplo AP, Favier J, Rustin P, et al. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. American journal of human genetics. 2001;69(6):1186–97. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimenez-Roqueplo AP, Favier J, Rustin P, et al. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. The Journal of clinical endocrinology and metabolism. 2002;87(10):4771–4. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- 10.Rapizzi E, Ercolino T, Canu L, et al. Mitochondrial function and content in pheochromocytoma/paraganglioma of succinate dehydrogenase mutation carriers. Endocrine-related cancer. 19(3):261–9. doi: 10.1530/ERC-11-0263. [DOI] [PubMed] [Google Scholar]
- 11.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Nakamura E, Yang H, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer cell. 2005;8(2):155–67. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhofer G, Pacak K, Huynh TT, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocrine-related cancer. 18(1):97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodenburg RJ. Biochemical diagnosis of mitochondrial disorders. Journal of inherited metabolic disease. 34(2):283–92. doi: 10.1007/s10545-010-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe N, Kamei S, Ohkubo A, et al. Urinary protein as measured with a pyrogallol red-molybdate complex, manually and in a Hitachi 726 automated analyzer. Clinical chemistry. 1986;32(8):1551–4. [PubMed] [Google Scholar]
- 16.Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 48(11):1739–49. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhofer G, Goldstein DS, Stull R, et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clinical chemistry. 1986;32(11):2030–3. [PubMed] [Google Scholar]
- 18.Passing H, Bablok A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. Journal of clinical chemistry and clinical biochemistry. 1983;21(11):709–20. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 19.Douwes Dekker PB, Hogendoorn PC, Kuipers-Dijkshoorn N, et al. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. The Journal of pathology. 2003;201(3):480–6. doi: 10.1002/path.1461. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Jimenez E, Gomez-Lopez G, Leandro-Garcia LJ, et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Molecular endocrinology (Baltimore, Md. 24(12):2382–91. doi: 10.1210/me.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmers HJ, Chen CC, Carrasquillo JA, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. Journal of the National Cancer Institute. 104(9):700–8. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro- deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. The Journal of clinical endocrinology and metabolism. 2009;94(12):4757–67. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmers HJ, Kozupa A, Chen CC, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25(16):2262–9. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 24.Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over- expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Human molecular genetics. 2005;14(15):2231–9. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhofer G, Walther MM, Huynh TT, et al. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. The Journal of clinical endocrinology and metabolism. 2001;86(5):1999–2008. doi: 10.1210/jcem.86.5.7496. [DOI] [PubMed] [Google Scholar]
- 26.Timmers HJ, Pacak K, Huynh TT, et al. Biochemically silent abdominal paragangliomas in patients with mutations in the succinate dehydrogenase subunit B gene. The Journal of clinical endocrinology and metabolism. 2008;93(12):4826–32. doi: 10.1210/jc.2008-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry JP, Sagne C, Bedet C, Gasnier B. The vesicular monoamine transporter: from chromaffin granule to brain. Neurochemistry international. 1998;32(3):227–46. doi: 10.1016/s0197-0186(97)00092-2. [DOI] [PubMed] [Google Scholar]
- 28.Sawada K, Echigo N, Juge N, et al. Identification of a vesicular nucleotide transporter. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5683–6. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- 30.Kopell WN, Westhead EW. Osmotic pressures of solutions of ATP and catecholamines relating to storage in chromaffin granules. The Journal of biological chemistry. 1982;257(10):5707–10. [PubMed] [Google Scholar]
- 31.Galli A, DeFelice LJ, Duke BJ, Moore KR, Blakely RD. Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. The Journal of experimental biology. 1995;198(Pt 10):2197–212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- 32.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nature reviews. 2003;4(1):13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhofer G, Huynh TT, Elkahloun A, et al. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. American journal of physiology. 2008;295(5):E1223–33. doi: 10.1152/ajpendo.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.