Abstract
Objective
We hypothesized that vascular amyloid contributes to chronic brain ischemia, therefore amyloid burden measured by Pittsburgh Compound B retention on PET (PiB-PET) would correlate with the extent of MRI white matter hyperintensities (WMHor leukoaraiosis) in patients with high vascular amyloid deposition (Cerebral Amyloid Angiopathy, CAA) but not high parenchymal amyloid deposition (Alzheimer’s Disease, AD; Mild Cognitive Impairment, MCI) or healthy elderly (HE).
Methods
Fourty-two non-demented CAA patients, 50 HE subjects and 43 AD/MCI patients had brain MRI and PiB-PET. Multivariate linear regression was used to assess the independent association between PiB retention and WMD volume controlling for age, gender, apolipoprotein E genotype, and vascular risk factors within each group.
Results
CAA patients were younger than HE and AD (68±10 vs 73.3±7 and 74±7.4, p<0.01) but had higher amounts of WMH (medians: 21ml vs 3.2ml and 10.8ml respectively, p<0.05 for both comparisons). Global PiB retention and WMH showed strong correlation (rho=0.52, p<0.001) in the CAA group but not in HE or AD. These associations did not change in the multivariate models. Lobar microbleed count, another marker of CAA severity also remained as an independent predictor of WMH volume.
Interpretation
Our results indicate that amyloid burden in CAA subjects (with primarily vascular amyloid) but not AD subjects (with primarily parenchymal amyloid) independently correlate with WMH volume. These findings support the idea that vascular amyloid burden directly contributes to chronic cerebral ischemia and highlights the possible utility of amyloid imaging as a marker of CAA severity.
INTRODUCTION
The severity and progression of white matter hyperintensities (WMH) on fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) are associated with major causes of morbidity/mortality in the elderly, including cognitive impairment, decreased mobility, stroke and depression.1–4 The prevalence and severity of WMH (or leukoaraiosis) increases with age and appear to be related to chronic cerebral small vessel ischemia.5, 6 The role of specific small vessel pathologies remains under exploration.7, 8 Hypertension (HTN) has been the most consistently identified vascular risk factor for WMH, whereas conflicting results have been reported regarding hyperlipidemia (HPL) and diabetes mellitus (DM).9–11 White matter disease is also commonly seen in neurodegenerative conditions of the elderly that involve amyloid deposition in cerebral parenchyma and vessels. Cerebral amyloid angiopathy (CAA), characterized by accumulation of amyloid beta (Aβ) proteins in the walls of cortical/leptomeningeal vessels, is increasingly associated with markers of ischemic brain injury in addition to its well recognized presentation of lobar intracerebral hemorrhages (ICH).9, 12 Leukoareosis is also commonly seen in patients with Alzheimer Disease (AD), an entity characterized by Aβ deposition primarily in the brain parenchyma as senile plaques. White matter injury in AD may play an additive role in promoting cognitive impairment.13, 14
The precise mechanism linking amyloid deposition in CAA and AD to WMH has not been established.12, 15, 16 The potential contributions of vascular and parenchymal amyloid pathologies to WMH are important to clarify, particularly as treatments aimed at reducing parenchymal amyloid can increase the severity of CAA.17 Pittsburgh Compound B (PiB) is a positron emission tomography (PET) ligand shown to label both vascular and parenchymal amyloid deposits, thus allowing in vivo quantification of CAA and AD pathology.18–21 If WMH in these conditions is primarily driven by vascular amyloid, then one might expect retention of PiB to correlate to white matter disease in CAA, where the amyloid deposits are primarily vascular, but not AD or mild cognitive impairment (MCI), where amyloid is primarily parenchymal. We tested the hypothesis that there is a dose-dependent relationship between PiB retention and chronic brain ischemia (measured by WMH volume) in patients diagnosed with CAA but not AD/MCI or healthy elderly (HE) without evidence of advanced CAA.
METHODS
Study Subjects
We performed a cross-sectional study of 42 CAA, 43 AD/MCI patients and 50 healthy elderly who underwent PiB PET scan and MRI. The CAA subjects were recruited from an ongoing single-center prospective longitudinal cohort study of the natural history of CAA.12 Detailed information including demographics, clinical status, risk factors, and characteristics of the presenting event were prospectively recorded at the time of cohort entry. None of the CAA or HE subjects had dementia and all CAA patients were free of symptoms suggestive of new stroke for 1 year prior to PiB-PET. All 42 subjects met criteria for probable CAA according to Boston criteria, including supporting CAA pathology in 14 subjects and presence of multiple strictly lobar hemorrhages or microbleeds in the remaining 28.22 CAA patients presented with symptomatic ICH (n = 23, 55%) or with other symptoms (n=19, 45%) such as seizures or gait problems. Age, global DVR and WMH volumes were not different between patients presenting with and without ICH (p>0.3 for all comparisons). Patients diagnosed with AD or MCI were recruited from an ongoing prospective study in the Massachusetts Alzheimer’s Disease Research Center. Participants were diagnosed with probable AD by National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria and MCI by Petersen criteria.23, 24 Healthy elderly subjects were enrolled from ongoing longitudinal studies in aging, all from the same center as the CAA and AD/MCI patients.25 AD, MCI, and HE subjects were excluded for the presence of any lobar microbleeds on T2*-weighted MRI in order to minimize the presence of accompanying CAA. Also, to minimize the impact of non-amyloid vascular disease on WMH, HE subjects were excluded for diabetes mellitus (DM) or for requiring more than one medication for hypertension or hyperlipidemia.
Demographic information (age at PiB PET and gender) and presence of vascular risk factors were ascertained by interview of patient and caregiver and review of medical records. HTN was defined by ongoing use of an antihypertensive medication or measurement of elevated blood pressures (systolic blood pressure >140 mm Hg or diastolic >90) on two separate readings in the hospital or outpatient clinic. DM was defined by ongoing use of a hypoglycemic agent. Hyperlipidemia (HPL) was determined based on use of a cholesterol-lowering drug. Apolipoprotein E (APOE) genotype was identified from blood samples of patients who gave consent for blood draw, by the PCR- restriction enzyme assay as previously described.26 This study was performed with the approval of and in accordance with the guidelines of the institutional review board of Massachusetts General Hospital and with informed consent of all subjects or authorized family members. Radiologic and genetic analyses were performed by separate study personal and the results recorded without knowledge of subjects’ clinical information.
Imaging Acquisition and Analysis
All subjects underwent a PiB-PET scan between October 2005 and December 2011 using procedures that have been described previously.18 PiB was prepared according to the method of Mathis et al.27 PET data were acquired using either of two PET cameras with a dynamic protocol, a Siemens/CTI ECAT HR+ (Knoxville, TN) or a GE PC4096 scanner (Milwaukee, WI).18 PiB was injected as a bolus (8.5 to 15mCi) and followed immediately by a 60-minute acquisition. The distribution volume ratio (DVR) was computed to express specific PiB retention using cerebellar cortex as the reference tissue. Mean Global DVR was calculated using a predefined region of interest (ROI) that included the full thickness of the cortex and immediate subcortical white matter in supratentorial regions.18 For the secondary analysis that combines PiB positive MCI subjects with AD patients, we chose a mean global DVR cutoff >1.4 to define PiB positivity, a recently suggested criterion for detecting individuals with preclinical AD. 28
Each patient underwent detailed structural MRI scans within one month of PiB-PET imaging. Research MRIs included FLAIR for quantification of WMH, susceptibility-weighted imaging (SWI) for detection of cerebral hemorrhages and Magnetization Prepared RApid Gradient Echo (MPRAGE) for estimation of total intracranial volume.29 Two T1-weighted sagittal scans (Multi-echo MPRAGE, 1×1×1mm voxel size), T2*-weighted 2D axial images (1×1mm in-plane resolution, 5mm slice thickness, TR/TE 750/24msec) and FLAIR 3D axial images (1×1×1mm) were acquired using the vendor-supplied 12-channel head coil. Cerebral microbleeds (CMB) were identified according to previously published guidelines.30 Quantitative analysis of the volume of WMH on FLAIR MRI sequences was performed using previously validated computer-assisted techniques.9 As in previous studies, WMH volume for CAA subjects was calculated based on the hemisphere without ICH while excluding other focal ischemic or hemorrhagic lesions in order to omit perilesional signal changes from the WMH measurement. This method proved to have a high interrater reliability in prior studies.9 Total intracranial volume (ICV) was obtained from MPRAGE scans using the Freesurfer neuroimaging analysis software’s segmentation algorithm as previously described.31 Quantitative measure of WMH was the main outcome measure of the study and both the volume obtained from FLAIR segmentation (WMH volume) and its ratio to total ICV (WMH percent of ICV) were used for analyses.
Statistical Analysis
Bivariate comparisons were performed using chi-square test for ratios and t-test or Wilcoxon rank-sum test for continuous variables depending on their distribution. Bivariate correlation analyses were performed to test the association between continuous variables, Spearman’s rho for variables that did not have a normal distribution. Multivariate linear regression analysis was used to study the association between global DVR and WMH after adjustment for other covariates, identified based on the results of bivariate analyses in this study and findings from previous reports. These covariates included age, gender, microbleeds, APOE genotype, and other vascular risk factors. Separate multivariate models were built for each group. Models developed using a forward selection method to reduce the number of variables did not differ substantially from those that included all covariates. Separate multivariate linear regression models that included an interaction term between the diagnostic category (CAA vs combinations of AD/MCI) and mean global DVR were built and analyzed using established methods 32 to confirm that the association between amyloid deposition and WMH volume was significantly stronger in patients with CAA. APOE genotypes were available for all CAA patients, 33 HE and 11 AD/MCI cases. For analyses involving APOE in AD/MCI and HE, multivariate models were first built without APOE; subsequent addition of APOE to the model did not change any of the observed associations. For multivariate models, presence/absence of ApoE ε4 was used as a binary variable. Appropriate arithmetic transformations were applied to the continuous variables that were non-normally distributed for use in multivariate models. All statistical analyses were performed using SPSS software. A threshold for significance of p<0.05 was used. All tests of significance were two-tailed.
RESULTS
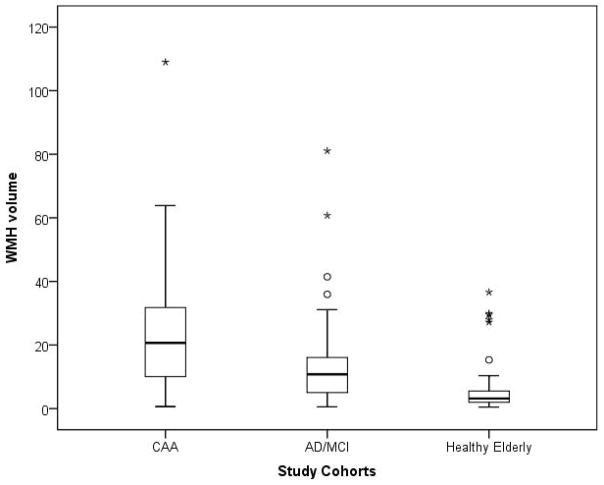
Demographic, genetic and imaging characteristics of the study population are presented in [Table 1]. Patients with CAA were younger than healthy elderly (p=0.004) and AD/MCI patients (p=0.002). Allele frequency of APOE ε4 was greater in both CAA and AD/MCI than HE (p<0.01 for both comparisons). In bivariate analyses, PiB retention was similar between CAA and AD/MCI patients (p=0.9) and both disease groups had higher mean DVR than HE (p<0.001 for both comparisons). In a multivariate linear regression model, higher PiB retention was associated with the diagnosis of CAA (p<0.001) and AD/MCI (p<0.001), age (p<0.001), presence of APOE ε4 (p=0.002) but not with gender or vascular risk factors such as HTN, HPL, or DM. Within the AD/MCI group, the AD subjects (n=18) and MCI subjects (n=25) differed in mean global PiB retention (1.46 for AD, 1.24 for MCI, p=0.007) but not age (mean 75 vs 73, p=0.7) or WMH volume (median 9.3 vs 10.8, p=0.9). After adjustment for age and other covariates, AD patients still had higher PiB retention than MCI (p=0.01). Patients with CAA had higher amounts of WMH volume than HE (p<0.001) and AD/MCI (p=0.002) cases [Figure 1]. This association between the presence of CAA and higher WMH volume remained independent (p=0.005) after adjustment for other relevant variables including the other diagnoses, age, gender, APOE ε4, CMB counts and vascular risk factors. Patients with AD/MCI had higher WMH volume when compared to HE (p=0.01) after adjustment for age, vascular risk factors and global DVR.
Table 1.
Demographic, genetic, biochemical, and radiographic characteristics of the subjects
| CAA, n=42 | AD/MCI, n=43 | Healthy Elderly, n=50 | |
|---|---|---|---|
| Age, mean (±SD) | 68 (10) | 74 (7.4) | 73.3 (7) |
| Female % | 26 | 40 | 58 |
| Hypertension % | 57 | 67 | 28 |
| Diabetes Mellitus % | 10 | 14 | 0 |
| Hyperlipidemia % | 38 | 74 | 14 |
| ApoE ε2, % allele frequency * | 13 | 0 | 9 |
| ApoE ε4, % allele frequency * | 37 | 32 | 8 |
| Global DVR, mean (±SD) | 1.33 (0.2) | 1.33 (0.27) | 1.17 (0.13) |
| Lobar CMB count, median (IQR) | 25 (5–57) | none | none |
| WMH volume in ml, median (IQR) | 21 (9.9–32) | 10.8 (5–16.3) | 3.2 (2–5.6) |
| WMH percent of ICV, median (IQR) | 1.2 (0.6–1.9) | 0.68 (0.3–0.9) | 0.2 (0.1–0.4) |
APOE genotype was available in all CAA patients, 33 HE and 11 AD/MCI cases.
DVR = distribution volume ratio
CMB = cerebral microbleed
WMH = white matter hyperintensities
ICV = intracranial volume
Figure 1. Comparison of WMH volume among the study cohorts.
Comparison of WMH volume among the three diagnostic categories. Patients with CAA had higher WMH volume than HE (p<0.001) and AD/MCI (p=0.002) subjects. Patients with AD/MCI had higher WMH volume when compared to HE (p=0.01). These associations remained significant after adjustment for relevant covariates.
Bivariate analysis of global DVR and WMH volumes showed a strong correlation (Spearman’s rho=0.52, p<0.001) in the CAA cohort [Figure 2]. This association remained independent (p=0.005) in a multivariate model that included age, gender, CMB counts, APOE ε4 status and vascular risk factors. Other predictors that remained associated with WMH in this model were age (p=0.02) and CMB count (p=0.02). Based on this model, a 10% increase in global DVR was associated with a 34% increase in WMH volume with other covariates held constant. Similar associations were seen expressing WMH as a percent of total intracranial volume rather than as an absolute volume. Representative PiB-PET and FLAIR images from 2 CAA patients illustrating the association between the severity of amyloid load and white matter disease are shown in [Figure 3].
Figure 2. Scatterplot of PiB retention and WMH in cerebral amyloid angiopathy (CAA).
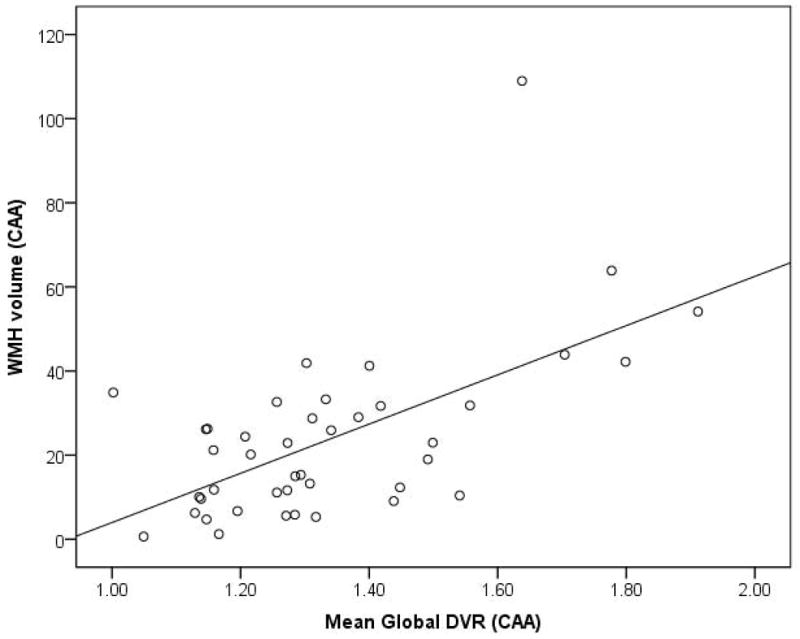
Scatterplot showing strong correlation between WMH and global DVR in cerebral amyloid angiopathy (CAA) (Spearman’s rho=0.52, p<0.001).
Figure 3. (A and B): Representative PiB PET and FLAIR MRIs showing correlation of amyloid deposition and WMH in 2 CAA patients.
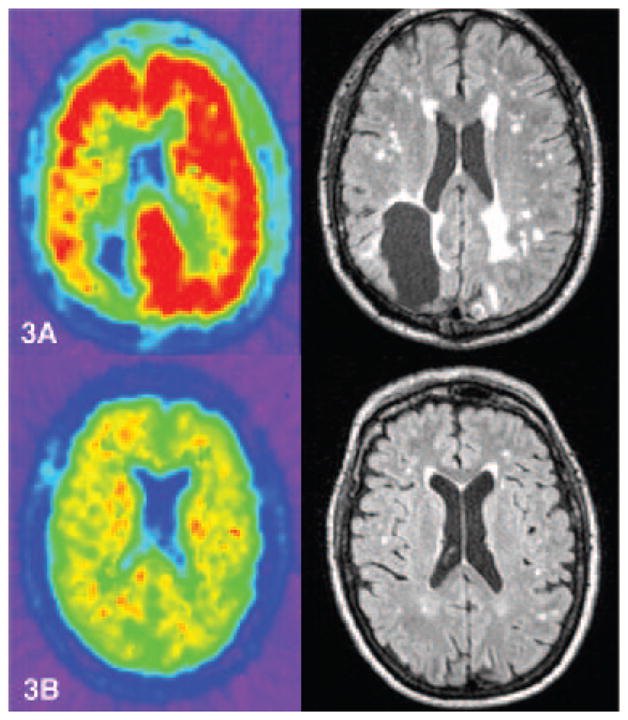
PiB PET (left) and FLAIR MRI (right) scans from 2 CAA patients with prior history of ICH, both had scans at 66 years of age. Both patients scored 30/30 on Mini-Mental Status Examination. Patient in panel 3A had a global DVR of 1.4 and WMH volume of 41ml. Patient in panel 3B had global DVR of 1.13 and WMH of 6.24ml.
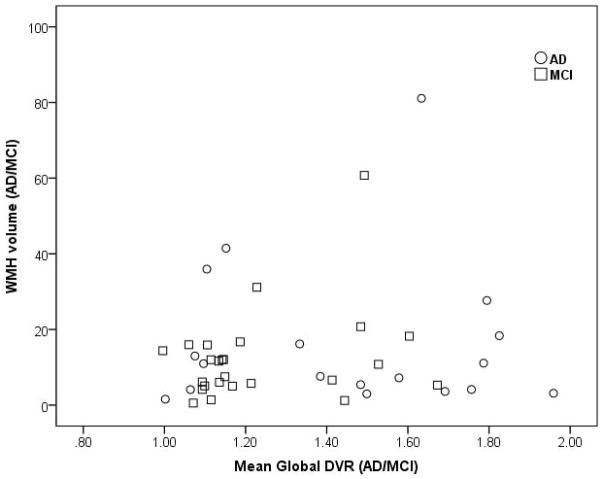
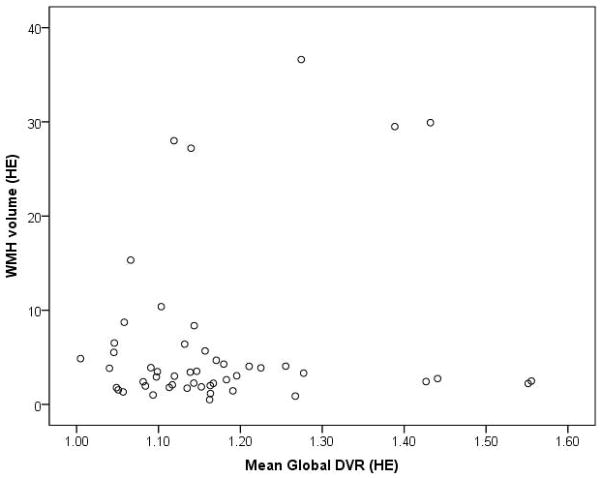
In contrast, global DVR and WMH were not correlated in AD/MCI (Spearman’s rho=0.11, p=0.47) [Figure 4] or HE (Spearman’s rho=0.01, p=0.95) [Figure 5] groups. Similar results were obtained expressing WMH as a percentage of ICV for the AD/MCI (Spearman’s rho=0.08, p=0.6) and HE cohorts (Spearman’s rho=0.02, p=0.9) as well as in multivariate regression models that included the above covariates. Similarly, repeating the analyses within the AD only or MCI only subjects or in the cognitively impaired subjects meeting criteria for AD and preclinical AD (comprised of all 18 AD subjects and 7 of the 25 MCI subjects with mean global DVR >1.4) did not yield any significant correlation between PiB retention and WMH volume. Age remained an independent predictor of WMH in all multivariate models of AD/MCI or HE subjects.
Figure 4. Scatterplot of PiB retention and WMH in patients with Alzheimer Disease (AD) and mild cognitive impairment (MCI).
Scatterplot showing no correlation between WMH and global DVR in patients with Alzheimer Disease (AD) and mild cognitive impairment (MCI) (Spearman’s rho=0.11, p=0.47).
Figure 5. Scatterplot of PiB retention and WMH in healthy elderly.
Scatterplot showing no correlation between WMH and global DVR in healthy elderly (Spearman’s rho=0.01, p=0.95).
To confirm the difference in the relationship of PiB retention to WMH volume between patients with predominantly vascular vs parenchymal amyloid pathology, we performed regression analyses of both subject groups with inclusion of an interaction term for diagnostic category x mean global DVR, along with the respective main effect terms. Controlling for other covariates (age, gender, CMB count, and hypertension), these models demonstrated significantly steeper increases in log-transformed WMH volume per unit of PiB retention in CAA relative to patients with primarily parenchymal amyloid pathology. For example, the model that included CAA vs the combined AD and PiB-positive MCI groups yielded a positive interaction coefficient of 0.95 (95% CI 0.14–1.7, p=0.02) demonstrating a significantly stronger positive correlation between PiB and WMH among CAA patients. Similar significant results were obtained in models that included all CAA and AD/MCI subjects or that included CAA and AD subjects only (p<0.05 for the interaction effect in all models).
DISCUSSION
Our data demonstrate a reasonably strong correlation between quantitative in vivo measures of brain amyloid load and white matter disease in subjects diagnosed with CAA, but not those with AD/MCI or healthy elderly controls. The association in the CAA group remained independent after adjustment for multiple covariates that may affect extent of white matter disease such as age, vascular risk factors, APOE genotype, and CMB counts. The contrast between the robust correlation in CAA and absent correlation in AD suggests that vascular, but not parenchymal amyloid burden may be an important contributor to white matter injury measured by the extent of FLAIR hyperintensities. The contrast between CAA and AD/MCI was further supported by models including an interaction term, which demonstrated a steeper association between white matter lesions and amyloid burden in CAA.
Multiple lines of evidence indicate that PiB-PET measures vascular as well as parenchymal Aβ. PiB has been found to label vascular amyloid in radiologic-pathologic correlation studies,33 and in a hereditary form of CAA devoid of fibrillar plaque amyloid.34 Sporadic, nondemented CAA patients also demonstrate higher PiB retention on PET compared to HE.18, 21 Further, increased PiB retention is associated with the presence of CAA-related hemorrhagic lesions in both a cross-sectional analysis 35 and a longitudinal study predicting future hemorrhages. 19
The current finding of association between amyloid burden and WMH highlights the pathophysiologic link between CAA and white matter injury. Although several mechanisms for WMH have been proposed,36 the most widely accepted mechanism is chronic ischemia due to small vessel diseases. Advanced CAA indeed appears to be an important contributor to small vessel dysfunction aside from its established role as a cause of ICH. Amyloid-related impairments in vascular reactivity have been demonstrated both in transgenic mouse models 37 and in CAA patients. In CAA patients, a functional transcranial Doppler study found decreased visual evoked flow velocity response in posterior cerebral arteries when compared to healthy controls,38 and a more recent functional MRI study showed delayed timing and smaller amplitude of the BOLD response to visual stimulation.29 The current results suggest that these amyloid-related mechanisms may act in a dose-dependent way to promote chronic microvascular ischemia and WMH in patients with CAA.
The absence of a similar association between PiB retention and WMH in AD or MCI suggests that amyloid burden in these subjects—likely primarily senile plaques—is not directly related to white matter injury. Although increased WMH is often observed in AD/MCI relative to HE (as in the current analysis), the mechanism is not well established.39 Possibilities include not only concomitant vascular pathologies such as CAA or arteriolosclerosis, but also nonvascular processes such as wallerian degeneration from AD-related neuronal injury. A recent autopsy study indeed found an association between neurofibrillary pathology and frontal white matter disease though extent of vascular amyloid pathology was not analyzed in this study.40 Another autopsy based study that used a simple binary measure for WMH (present/absent) did not demonstrate an association between brain amyloid load and presence of WMH in non-demented people who died of non-neurological causes.16 Our current findings do not support a direct contribution from parenchymal Alzheimer pathology to WMH. As Alzheimer plaque burden is only loosely related to other Alzheimer pathologies such as neurofibrillary lesions, however, our results do not exclude the possibility that other AD-related mechanisms might play a role in WMH. In addition to the downstream effects of neuronal injury, other potential mechanisms for AD-related white matter damage include leukocyte plugging of capillaries, alteration of thrombosis by the interaction of fibrinogen and Aβ, and convergence of hematologic and inflammatory pathways in AD.41–43
Our study has several limitations. The lack of association between PiB retention and WMH volume in the HE and AD/MCI cohorts might be due to the relatively small sample sizes. We note, however, that a slightly smaller group of CAA subjects yielded a strong and independent relationship. A second important limitation is the inability of PiB-PET to distinguish between vascular and plaque amyloid, leading to the likelihood that a portion of the PiB signal in CAA subjects reflected plaque deposits and a portion in the AD/MCI subjects was vascular. We would expect this type of “cross contamination” to reduce rather than enhance the contrast between the CAA and AD/MCI groups, however. We also note that the lack of association between PiB retention and WMH in the AD/MCI and HE groups helps exclude spurious cause-effect relationships between PiB and WMH, such as enhanced PiB retention in regions of white matter damage. No association between WMH and a global measure of PiB uptake was found in a recently published study of 54 clinically normal elderly adults, further supporting our findings in the HE population. 44
In vivo amyloid imaging has revolutionized research into the pathogenic mechanisms of AD and CAA. In the case of CAA, it allows detection in living subjects of not only the manifestations of small vessel brain disease such as WMH or CMB, but also of the small vessel pathology itself. The current study supports further use of amyloid imaging as a marker of overall vascular amyloid burden in patients diagnosed with CAA. The results also support the important possibility that reducing vascular amyloid burden might be a rational approach for limiting injury to the white matter.
Acknowledgments
This work was supported by grants from the National Institutes of Health (T32NS048005, R01 AG026484).
References
- 1.Oksala NK, Oksala A, Pohjasvaara T, et al. Age related white matter changes predict stroke death in long term follow-up. Journal of neurology, neurosurgery, and psychiatry. 2009;80:762–766. doi: 10.1136/jnnp.2008.154104. [DOI] [PubMed] [Google Scholar]
- 2.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Bmj. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 4.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 5.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 6.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. Journal of neurology, neurosurgery, and psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 8.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 9.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Conde J, Biffi A, Rahman R, et al. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41:437–442. doi: 10.1161/STROKEAHA.109.563502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovasc Dis. 2002;13 (Suppl 2):7–10. doi: 10.1159/000049143. [DOI] [PubMed] [Google Scholar]
- 12.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 13.Swartz RH, Stuss DT, Gao F, Black SE. Independent cognitive effects of atrophy and diffuse subcortical and thalamico-cortical cerebrovascular disease in dementia. Stroke. 2008;39:822–830. doi: 10.1161/STROKEAHA.107.491936. [DOI] [PubMed] [Google Scholar]
- 14.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 15.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 16.Rutten-Jacobs LC, de Leeuw FE, Geurts-van Bon L, et al. White Matter Lesions Are Not Related to beta-Amyloid Deposition in an Autopsy-Based Study. Current gerontology and geriatrics research. 2011;2011 doi: 10.1155/2011/826862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uro-Coste E, Russano de Paiva G, Guilbeau-Frugier C, et al. Cerebral amyloid angiopathy and microhemorrhages after amyloid beta vaccination: case report and brief review. Clin Neuropathol. 2010;29:209–216. doi: 10.5414/npp29209. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 19.Gurol ME, Dierksen G, Betensky R, et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. 2012;79:320–326. doi: 10.1212/WNL.0b013e31826043a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 21.Ly JV, Donnan GA, Villemagne VL, et al. 11C-PIB binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology. 2010;74:487–493. doi: 10.1212/WNL.0b013e3181cef7e3. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 25.Becker JA, Hedden T, Carmasin J, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. The New England journal of medicine. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 27.Mathis CA, Wang Y, Holt DP, et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. Journal of medicinal chemistry. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 28.Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79:1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumas A, Dierksen GA, Gurol ME, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72:76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. The Lancet Neurology. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuro Image. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Jaccard J, Turrisi R. Interaction effects in multiple regression. 2. vii. Thousand Oaks, Calif: Sage Publications; 2003. p. 92. [Google Scholar]
- 33.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg SM, Grabowski T, Gurol ME, et al. Detection of isolated cerebrovascular beta-amyloid with Pittsburgh compound B. Ann Neurol. 2008;64:587–591. doi: 10.1002/ana.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dierksen GA, Skehan ME, Khan MA, et al. Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy. Ann Neurol. 2010;68:545–548. doi: 10.1002/ana.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 37.Niwa K, Younkin L, Ebeling C, et al. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith EE, Vijayappa M, Lima F, et al. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology. 2008;71:1424–1430. doi: 10.1212/01.wnl.0000327887.64299.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polvikoski TM, van Straaten EC, Barkhof F, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–2078. doi: 10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaffer CB. Leukocyte Plugging of Capillaries Reduces Brain Blood Flow in Mouse Models of Alzheimer’s Disease. In: Fillit H, editor. Targeting the Vasculature in Alzheimer’s Disease and Vascular Cognitive Impairment. New York: New York Academy of Sciences; 2012. [Google Scholar]
- 42.Ahn HJ, Zamolodchikov D, Cortes-Canteli M, et al. Alzheimer’s disease peptide beta-amyloid interacts with fibrinogen and induces its oligomerization. Proc Natl Acad Sci U S A. 2010;107:21812–21817. doi: 10.1073/pnas.1010373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suo Z, Citron BA, Festoff BW. Thrombin: a potential proinflammatory mediator in neurotrauma and neurodegenerative disorders. Current drug targets. Inflammation and allergy. 2004;3:105–114. doi: 10.2174/1568010043483953. [DOI] [PubMed] [Google Scholar]
- 44.Marchant NL, Reed BR, DeCarli CS, et al. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol Aging. 2012;33:1006, e1025–1036. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]