Abstract
Syk is a 72 kDa non-receptor tyrosine kinase that is best characterized in hematopoietic cells. While Syk is pro-tumorigenic in some cancer cell types, it also has been reported as a negative regulator of metastatic cell growth in others. An examination of the RelA (p65) subunit of NF-κB expressed in MCF7 breast cancer cells indicated that either treatment with pervanadate or stable expression of Syk protected RelA from calpain-mediated proteolysis. Similar results were observed with the tyrosine phosphatase, PTP1B, another sensitive calpain substrate. The activity of calpain in MCF7 cell lysates was inhibited by both treatment with hydrogen peroxide and expression of Syk, the former due to oxidative inactivation of calpain and the latter to enhanced expression of calpastatin (CAST), the endogenous calpain inhibitor. The level of CAST was elevated in the cytosolic fraction of Syk-positive breast cancer cells resulting in more CAST present in complex with calpain in cell lysates. The high levels of CAST coincided with elevated basal levels of calcium—and of intracellular calpain activity—in Syk-expressing cells resulting from decreased levels of Bcl-2, an inhibitor of IP3-receptor-mediated calcium release. The inhibition of cellular calpain stimulated the Syk-mediated enhancement of NF-κB induced by TNF-α, enhanced tyrosine phosphorylation resulting from integrin crosslinking, and increased the localization of Syk to the plasma membrane.
Keywords: Syk, Calpain, Calpastatin, PTP1B, NF-κB, Bcl-2
1. Introduction
Syk is a 72 kDa protein-tyrosine kinase that is widely distributed among hematopoietic cells and is found as well in several non-immune cell types including many epithelial cells [1–5]. Syk can be activated through the crosslinking of various cell surface receptors including those for antigens on lymphocytes, receptors for the Fc regions of antigen-IgG and -IgE complexes on monocytes, macrophages and mast cells [1, 2], and integrins in both immune and non-immune cells [6–9]. In B cells, for example, Syk plays a critical role in coupling the antigen receptor (BCR) to downstream pathways that regulate activation, differentiation and proliferation. Consequently, impaired differentiation of B-lineage cells is observed in SYK knockout mice [10. 11]. The pro-inflammatory cytokine, tumor necrosis factor-α (TNF-α), also is reported to activate Syk in various cell types including Jurkat T cells and epithelial cells where the expression of Syk enhances the TNF-induced activation of NF-κB [12–14].
In cancer cells, Syk has been described as both an enhancer and suppressor of tumorigenesis. The growth and survival of subsets of adult myeloid leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma, retinoblastoma and pancreatic carcinoma are dependent on Syk [15–20]. In contrast, Syk was identified as a tumor suppressor in breast cancer when a correlation between allelic loss of the human SYK locus on chromosome 9q22 and lymph node metastasis of primary breast cancer was reported [21]. While Syk is expressed in normal human breast epithelium, its level is decreased or lost in highly malignant and invasive breast cancer cells [3] due to hypermethylation of a CpG-rich fragment in the 5’-regulatory region of the gene [22]. The overexpression of a kinase-deficient mutant of Syk or the downregulation of Syk expression results in increased anchorage-independent growth, and motility [3, 23]. The tumor suppressing function of Syk has been attributed to its ability to interrupt normal cell division through its effects on mitosis, its ability to repress transcription through interactions with Sp1 and its ability to inhibit motility and promote cell-cell interactions [3, 24–26].
Cell motility and adhesion also are modulated by the calpain-calpastatin system, which comprises three molecules: two Ca2+-dependent proteases, µ-calpain and m-calpain, and calpastatin (CAST), which is the only known endogenous specific inhibitor of calpain. Both µ-and m-calpain are heterodimers comprising an identical 28-kDa regulatory subunit and a 76–80 kDa catalytic subunit. The catalytic subunits share 55–65% sequence similarity. CAST has a large number of isoforms of different molecular weights that are expressed in a species- and tissue-specific manner as a result either of the use of different promoters or alternative splicing of the CAST gene transcript [27–30].
A variety of intracellular proteins have been identified as substrates for calpain including the cytoskeletal proteins β-catenin, E-cadherin [31] and β-spectrin [32]; kinases and phosphatases including focal adhesion kinase (FAK) [33], protein kinase C [34], and protein tyrosine phosphatase 1B (PTP1B) [35, 36]; arrestin [37]; and several transcription factors including c-Jun, c-Fos [38, 39] and p53 [40]. As a result, the calpain system plays multiple roles under different physiological scenarios including, but not limited to, modulation of cell motility, regulation of signal transduction, regulation of gene expression, control of cell cycle, and regulation of apoptosis. Under normal conditions, the activity of calpain is tightly regulated by the intracellular concentration of calcium, its level of expression, post-translational modifications [41, 42], and the balance between the level of the protease and that of its endogenous inhibitor CAST [43]. Dysregulation of the calpain system is related to a wide range of pathologies such as muscular dystrophies [44], myocardial infarcts [45], neural degenerative diseases [46], and tumor invasion [47]. Even though limited, documentation in the literature has suggested some potential for reciprocal regulation between Syk and the calpain system [48, 49]. Given the critical role that calpain plays in cancer metastasis, the potential interactions between Syk and calpain could provide a better understanding of the tumor promoting and suppressing functions of Syk in human breast epithelial cells.
2. Materials and methods
2.1. Plasmids and DNA constructs
The series of Syk-expressing constructs (wild type and catalytically inactive (kinase dead) Syk) with a C-terminal EGFP tag was generated in the pEGFP-N2 vector (BD Biosciences, CA) as described previously [50]. The mouse RelA cDNA was cloned into cFLAG-pcDNA backbone between the HindIII and XhoI restriction sites. A pCMV-SPORT6 plasmid overexpressing untagged human calpastatin (I.M.A.G.E. Clone ID: 3878564) was purchased from American Type Culture Collection (ATCC). The calpastatin cDNA was then amplified by PCR and cloned into the pCMV-myc vector (BD Biosciences) between the SalI sites to generate a myc-tagged calpastatin construct (pCMV-myc-CAST). The NF-κB-driven firefly luciferase reporter pNF-κBluc was obtained from Stratagene. The internal control plasmid pRL-TK was purchased from Promega. The GCaMP3 (plasmid #22692), RelA-FLAG (plasmid #20012) and FLAG-Bcl-2 (plasmid #18003) expression plasmids were obtained from Addgene.
2.1. Cell culture
Three lines of MCF7 cells were used in this study. One was Syk-deficient, purchased from BD Biosciences (MCF7-BD) [14]; another was positive for endogenous Syk, obtained from American Type Culture Collection (ATCC) (MCF7-ATCC). The MCF7-Syk cell line was established by stably reconstituting MCF7-BD cells with Syk-EGFP. MDA-MB-231 breast cancer cells were obtained from ATCC. A line of MDA-MB-231 cells expressing Syk-EGFP in response to tetracycline were described previously [26]. All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 7.5% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin.
2.2. Antibodies and other reagents
Primary antibodies against Syk (N19), Myc (9E10), HA (Y-11), calpain 4 (capn4) (P-1), and RelA (F-6) were obtained from Santa Cruz Biotechnology. Anti-CAST (for Western blotting), anti-calpain 1 (capn1), and anti-PARP antibodies were from Cell Signaling Technology. Anti-GAPDH antibody was from Ambion. Anti-phosphotyrosine antibody (4G10), anti-CAST (for immunostaining), mouse anti-human CD29 (integrin β1), and protein A-Sepharose beads covalently conjugated with 4G10 for immunoprecipitation were purchased from Millipore. Monoclonal anti-FLAG M2 antibody was purchased from Sigma-Aldrich. GFP-Trap-A beads for immunoprecipitation of GFP-fusion proteins were purchased from Chromotek. Both goat anti-rabbit and goat anti-mouse horseradish peroxidase-conjugated secondary antibodies for Western blotting were from Thermo Fischer Scientific. Alexa Flour 594 labeled goat anti-mouse IgG for immunostaining was obtained from Invitrogen. Calpeptin and staurosporine were purchased from Sigma-Aldrich. Calpain 1 purified from human erythrocytes and the recombinant CAST peptide were obtained from Calbiochem. t-Butoxycarbonyl-leucine-methionine-7-amino-4-chloromethylcoumarin (t-Boc-Leu-Met-CMAC) was purchased from Invitrogen. Recombinant human TNF-α was purchased from R&D Systems. Calpain-Glo Protease Assay and Dual-Luciferase Reporter Assay Systems were from Promega. ProteSEEKER protease inhibitor kit was obtained from G-Biosciences. Rhod-3 imaging kit was purchased from Invitrogen.
2.3. Immunoprecipitation
For immunoprecipitation experiments, cells were lysed in buffer containing 50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 1% NP-40, 1 mM phenylmethylsulfonylfluoride (PMSF), 1 mM Na3VO4, and 0.2% (v/v) protease inhibitor cocktail. Pre-cleared lysate was incubated with protein G-Sepharose beads pre-mixed with primary antibody and agitated at 4°C for 4 h. The immune complexes were washed four times with lysis buffer and resuspended in SDS sample buffer. In some experiments, cells were treated with sodium orthovanadate (50 µM) mixed with H2O2 (50 mM) to generate pervanadate (PV), which was added to the culture medium for a 15 min incubation on ice just before cells were harvested. Proteins were resolved by SDS-PAGE and detected by Western blotting using the appropriate primary antibody and horseradish peroxidase-conjugated secondary antibody using enzyme-linked chemiluminescence (ECL) reagents (PerkinElmer).
2.4. Immunofluorescence
Cells seeded on coverslips were fixed in 3.7% formaldehyde, permeablized and blocked in phosphate-buffered saline (PBS) containing 0.5% Triton X-100 and 5% goat serum and stained with anti-CAST primary antibody. Bound antibody was detected with Alexa Fluor® 594 goat anti-mouse IgG (1:1000). 4’,6’-Diamidino-2-phenylindole (DAPI) was added for the last 5 min. Slides were examined by an Olympus BH2-RFCA fluorescence microscope with 60X objective equipped with a Sony DXC-950 3CCD color camera and Northern Eclipse 5.0 software from Empix Imaging (Mississauga, Canada).
2.5. Subcellular fractionation
MCF7-BD or MCF7-Syk cells were incubated on ice for 10 min in buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM PMSF, 1 mM Na3VO4, 0.2% protease inhibitor cocktail). NP-40 was added to a final concentration of 0.2% to lyse cells. Lysates were centrifuged at 18,400 × g for 30 s. The supernatant was saved as the cytosol/membrane fraction. The nuclear pellet was washed twice with buffer A and then resuspended in 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1mM EGTA, 1 mM DTT, 1 mM PMSF, 1 mM Na3VO4, and 0.2% protease inhibitor cocktail. The supernatant of a sample centrifuged at 18,400 × g for 5 min was removed as the nuclear fraction.
2.6. In vitro proteolysis assay of RelA-FLAG
A plasmid encoding FLAG-tagged RelA was transiently transfected into cells using FuGENE 6 transfection reagent. Cells were lysed as described above. In some experiments, whole cell lysates were harvested in buffer containing 1% SDS, 50 mM Tris-HCl, pH 8.0, and 150 mM NaCl. Extracts were then reconstituted with lysis buffer containing 1% NP-40 to dilute the final concentration of SDS to 0.1%. Protein G-Sepharose beads pre-incubated with anti-FLAG (M2) antibody were used to immunoprecipitate RelA-FLAG. The resulting immune complex was resuspended in proteolysis buffer (25 mM Tris-HCl, pH 7.5, 10 mM KCl, 10 mM CaCl2, 0.1% Triton X-100) and incubated with 1 µg purified human calpain 1 with or without 20 µM recombinant human CAST peptide at 37°C for 30 min with gentle shaking. The reaction was terminated by addition of an equal volume of SDS-sample buffer.
2.7. Dual reporter luciferase assay
MCF7-BD cells were seeded into a 24-well plate (1 × 105 cells/well) and co-transfected with 0.2 µg/well pNF-κB-Luc reporter plasmid and 0.02 µg/well pRL-TK internal control plasmid, together with indicated amounts of other expression plasmids. Six hours after transfection, 10 ng/ml recombinant human TNF-α was applied to the cell culture for an 18 h incubation. Activities of both the firefly luciferase driven by an NF-κB specific promoter and the Renilla luciferase control driven by a TK promoter were measured with a luminometer. The relative luciferase units (RLU) were calculated as the ratio between the firefly and Renilla activity readings.
2.8. In vitro calpain protease assay
To measure the activity of calpain in cell lysates, calpain was immunoprecipitated with a capn 4 antibody from the lysates of MCF7 and MDA-MB-231 cells, treated or not with 50 µM pervanadate. The resulting immune complex was resuspended in calpain activity assay buffer (Calbiochem) and incubated with 50 µl calpain-Glo reagent (Promega) at room temperature in the dark for 15 min with or without 5 µM recombinant CAST peptide (Calbiochem). The luminescence generated from each sample was measured by a luminometer. Calpain-specific activity in the lysates was calculated as the readout difference between samples from the same immune complex, incubated with or without CAST.
2.9. Intracellular calcium imaging
To evaluate qualitatively the calcium level in living cells, a Rhod-3 imaging kit (Invitrogen) was used. MCF7-BD and MCF7-Syk cells were seeded onto coverslips in a 6-well plate. Rhod 3-AM (10 µM) was loaded into the cells passively for 30 min in the dark at room temperature in the presence of 1× PowerLoad concentrate and 2.5 mM probenecid. Cells were incubated for 30 min in the dark at room temperature, washed once with DMEM, fixed in 3.7% formaldehyde and examined under the fluorescence microscope. MCF7-BD or MDA-MB-231 cells were transfected with plasmids encoding the GCaMP3 calcium indicator [51] and FLAG-Bcl-2 as indicated. Cells were placed in a black-walled 96-well plate and assayed for calcium using a Synergy 4 plate reader and Gen5 software (BioTek).
2.10. Integrin crosslinking
MCF7 cells were serum starved overnight, collected in Hank’s balanced salt solution (HBSS) supplemented with 10 mM EDTA and washed twice with serum free medium. MCF7 cells in suspension in serum-free DMEM were incubated with 2.5 µg/ml mouse anti-human integrin β1 on ice for 30 min, followed by goat anti-mouse secondary antibody at 37°C for 15 min. Cells were harvested in 1% NP-40 lysis buffer and lysates examined by SDS-PAGE and Western blotting with anti-phosphotyrosine (4G10).
2.11. Spreading assay on fibronectin
To prepare fibronectin coated coverslips, 1 ml DMEM containing 20 µg/ml fibronectin was added to each sterilized coverslip placed in a 6-well plate. The plate was rocked at room temperature for 1 h and stored at 4 °C overnight. MCF7-Syk cells were pre-treated with calpeptin (20 µM) or DMSO carrier alone and serum-starved overnight, removed from the plate using HBSS buffer supplemented with 10 mM EDTA, and plated on fibronectin-coated coverslips for 1 h at 37°C. Attached, spread cells were fixed by 3.7% formaldehyde and examined under the fluorescence microscope.
3. Results
3.1. The expression of Syk inhibits partial proteolysis of RelA in MCF7 cell lysates
Syk plays an enhancing role in the TNF-α induced activation of NF-κB in MCF7 cells [14]. In human umbilical endothelial cells, treatment with TNF-α induces the tyrosine phosphorylation of RelA and activation of NF-κB [52]. In this system, Syk was reported to interact directly with endogenous RelA and phosphorylate it on tyrosine [53]. To investigate a similar interaction between Syk and RelA in breast epithelial cells, we co-transfected plasmids encoding a C-terminally EGFP-tagged Syk and a C-terminally FLAG-tagged RelA into a line of MCF7 cells (MCF7-BD) that lack endogenous Syk. However, we were unable to detect through co-immunoprecipitation assays an interaction between Syk-EGFP and either RelA-FLAG or endogenous RelA or the tyrosine phosphorylation of RelA or RelA-FLAG (Supplementary Fig. S1 and S2).
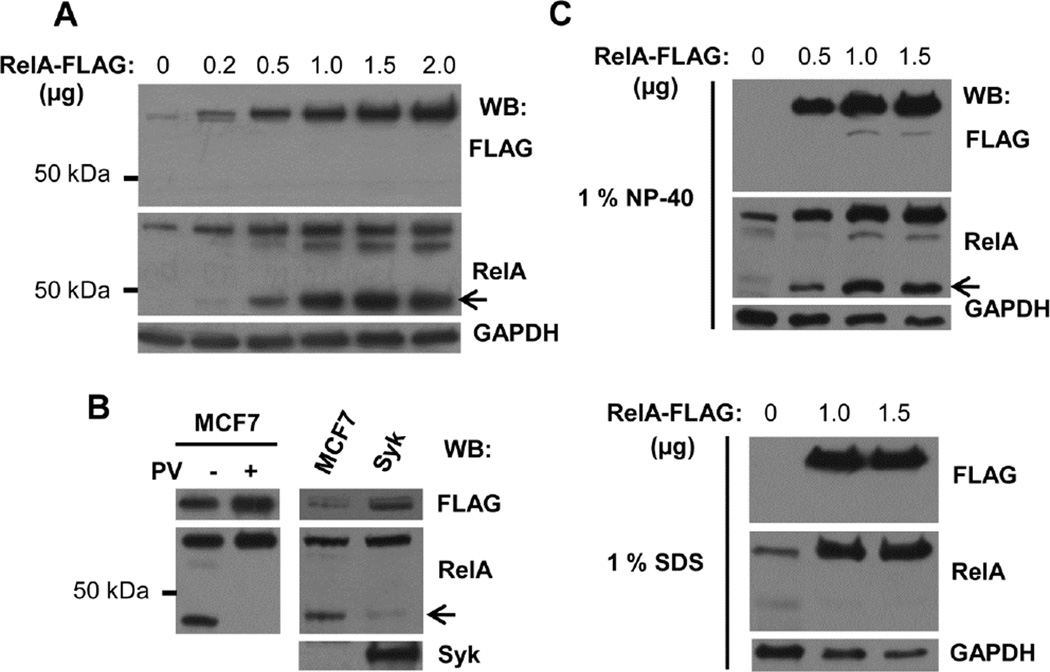
Western blots of lysates from cells transfected to express RelA-FLAG revealed, in addition to the 70 kDa full-length RelA-FLAG, two additional bands, a prominent band migrating at 45 kDa and a minor band near 60 kDa whose intensities increased with increasing amounts of RelA-FLAG plasmid. This suggested that RelA-FLAG was susceptible to proteolysis (Fig. 1A). These two bands were not recognized by an anti-FLAG antibody, indicating that the partial proteolysis occurred at the C-terminus where the FLAG tag was located. Interestingly, when the expression of Syk (as Syk-EGFP) was restored to the Syk-deficient cells, the extent of proteolysis of RelA-FLAG was reduced significantly as compared to the Syk-deficient cells (Fig. 1B).
Fig. 1.
RelA-FLAG is proteolytically processed at its C-terminus in cell lysates. (A) Increasing amounts of RelA-FLAG expression plasmid were transfected into MCF7-BD cells. Cell lysates (1% NP-40) were resolved by SDS-PAGE, followed by Western blot using antibodies against FLAG and RelA. (B) RelA-FLAG was transiently overexpressed in MCF7-BD (MCF7) and MCF7-Syk (Syk) cells. Cells were treated or not treated with 50 µM pervanadate (PV) on ice for 15 min. Lysates were analyzed by Western blotting using antibodies against FLAG, RelA, and Syk. (C) Increasing amounts of RelA-FLAG expression plasmid were transfected into MCF7-BD cells. Cells were harvested in lysis buffer containing either 1% NP-40 (top panel) or 1% SDS (bottom panel). Cell lysates were analyzed by Western blotting using antibodies against FLAG and RelA. GAPDH served as a protein loading control. Arrows indicate the major truncated RelA polypeptide.
To determine if the cleavage of RelA-FLAG had occurred prior to or subsequent to cell lysis, we examined the appearance of the truncated RelA-FLAG polypeptides in lysates prepared with buffer containing either 1% NP-40 or 1% SDS. The RelA fragments were dramatically reduced in level in cell lysates harvested in lysis buffer containing SDS, indicating that this partial proteolysis of RelA-FLAG was primarily a post-lysis event (Fig. 1C).
3.2. RelA is a substrate of calpain
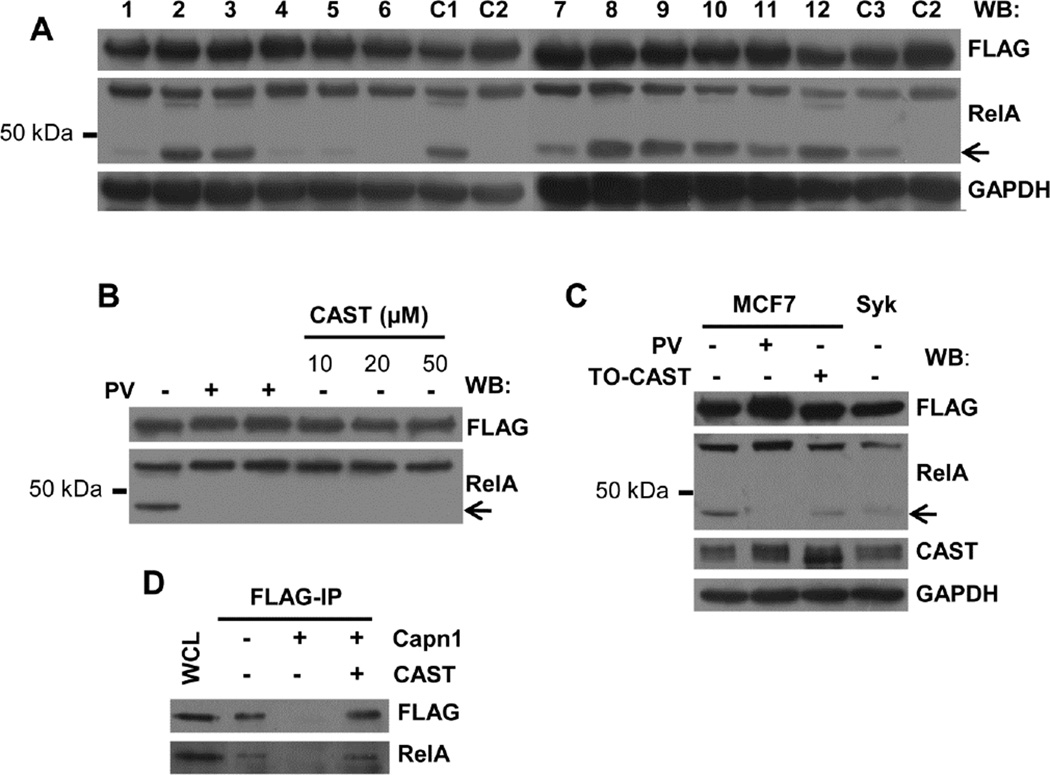
To characterize further the proteolysis of RelA, we treated lysates of MCF7-BD cells in which RelA-FLAG was overexpressed with a variety of different protease inhibitors. The production of the 45 kDa truncated polypeptide was decreased in samples treated with antipain, chymostatin, E-64, or leupeptin, all of which are inhibitors of cysteine proteases (Fig. 2A). Proteolysis also was blocked by the addition of EDTA. These observations indicated the involvement of a cysteine protease whose activity was dependent on metal ions. This suggested the possible involvement of calpain, a calcium-dependent cysteine protease.
Fig. 2.
The post-lysis proteolysis of RelA is catalyzed by calpain. (A) Lysates (1% NP-40) from MCF7-BD cells expressing RelA-FLAG were harvested using buffer supplemented without (C1) or with different protease inhibitors (1, antipain; 2, aprotinin; 3, bestatin; 4, chymostatin; 5, E-64; 6, EDTA; 7, leupeptin; 8, pepstatin; 9, AEBSF; 10, phosphoramidon; 11, PMSF; 12, Z-VAD-FMK). Additional controls were lysates of PV-treated cells harvested in standard lysis buffer (C2) and lysates of DMSO-treated cells harvested in standard lysis buffer as the vehicle control for Z-VAD-FMK (C3). Anti-FLAG and RelA antibodies were used for Western blotting. GAPDH served as a loading control. The arrow indicates the major truncation product of RelA. (B) MCF7-BD cells expressing RelA-FLAG were treated with or without pervanadate (PV) as indicated. Increasing concentrations of recombinant human CAST peptide was included in the lysis buffer used for untreated cells. Lysates were analyzed by Western blotting using anti-FLAG and anti-RelA. The arrow indicates the major truncation product of RelA. (C) MCF7-BD (MCF7) or MCF7-Syk (Syk) cells expressing RelA-FLAG were either treated with or without pervanadate (PV) or were transiently transfected with a CAST expression plasmid (TO-CAST). Lysates were Western blotted using antibodies against FLAG, RelA, and CAST. GAPDH served as the loading control. (D) Whole cell lysates (WCL) were collected from MCF7-BD cells expressing RelA-FLAG using extraction buffer containing 1% SDS. RelA-FLAG was immunoprecipitated using anti-FLAG antibody and incubated at 37 °C for 30 min with either 1 µg purified calpain 1 alone or together with 20 µM recombinant CAST peptide. Both the cell lysate and the immune complexes were resolved by SDS-PAGE and Western blotted using antibodies against FLAG, and RelA.
To determine if calpain was responsible for the proteolysis of RelA-FLAG, we added a recombinant peptide inhibitor derived from human CAST domain I to the lysate of MCF7-BD cells expressing RelA-FLAG and examined the amount of the truncated RelA polypeptide that was formed. The addition of the CAST-derived peptide greatly decreased the proteolysis of RelA-FLAG (Fig. 2B). Similarly, when the level of cellular CAST in MCF7-BD cells was elevated by transient transfection with a full-length CAST expression plasmid, we observed a significant reduction in the generation of the 45 kDa RelA polypeptide in cell lysates (Fig. 2C). Finally, µ-calpain could readily digest immunoprecipitated RelA-FLAG and this proteolysis was rescued by the addition of the recombinant CAST peptide (Fig. 2D).
3.3. Inactivation of calpain by hydrogen peroxide
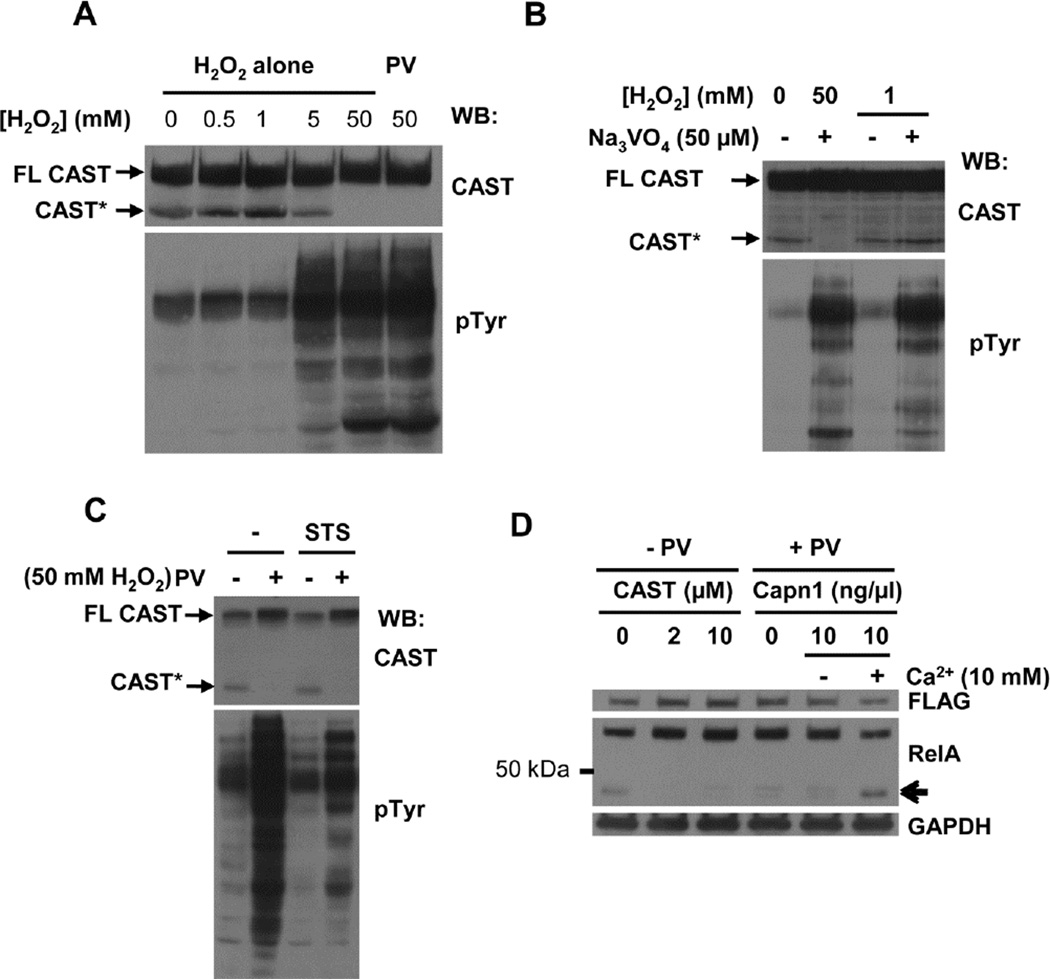
The pre-treatment of cells with pervanadate, a mixture of H2O2 and sodium orthovanadate, abrogated the appearance of the RelA proteolytic product in cell lysates (Fig. 1B, Fig. 2B and C). The sensitivity of the calpain active site cysteine to oxidation, which inhibits its proteolytic activity [54–56], could explain the loss of protease activity in lysates from pervanadate-treated cells. To test this, we treated MCF7-BD cells with different concentrations of hydrogen peroxide and monitored the appearance of truncated CAST migrating as a 70 kDa polypeptide on SDS-polyacrylamide gels as an indicator of calpain activity in cell lysates since CAST is not only an inhibitor of calpain, but also an in vitro substrate. The treatment of cells with increasing concentrations of H2O2 decreased the appearance of the 70 kDa CAST fragment in the cell lysates, consistent with oxidative inhibition of calpain (Fig. 3A).
Fig. 3.
Inhibition of calpain activity by hydrogen peroxide. (A) MCF7-BD cells were pretreated with different concentrations of H2O2 or 50 µM PV (containing 50 mM H2O2). Lysates (1% NP-40) were analyzed by Western blotting with antibodies against CAST and phosphotyrosine (pTyr). Arrows indicate full-length (FL-CAST) and truncated (CAST*) CAST. (B) MCF7-BD cells were pretreated with H2O2 (0, 1 or 50 mM) with or without 50 µM Na3VO4. Lysates (1% NP-40) were analyzed by Western blotting with antibodies against CAST and phosphotyrosine (pTyr). (C) MCF7-BD cells pretreated with DMSO (−) or 1 µM staurosporine (STS) were treated on ice for 15 min with pervanadate (PV). Cell lysates (1% NP-40) were analyzed by Western blotting with antibodies against CAST and phosphotyrosine (pTyr). Arrows indicate full-length (FL-CAST) or truncated (CAST*) CAST. (D) Lysates from MCF7-BD cells pretreated without (−PV) or with (+PV) pervanadate were prepared in lysis buffer containing a recombinant CAST peptide (0, 2, or 10 µM) or 10 ng/µl purified calpain 1 with or without 10 mM CaCl2. Lysates were analyzed by Western blotting using antibodies against FLAG and RelA. GAPDH served as the loading control. The arrow indicates the major truncation product of RelA.
It is known that the treatment of cells with H2O2 also inhibits the activity of protein-tyrosine phosphatases (PTPs) and it has been suggested that calpain is inhibited as a consequence of the inhibition of PTP activity [57]. The decrease in calpain activity observed in lysates of cells treated with H2O2 occurred concomitantly with an increase in protein-tyrosine phosphorylation, which is consistent with an inhibition of PTP activity (Fig. 3A). However, the treatment of cells with a lower concentration of H2O2 (≤1 mM) was sufficient neither to inhibit calpain activity nor to induce extensive protein-tyrosine phosphorylation (Fig. 3B). When a small amount of sodium orthovanadate was added to the 1 mM H2O2 to generate pervanadate, a mixture of potent PTP inhibitors, protein tyrosine phosphorylation was elevated, but calpain activity was not affected (Fig. 3B). Thus, the inhibition of calpain under oxidizing conditions was independent of the inhibition of PTP activity. Furthermore, a reduction in overall tyrosine phosphorylation resulting from the addition to cells of the kinase inhibitor staurosporine failed to restore the calpain activity to lysates of cells pretreated with pervanadate (Fig. 3C). The addition of purified human erythrocyte calpain 1 (µ-capn) plus calcium to the lysate of pervanadate-pretreated cells restored the appearance of the 45 kDa truncated RelA polypeptide (Fig. 3D).
3.4. Syk and pervanadate decrease calpain activity in immune complexes
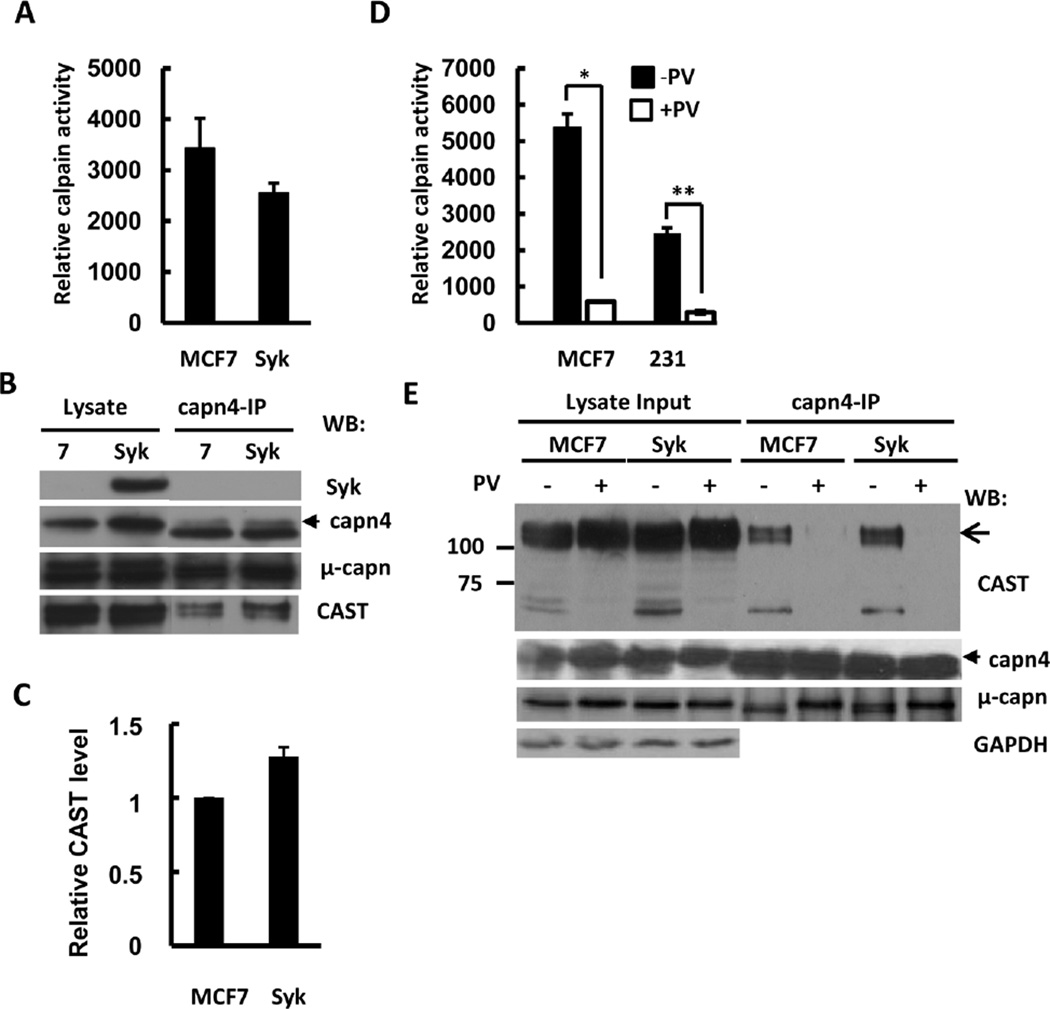
Since the expression of Syk or the treatment of cells with pervanadate (containing a high concentration of hydrogen peroxide) reduced the partial proteolysis of calpain substrates in cell lysates, we evaluated the effect of each on the proteolytic activity of calpain using an exogenous substrate. Endogenous calpain was immunoprecipitated from lysates of MCF7-BD or MCF7-Syk cells. A luminogenic calpain substrate (Suc-LLVY-aminoluciferin) was used to measure the activity of the resulting calpain-containing immune complexes. As shown in Fig. 4A, the expression of Syk resulted in a significant reduction in calpain activity. An even larger decrease in activity was observed in immune complexes isolated from MCF7-BD cells or MDA-MB-231 cells that had been pretreated with pervanadate (Fig. 4D).
Fig. 4.
Regulation of calpain activity and CAST level by Syk. (A) Calpain was immunoprecipitated from 1% NP-40 lysates of MCF7-BD (MCF7) or MCF7-Syk (Syk) cells with anti-calpain 4 antibodies and examined for protease activity using the Calpain-Glo protease assay in the presence or absence of 5 µM recombinant human CAST peptide. Levels of CAST-inhibitable activity are shown in relative light units. The data represent means ± SE (n = 6). (B) Lysates and anti-calpain 4 immune complexes (capn4-IP) from the experiment in panel A were analyzed by Western blotting with antibodies against Syk, calpain 4 (capn4), µ-calpain (µ-capn) and CAST. (C) The relative level of CAST in the anti-calpain 4 immune complexes was compared between MCF7-BD (MCF7) and MCF7-Syk (Syk) cells. The level of CAST in the complexes from MCF7-BD cells was normalized to a value of 1.0. The data represent the mean ± SE (n = 3). (D) Calpain was immunoprecipitated from 1% NP-40 lysates of MCF7-BD (MCF7) or MDA-MB-231 (231) cells with anti-calpain 4 antibodies and examined for protease activity using the Calpain-Glo protease assay in the presence or absence of 5 µM recombinant human CAST peptide. Relative levels of CAST-inhibitable activity are shown. The data represent means ± SE (n = 4). *P<0.05 when compared to non-treated control; **P<0.01 when compared to non-treated control. (E) Calpain was immunoprecipitated with anti-calpain 4 antibodies from 1% NP-40 lysates of MCF7-BD (MCF7) or MCF7-Syk (Syk) cells that were pretreated without or with pervanadate (PV). Lysates and anti-calpain 4 immune complexes (capn4-IP) were analyzed by Western blotting with antibodies against Syk, CAST, calpain 4 (capn4), µ-calpain (µ-capn) and CAST.
A major mode of regulation of calpain, other than the binding of calcium, is the extent of its association with its endogenous inhibitor, CAST. To examine an association between the protease and its inhibitor, we immunoprecipitated the complex using antibodies against the small regulatory subunit of calpain, capn4. More CAST was present in the complex with capn4 in lysates from MCF7-Syk cells as compared to MCF7-BD cells, consistent with a lower level of protease activity in these complexes (Fig. 4B and C). CAST associated robustly with capn4 in immune complexes isolated from MCF7-BD or MCF7-Syk cells, but not from cells that were treated with pervanadate under conditions that led to the inhibition of calpain (Fig. 4E). The large catalytic subunit of µ-calpain that was present in the anti-capn4 immune complexes migrated as a single band in both the lysate and in immune complexes from pervanadate-treated cells, but as a doublet in the immune complexes from control cells. Since a shorter form of the protease is generated by autolysis, the capn1 doublet observed in the immune complex from untreated cells likely arose from the generation of the smaller, autolyzed form. If EDTA/EGTA was added to cell lysates to chelate calcium, no CAST was detected in the immune complexes and both the interactions between calpain and CAST or between the large and small subunits of calpain were abolished (Fig. S3).
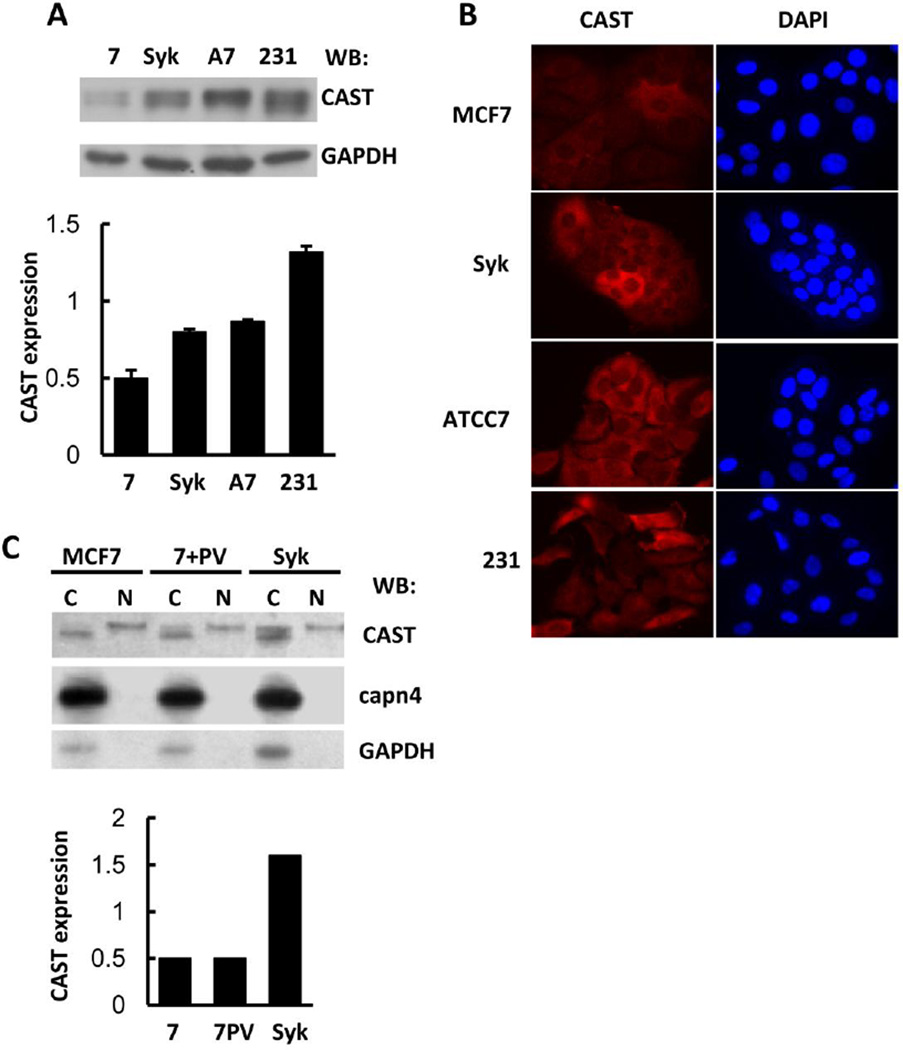
3.5. CAST is more highly expressed in Syk-positive breast cancer cells, particularly in the cytosolic fraction
To investigate factors that determined the difference in calpain activity between lysates of Syk-negative and -positive cells, we examined the relative expression levels of the protease and its endogenous inhibitor. Analyses by Western blotting revealed no significant difference in the expression level of calpain between MCF7-BD and MCF7-Syk cells. However, a substantial difference was observed in the expression level of CAST with a higher level in the MCF7-Syk cells as compared to the Syk-negative MCF7-BD cells (Fig. 5A). We also examined the expression of CAST in MCF7-ATCC, which is a line of MCF7 cells that expresses normal levels of endogenous Syk [14]. The Syk-positive MCF7-ATCC cells expressed CAST at a level comparable to MCF7-Syk cells, which was higher than that observed in MCF7-BD cells. We also examined MDA-MB-231, which is a highly metastatic breast cancer cell line that lacks any expression of Syk. These cells expressed the highest level of CAST among the cell lines examined. Correspondingly, they also exhibited a lower level of calpain activity in anti-calpain immune complexes as compared to MCF7-BD cells (Fig. 4D). These differences in the expression of CAST were confirmed by immunofluorescence analysis of all four cells using antibodies against the endogenous protein (Fig. 5B). Thus, the level of calpain activity measured in lysates from cells or in anti-calpain immune complexes varies between different cell types, but is inversely related to the level of expression of CAST within the cell.
Fig. 5.
Expression of CAST in breast cancer cell lines. (A) Lysates (1% NP-40) of MCF7-BD (7), MCF7-Syk (Syk), MCF7-ATCC7 (A7), and MDA-MB-231 (231) cells were analyzed by Western blotting using an anti-CAST antibody. GAPDH was detected as a loading control. The expression level of endogenous CAST was normalized to that of GAPDH. Data represent means ± SE (n=3 for each measurement). (B) Endogenous CAST in MCF7-BD (MCF7), MCF7-Syk (Syk), MCF7-ATCC (ATTC7), and MDA-MB-231 (231) cells was detected using an anti-CAST antibody and a fluorescently tagged secondary antibody. DAPI was used to stain the nuclei. (C) Cytosolic (C) and nuclear (N) fractions of MCF7-BD cells untreated (MCF7) or pretreated (7 + PV) with pervanadate (PV) and MCF7-Syk cells (Syk) were analyzed by Western blotting using antibodies against CAST, calpain 4 (capn4) and GAPDH. The intensity of the upper CAST band in the cytosolic fraction was normalized to that of the nuclear CAST band to indicate the fold increase in the cytosolic amount of the slower-migrating CAST polypeptide.
When measuring the intracellular level of CAST, we also examined its subcellular distribution through cell fractionation. Cells were divided into a detergent soluble (cytosol plus membrane) and a detergent insoluble fraction that contained the nuclei. CAST was found in both fractions while capn4 was found exclusively in the cytosolic fraction (Fig. 5C). The two components of the CAST protein doublet that was visible in Western blots of whole cell lysates were differentially distributed between the two fractions with the lower, more rapidly migrating band present only in the cytosolic fraction and the upper band predominantly in the nuclear fraction. The increased amount of CAST that was present in cells expressing Syk was in the soluble fraction.
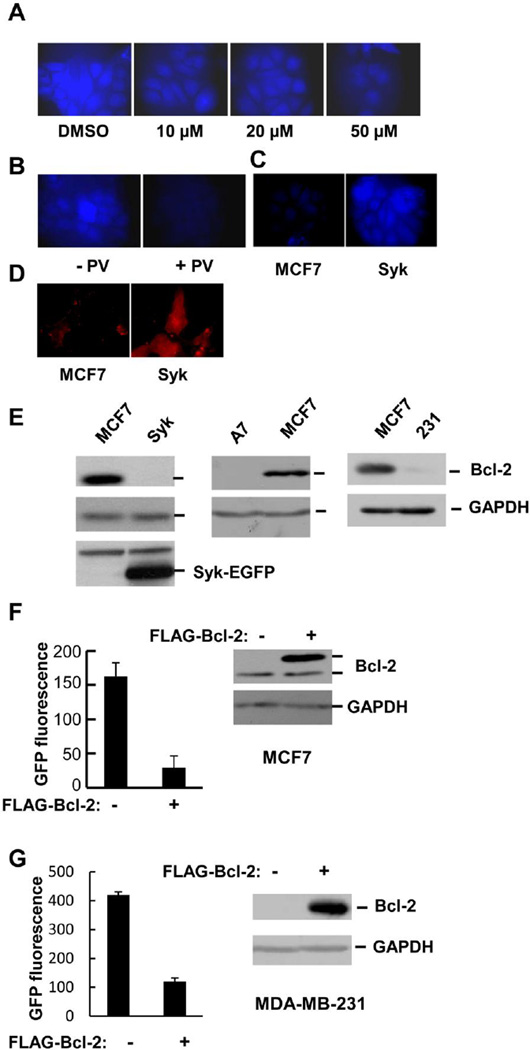
3.6. Intracellular calcium levels are high and Bcl-2 levels low in Syk-expressing cells
Since cells expressing higher levels of CAST exhibited lower levels of calpain activity in lysates or immune complexes, we asked whether or not calpain activity in the intact cells also was reduced to a similar extent. For this assay, we used a cell permeable calpain substrate (t-Boc-Leu-Met-CMAC) whose cleavage can be detected by fluorescence microscopy. The treatment of cells with increasing concentrations of calpeptin, a calpain inhibitor, led to a decrease in fluorescence intensity as did the treatment of cells with pervanadate, confirming the dependency of the assay on calpain activity (Fig. 6A and B). Surprisingly, a 5-fold increase in fluorescence intensity was observed in MCF7-Syk cells as compared to Syk-negative MCF7-BD cells despite the higher levels of endogenous CAST (Fig. 6C). Since calcium is the most important regulator of calpain activity, we compared the relative levels of intracellular calcium in the Syk-deficient MCF7-BD and Syk-expressing MCF7-Syk cells. An examination of cells passively loaded with Rhod-3 revealed an approximately 4-fold increase in fluorescence in the MCF7-Syk cells as compared to Syk-deficient MCF7-BD cells, indicating that the level of calcium in the Syk-expressing cells was higher than in Syk-deficient MCF7 cells (Fig. 6D). This observation provides an explanation for the higher calpain activity within live MCF7-Syk cells despite the higher level of CAST.
Fig. 6.
Elevated levels of calpain and calcium in Syk-expressing cells. (A) MCF7-BD cells pretreated with increasing concentrations of calpeptin were incubated with 10 µM BOC-Leu-Met-CMAC at 37 °C for 1 h before fixation and examination by fluorescence microscopy. (B) MCF7-BD cells were either pretreated or not with 50 µM PV and then with 50 µM BOC-Leu- Met-CMAC at 37 °C for 15 min. Fixed cells were then examined under the fluorescence microscope. (C) MCF7-BD (MCF7) and MCF7-Syk (Syk) cells were incubated with 10 µM BOC-Leu-Met-CMAC at 37 °C for 1 h, fixed and examined by fluorescence microscopy. (D) MCF7-BD (MCF7) and MCF7-Syk (Syk) cells loaded with Rhod-3 AM were fixed and examined by fluorescence microscopy. (E) Lysates from MCF7-BD (MCF7), MCF7-Syk (Syk) MCF7-ATCC (A7) and MDA-MB-231 cells were analyzed by Western blotting with antibodies against Bcl-2, GAPDH and Syk. (F) MCF7-BD cells were transfected with the GCaMP3 expression plasmid along with either an empty vector (−) or FLAG-Bcl-2 expression plasmid. Lysates were analyzed by Western blotting using antibodies against Bcl-2 and GAPDH. Fluorescence was measured with a plate reader. Values represent the means ± SE (n = 3). (G) MDA-MB-231 cells were analyzed as described in panel F.
The level of free intracellular calcium is, in part, a product of its release from intracellular stores in the endoplasmic reticulum. The release of calcium is mediated by IP3 receptors, which are, in turn, negatively regulated by the anti-apoptotic protein, Bcl-2 [58, 59]. To determine if Bcl-2 might contribute to the differences in intracellular calcium observed between the Syk-deficient versus Syk-positive cells, we examined the relative levels of the protein in the two cell types. Interestingly, the level of Bcl-2 was much higher in the Syk-deficient MCF7-BD than in the MCF7-Syk cells (Fig. 6E). A similar difference in expression was observed between the MCF7-BD cells and the MCF7-ATCC cells that express endogenous Syk. To verify a role for Bcl-2 in the regulation of intracellular calcium in breast cancer cells, we transfected MCF7-BD cells with a plasmid coding for the calcium-indicator protein GCaMP3 along with an expression plasmid for FLAG-tagged Bcl-2 or an empty vector. The expression of FLAG-Bcl-2 resulted in a substantial decrease in the intracellular concentration of calcium, consistent with an inhibitory role for Bcl-2 in calcium release from the ER in MCF7 cells (Fig. 6F).
Since the level of CAST also was higher in MDA-MB-231 than in MCF7-BD cells, we predicted that the level of Bcl-2 would be low in these cells, similar to what was observed for MCF7-Syks cells. Indeed, Western blotting analyses of cell lysates revealed a much lower level of Bcl-2 in MDA-MB-231 cells as compared to MCF7-BD cells (Fig. 6E). The expression of exogenous Bcl-2 in these cells again dramatically lowered the level of intracellular calcium as measured by GCaMP3 fluorescence (Fig. 6G).
3.7. Inhibition of calpain increases TNF-α induced activation of NF-κB
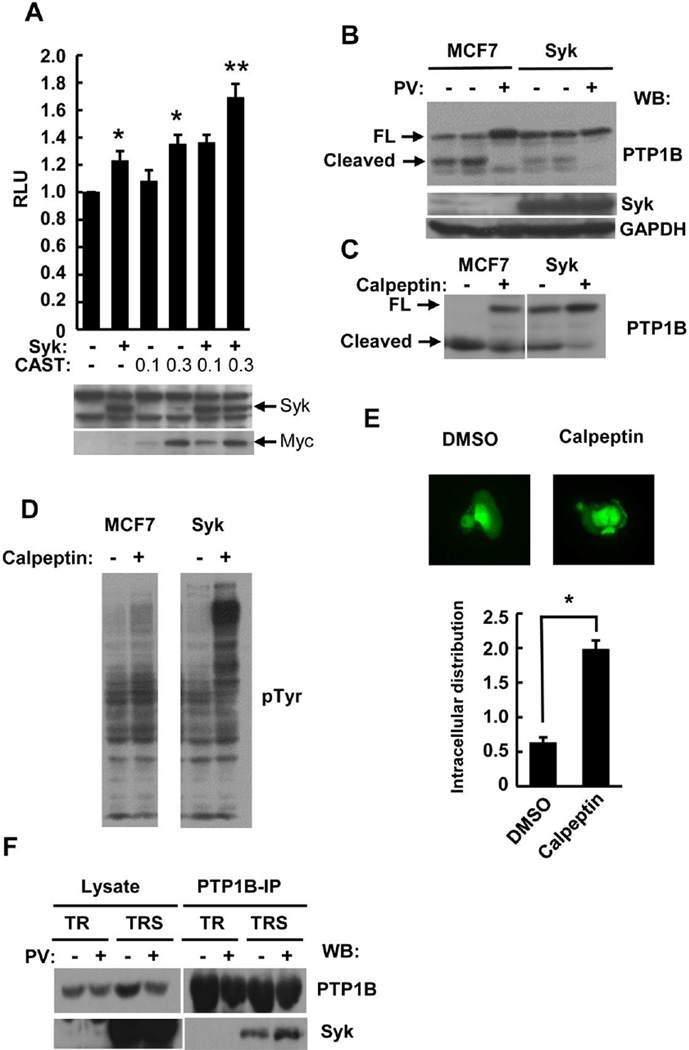
Based on the observed connections between Syk and the calpain system, we asked how the inhibition of calpain might affect known Syk-regulated pathways in epithelial cells. Since the re-expression of Syk in MCF7-BD cells enhances the TNF-α induced activation of NF-κB [14], we asked if calpain might be involved in this regulation. MCF7-BD cells were transiently transfected with an expression plasmid for either EGFP or Syk-EGFP, an NF-κB-driven luciferase reporter plasmid, a TK-luciferase internal control plasmid, and either a plasmid encoding CAST or an empty vector. The expression of Syk-EGFP or CAST alone enhanced the TNF-α induced activation of NF-κB while a combination of CAST and Syk resulted in a substantial increase in activation (Fig. 7A). This suggests that, by inhibiting calpain activity, CAST plays a positive role in TNF-α induced NF-κB activation in MCF7 cells.
Fig. 7.
Effect of calpain on TNF-α and integrin signaling. (A) MCF7-BD cells were transfected with the control and NF-κB-luciferase reporter plasmids and either empty vectors or expression plasmids for Syk-EGFP (Syk) and/or myc-CAST (CAST) (0.1 or 0.3 µg). Cells were stimulated with 10 ng/ml TNF-α at 37°C for 18 h and assayed for luciferase expression. Lysates were analyzed by Western blotting with anti-Syk and anti-myc epitope antibodies. Data are normalized to vector only controls and represent the ± SE of three independent experiments performed in triplicate. *P<0.05; ***P<0.001. (B) Lysates (1% NP-40) of MCF7-BD (MCF7) and MCF7-Syk (Syk) cells pretreated without or with pervanadate (PV) were analyzed by Western blotting with antibodies against PTP1B, Syk and GAPDH. (C) Lysates (1% NP-40) of MCF7-BD (MCF7) and MCF7-Syk (Syk) cells pretreated without or with calpeptin were analyzed by Western blotting with antibodies against PTP1B. Arrows indicate migration positions of full-length (FL) and cleaved PTP1B. (D) Lysates from MCF7-BD (MCF7) or MCF7-Syk (Syk) cells pre-treated with or without calpeptin and then treated in suspension with anti-β1-integrin were analyzed by Western blotting using an antibody against phosphotyrosine (pTyr). (E) MCF7-Syk cells were pretreated with 20 µM calpeptin or DMSO for 24 h were detached from plates and then allowed to spread on fibronectin (2 µg/ml) coated coverslips at room temperature for 1 h. Cells were fixed and examined under the fluorescence microscope. The distribution of Syk-EGFP at the cell edge was normalized to that in the cytosol and plotted as a histogram. Data represent means ± SE (n=30 for each measurement). *P<0.0001 when compared to the DMSO control. (F) PTP1B was immunoprecipitated from lysates of MDA-MB-231 cells lacking Syk (TR) or induced to express Syk-EGFP (TRS) under control of a Tet- responsive promoter and pre-treated with or without pervanadate (PV). Lysates and immune complexes were analyzed by Western blotting with antibodies against PTP1B and Syk.
3.8. PTP1B is a substrate of calpain
In addition to RelA, a number of other cellular proteins have been described that are uniquely sensitive to calpain-mediated cleavage. One example is the protein-tyrosine phosphatase, PTP1B. To determine if PTP1B was susceptible to proteolysis by calpain in breast epithelial cells, we compared lysates from cells either expressing or lacking Syk. MCF7-BD and MCF7-Syk cells were pretreated without or with pervanadate under conditions where it inhibited calpain activity. As shown in Fig. 7B, the cleavage of PTP1B to generate the smaller, more rapidly migrating form was readily observed in lysates from Syk-deficient cells. This cleavage again was reduced in lysates from cells expressing the kinase and in lysates of cells treated with pervanadate and was blocked by calpain (Fig. 7C). Thus, PTP1B, like RelA, was sensitive to calpain-mediated cleavage in cell lysates and the stable expression of Syk in MCF7 cells partially inhibited its cleavage due to increased CAST expression.
In epithelial cells, the cross-linking of integrins leads to the activation of Syk [12, 26]. Interestingly, integrin engagement in both platelets and breast cancer cells also results in the cleavage of PTP1B to generate the smaller, catalytically more active fragment [35, 36, 60]. To begin to investigate the effect of calpain on Syk-mediated integrin signaling, the cellular level of tyrosine phosphorylation was monitored in MCF7-BD and MCF7-Syk cells following integrin crosslinking in the presence or absence of the cell permeable calpain inhibitor calpeptin. The integrin-stimulated phosphorylation of proteins on tyrosine was enhanced by the addition of calpeptin in the Syk-expressing cells (Fig. 7D). In human umbilical vein endothelial cells (HUVEC), more Syk was observed in the membrane fraction following the inhibition of calpain [48]. To investigate the influence of calpain inhibition on the intracellular localization of Syk in MCF7-Syk cells, we pretreated MCF7-Syk cells with calpeptin for 24 h and then plated these on fibronectin-coated coverslips. After 1 h, cells were fixed and the localization of Syk-EGFP was examined under the fluorescence microscope. Consistent with our previous observations, Syk was found to be expressed in both the cytosol and the nucleus [26]. When calpain was inhibited by calpeptin, a 4-fold increase in the amount of Syk present at the edge of spreading cells was observed as compared to the control, DMSO-treated cells (Fig. 7E). To determine if Syk and PTP1B might be directly or indirectly associated with one another, we immunoprecipitated PTP1B from a line of MDA-MB-231 cells in which the expression of Syk (as Syk-EGFP) could be induced by tetracycline. Indeed, Syk-EGFP could be readily visualized by Western blotting in anti-PTP1B immune complexes isolated from lysates of cells induced to express the kinase (Fig. 7F).
4. Discussion
In hematopoietic cells, Syk plays a pivotal role in coupling a variety of different membrane-associated receptors to diverse intracellular signaling pathways, leading to enhanced proliferation and differentiation. It also plays a pro-survival role in a variety of malignancies [15–17, 19]. In MCF7 human breast adenocarcnoma cells, Syk enhances the TNF-α induced activation of NF-κB and protects cells from apoptosis [14]. To investigate how Syk enhances TNF-α induced NF-κB activation, we explored a potential interaction between Syk and RelA, a subunit of NF-κB. Although neither an interaction with Syk nor the tyrosine phosphorylation of RelA could be detected, we did find that overexpressed RelA is partially proteolyzed in lysates of MCF7 cells and that the stable expression of Syk decreases this proteolysis. In a series of experiments using a variety of different protease inhibitors, we find that the proteolysis of RelA is catalyzed by calpain, a widely expressed cysteine protease that is tightly regulated by calcium. The identification of calpain as the responsible protease also was supported by its sensitivity to inactivation through oxidation. The pre-treatment of cells with pervanadate inhibited the subsequent recovery of active calpain in cell lysates. This effect required high concentrations of H2O2 and was independent of the pervanadate-induced inhibition of PTP activity, suggesting an inhibitory mechanism involving direct oxidative modification of the cysteine-containing catalytic triad. This is consistent with previous reports that calpain can be inhibited by H2O2-triggered oxidative stress both in vitro and in vivo [54, 55]. An analysis of H2O2-oxidized µ-calpain purified from porcine skeletal muscle by mass spectrometry revealed an intramolecular disulfide bond between the active site cysteine (Cys115) and Cys108 [56].
The different levels of calpain activity present in MCF7 cell lysates is dictated by the level of expression in the cell of its inhibitor, CAST; and the expression level of CAST is affected by the presence or absence of Syk in the cells. Conventional MCF7 cells (MCF7- ATCC) that express normal levels of the kinase express higher levels of CAST than do Syk-deficient MCF7-BD cells. However, the amount of CAST in the MCF7-BD cells is restored to near normal levels by the re-expression of the kinase. No obvious change in calpain expression is observed between Syk-negative and -positive cells. CAST levels are also higher in MDA-MB-231 cells than in MCF7-BD cells even though these cells lack endogenous Syk. However, these cells do express the EGF receptor tyrosine kinase at high levels, which could possibly be coupled to the enhanced expression of CAST if this is a tyrosine kinase-regulated event. In all cases, the level of calpain activity in cell lysates and in immune complexes is inversely related to the level of CAST expressed in the cell and likely, therefore, reflects the formation of inactive calpain-CAST complexes once cells are lysed.
The increased amount of CAST found in the Syk-expressing cells was present primarily in the soluble fraction of the cell. Although encoded by a single gene, CAST is expressed as multiple isoforms due to alternative splicing events that most frequently result in the elimination of regions encoded by exon 3 or exons 3 and 5. As consistently observed by Western blotting, the endogenous CAST protein in breast cancer cells migrates on SDS-PAGE as multiple bands, consistent with the presence of more than a single isoform. The more rapidly migrating forms are present in the soluble fraction while the most slowly migrating form is found predominantly in the insoluble fraction that contains nuclei. Whether or not this CAST isoform is a nuclear protein or is instead present in other insoluble compartments or aggregates that are present in the “nuclear” fraction remains to be clearly defined. A correlation between the inhibitory efficiency of CAST and its localization to the cytoplasm or within aggregates has been reported in various cell lines [43, 61]. Soluble, non-aggregated CAST binds and inhibits calpain more efficiently. Since the expression of Syk significantly increases the amount of CAST present in the soluble fraction, the same fraction in which calpain is found to reside, more CAST can associate with calpain in lysates generated from Syk-positive as compared to Syk-negative cells.
Despite the higher level of CAST, the level of intracellular calpain activity in live, intact cells is elevated in those expressing Syk. This increased activity correlates with increased basal levels of intracellular calcium, the positive regulator of calpain that induces the dissociation of calpain and CAST. It is likely that this elevated level of CAST is a consequence of the increased calpain activity as CAST expression at the transcriptional level has been correlated with the cellular requirements for calpain inhibition [43]. The elevated level of calcium in the Syk-expressing cells correlates, in turn, with a reduced expression of Bcl-2. We find that, in MCF7 cells expressing normal levels of endogenous Syk, intracellular levels of Bcl-2 are low. In contrast, levels of Bcl-2 protein are much higher in Syk-deficient MCF7 cells, but are again reduced when Syk (as Syk-EGFP) is expressed. Bcl-2 interacts directly with the regulatory domain of the IP3 receptor through its BH4 domain to inhibit calcium release from the ER [62] and elevating its level through the expression of exogenous FLAG-Bcl-2 reduces the intracellular concentration of calcium in MCF7 cells. Similarly, MDA-MB-231 cells, which also have elevated levels of CAST, also have low levels of Bcl-2 relative to those in MCF7-BD cells. The expression of exogenous Bcl-2 in these cells also reduces the intracellular level of calcium. Thus, differences in the cellular level of Bcl-2 provide a reasonable explanation for the observed differences in calcium levels, which, in turn, modulate calpain activity and the levels of CAST expression.
While the proteolysis of both RelA and PTP1B by calpain are observed most readily in cell lysates, it is certainly possible that both are also subject to regulation by proteolysis within the cell. Since calpain activity is higher in Syk-expressing cells, we monitored possible effects of calpain inhibitors on Syk-modulated signaling events. Previously, both calpain and Syk have been implicated in the regulation of pathways leading to the activation of NF-κB [12–14, 63–65]. We found that the inhibition of calpain activity in intact cells through the expression of CAST does modulate the transcriptional activity of NF-κB induced by the treatment of cells with TNF-α. Syk enhances TNF-α induced NF-κB activation in MCF7 breast cancer cells and the overexpression of CAST alone also has a positive effect on TNF-α induced NF-κB activation. The expression of both CAST and Syk together leads to an even larger increase in signaling.
An important role for Syk in epithelial cells is its participation in the integrin signaling pathway. The ligation of cell surface integrin receptors enhances tyrosine phosphorylation of cellular proteins, in part through the activation of Syk [3, 7, 8, 26, 66, 67]. PTP1B has been implicated as a key negative regulator of integrin-induced signaling and is activated by calpain cleavage and integrin ligation [35, 36, 49, 60, 68, 69]. Interestingly, in Syk-expressing cells, integrin-mediated tyrosine-phosphorylation is enhanced by the inhibition of calpain by calpeptin. Thus, it is reasonable to speculate that the increase in integrin-stimulated tyrosine phosphorylation in Syk-expressing cells by calpeptin is a result of downregulated PTP1B activity. PTP1B also has been identified by mass spectrometry as a substrate for Syk in Syk-transfected MDA-MB-231 cells [70] and we observe an interaction between the two proteins. More Syk was detected at the cell edge in spreading cells that were treated with calpeptin, indicating an induced accumulation of the kinase at its sites of activation that might lead to its prolonged activation. Indeed, it has been reported that following β3-integrin ligation, calpain is activated and proteolyzes the cytoplasmic tail of the integrin receptor that provides important binding sites for Syk [69]. Interestingly, a reduced expression of CAST is associated with the early stages of metastatic behavior in breast cancer cells [71], consistent with a role for calpain in the regulation of motility via the enhanced disassembly of focal adhesions [72–74]. This is interesting as the expression of Syk both enhances the expression of CAST and inhibits the motility of breast cancer cells and reduces their metastatic behavior [3, 75].
Taken together, results reported here demonstrated that Syk regulates calpain activity in MCF7 cells by modulating the intracellular concentrations of calcium and upregulating the expression of CAST. Inhibition of calpain enhances signaling events that are regulated by Syk, including TNF-α induced activation of NF-κB, as well as integrin ligation induced tyrosine phosphorylation. The reciprocal regulation between Syk and calpain-CAST proteolytic system provides novel insights into the tumor suppressing and promoting functions of Syk in breast epithelial cells.
Supplementary Material
Highlights.
Lysates of Syk-expressing cells have reduced calpain activity
Syk-expressing cells have elevated levels of calpastatin
Bcl-2 regulates basal levels of intracellular calcium
Calpain inhibitors enhance Syk-mediated signaling
Acknowledgements
This work was supported by United States Public Health Services grants CA115465 and CA037372 awarded by the National Cancer Institute. S.Y. was supported by the National Cancer Institute Cancer Prevention Internship Program R25 CA128770 (D. Teegarden).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geahlen RL. Syk and pTyr’d: Signaling through the B cell antigen receptor. Biochim. Biophys. Acta. 2009;1793:1115–1127. doi: 10.1016/j.bbamcr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mócsai A, Ruland J, Tybulewicz VLJ. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E, Blancato JK, Vezza PR, McLeskey SW, Mangeat PH, Mueller SC. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 4.Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem. Biophys. Res. Commun. 2001;288:495–498. doi: 10.1006/bbrc.2001.5788. [DOI] [PubMed] [Google Scholar]
- 5.Hoeller C, Thallinger C, Pratscher B, Bister MD, Schicher N, Loewe R, Heere-Ress E, Roka F, Sexl V, Pehamberger H. The non-receptor-associated tyrosine kinase Syk is a regulator of metastatic behavior in human melanoma cells. J. Invest. Derm. 2005;124:1293–1299. doi: 10.1111/j.0022-202X.2005.23685.x. [DOI] [PubMed] [Google Scholar]
- 6.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J. Biol. Chem. 1996;271:18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 7.Miranti CK, Leng L, Maschberger P, Brugge JS, Shattil SJ. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr. Biol. 1998;8:1289–1299. doi: 10.1016/s0960-9822(07)00559-3. [DOI] [PubMed] [Google Scholar]
- 8.Woodside DG, Obergfell A, Leng L, Wilsbacher JL, Miranti CK, Brugge JS, Shattil SJ, Ginsberg MH. Activation of Syk protein tyrosine kinase through interaction with integrin beta cytoplasmic domains. Curr. Biol. 2001;11:1799–1804. doi: 10.1016/s0960-9822(01)00565-6. [DOI] [PubMed] [Google Scholar]
- 9.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VLJ. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 12.Ulanova M, Puttagunta L, Marcet-Palacios M, Duszyk M, Steinhoff U, Duta F, Kim MK, Indik ZK, Schreiber AD, Befus AD. Syk tyrosine kinase participates in beta1-integrin signaling and inflammatory responses in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L497–L507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]
- 13.Takada Y, Aggarwal BB. TNF activates Syk protein tyrosine kinase leading to TNF-induced MAPK activation, NF-kappaB activation, and apoptosis. J. Immunol. 2004;173:1066–1077. doi: 10.4049/jimmunol.173.2.1066. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Geahlen RL. The protein-tyrosine kinase Syk interacts with TRAF-interacting protein TRIP in breast epithelial cells. Oncogene. 2009;28:1348–1356. doi: 10.1038/onc.2008.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young RM, Hardy IR, Clarke RL, Lundy N, Pine P, Turner BC, Potter TA, Refaeli Y. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood. 2009;113:2508–2516. doi: 10.1182/blood-2008-05-158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K, Schinzel AC, Ross L, Galinsky I, Davis TN, Silver SJ, Root DE, Stone RM, DeAngelo DJ, Carroll M, Hahn WC, Carr SA, Golub TR, Kung AL, Stegmaier K. Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell. 2009;16:281–294. doi: 10.1016/j.ccr.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M, Chevalier N, Vallat L, Timmer J, Gribben JG, Jumaa H, Veelken H, Dierks C, Zirlik K. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baudot AD, Jeandel PY, Mouska X, Maurer U, Tartare-Deckert S, Raynaud SD, Cassuto JP, Ticchioni M, Deckert M. The tyrosine kinase Syk regulates the survival of chronic lymphocytic leukemia B cells through PKC-δ and proteasome-dependent regulation of Mcl-1 expression. Oncogene. 2009;28:3261–3273. doi: 10.1038/onc.2009.179. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Benavente CA, McEvoy J, Flores-Otero J, Ding L, Chen X, Ulyanov A, Wu G, Wilson M, Wang J, Brennan R, Rusch M, Manning AL, Ma J, Easton J, Shurtleff S, Mullighan C, Pounds S, Mukatira S, Gupta P, Neale G, Zhao D, Lu C, Fulton RS, Fulton LL, Hong X, Dooling DJ, Ochoa K, Naeve C, Dyson NJ, Mardis ER, Bahrami A, Ellison D, Wilson RK, Downing JR, Dyer MA. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481:329–334. doi: 10.1038/nature10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minobe K, Onda M, Iida A, Kasumi F, Sakamoto G, Nakamura Y, Emi M. Alle lic losson chromosome 9q is associated with lymph node metastasis of primary breast cancer. Jpn. J. Cancer Res. 1998;89:916–922. doi: 10.1111/j.1349-7006.1998.tb00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, Mendez R, Sahin A, Dai JL. Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer Res. 2001;61:5558–5561. [PubMed] [Google Scholar]
- 23.Sung YM, Xu X, Sun J, Mueller D, Sentissi K, Johnson P, Urbach E, Seillier-Moiseiwitsch F, Johnson MD, Mueller SC. Tumor suppressor function of Syk in human MCF10A in vitro and normal mouse mammary epithelium in vivo. PLoS One. 2009;4:e7445. doi: 10.1371/journal.pone.0007445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyss Z, Montcourrier P, Vidal B, Anguille C, Merezegue F, Sahuquet A, Mangeat PH, Coopman PJ. The Syk tyrosine kinase localizes to the centrosomes and negatively affects mitotic progression. Cancer Res. 2005;65:10872–10880. doi: 10.1158/0008-5472.CAN-05-1270. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Devarajan E, He J, Reddy SP, Dai JL. Transcription repressor activity of spleen tyrosine kinase mediates breast tumor suppression. Cancer Res. 2005;65:10289–10297. doi: 10.1158/0008-5472.CAN-05-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Shrikhande U, Alicie BM, Zhou Q, Geahlen RL. Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells. Mol. Cancer Res. 2009;7:634–644. doi: 10.1158/1541-7786.MCR-08-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WJ, Ma H, Takano E, Yang HQ, Hatanaka M, Maki M. Molecular diversity in amino-terminal domains of human calpastatin by exon skipping. J. Biol. Chem. 1992;267:8437–8442. [PubMed] [Google Scholar]
- 28.Parr T, Sensky PL, Bardsley RG, Buttery PJ. Calpastatin expression in porcine cardiac and skeletal muscle and partial gene structure. Arch. Biochem. Biophys. 2001;395:1–13. doi: 10.1006/abbi.2001.2546. [DOI] [PubMed] [Google Scholar]
- 29.Cong M, Thompson VF, Goll DE, Antin PB. The bovine calpastatin gene promoter and a new N-terminal region of the protein are targets for cAMP-dependent protein kinase activity. J. Biol. Chem. 1998;273:660–666. doi: 10.1074/jbc.273.1.660. [DOI] [PubMed] [Google Scholar]
- 30.Takano J, Watanabe M, Hitomi K, Maki M. Four types of calpastatin isoforms with distinct amino-terminal sequences are specified by alternative first exons and differentially expressed in mouse tissues. J. Biochem. 2000;128:83–92. doi: 10.1093/oxfordjournals.jbchem.a022733. [DOI] [PubMed] [Google Scholar]
- 31.Rios-Doria J, Day KC, Kuefer R, Rashid MG, Chinnaiyan AM, Rubin MA, Day ML. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J. Biol. Chem. 2003;278:1372–1379. doi: 10.1074/jbc.M208772200. [DOI] [PubMed] [Google Scholar]
- 32.Löfvenberg L, Backman L. Calpain-induced proteolysis of beta-spectrins. FEBS Lett. 1999;443:89–92. doi: 10.1016/s0014-5793(98)01697-4. [DOI] [PubMed] [Google Scholar]
- 33.Cooray P, Yuan Y, Schoenwaelder SM, Mitchell CA, Salem HH, Jackson SP. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 1996;318:41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J. Biol. Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- 35.Frangioni JV, Oda A, Smith M, Salzman EW, Neel BG. Calpain-catalyzed cleavage and subcellular relocation of protein phosphotyrosine phosphatase 1B (PTP-1B) in human platelets. EMBO J. 1993;12:4843–4856. doi: 10.1002/j.1460-2075.1993.tb06174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuchay SM, Kim N, Grunz EA, Fay WP, Chishti AH. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol. Cell. Biol. 2007;27:6038–6052. doi: 10.1128/MCB.00522-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azarian SM, King AJ, Hallett MA, Williams DS. Selective proteolysis of arrestin by calpain. J. Biol. Chem. 1995;270:24375–24384. doi: 10.1074/jbc.270.41.24375. [DOI] [PubMed] [Google Scholar]
- 38.Hirai S, Kawasaki H, Yaniv M, Suzuki K. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett. 1991;287:57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- 39.Pariat M, Salvat C, Bebien M, Brockly F, Altieri E, Carillo S, Jariel-Encontre I, Piechaczyk M. The sensitivity of c-Jun and c-Fos proteins to calpains depends on conformational determinants of the monomers and not on formation of dimmers. Biochem. J. 2000;345:129–138. [PMC free article] [PubMed] [Google Scholar]
- 40.Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol. Cell. Biol. 1997;17:460–468. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol. Cell. Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell. Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Averna M, De Tullio R, Capini P, Salamino F, Pontremoli S, Melloni E. Changes in calpastatin localization and expression during calpain activation: a new mechanism for the regulation of intracellular Ca(2+)-dependent proteolysis. Cell. Mol. Life Sci. 2003;60:2669–2678. doi: 10.1007/s00018-003-3288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tidball JG, Spencer MJ. Calpains and muscular dystrophies. Int. J. Biochem. Cell. Biol. 2000;32:1–5. doi: 10.1016/s1357-2725(99)00095-3. [DOI] [PubMed] [Google Scholar]
- 45.Sandmann S, Yu M, Unger T. Transcriptional and translational regulation of calpain in the rat heart after myocardial infarction-effects of AT(1) and AT(2) receptor antagonists and ACE inhibitor. Br. J. Pharmacol. 2001;132:767–777. doi: 10.1038/sj.bjp.0703860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer's disease brains. Neurosci. Lett. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- 47.Mamoune A, Luo J-H, Lauffenburger DA, Wells A. Calpain-2 as a target for limiting prostate cancer invasion. Cancer Res. 2003;63:4632–4640. [PubMed] [Google Scholar]
- 48.Gonscherowski V, Becker BF, Moroder L, Motrescu E, Gil-Parrado S, Gloe T, Keller M, Zahler S. Calpains: a physiological regulator of the endothelial barrier? Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2035–H2042. doi: 10.1152/ajpheart.00772.2004. [DOI] [PubMed] [Google Scholar]
- 49.Maile LA, Aday AW, Busby WH, Sanghani R, Veluvolu U, Clemmons DR. Modulation of integrin antagonist signaling by ligand binding of the heparin-binding domain of vitronectin to the alphaVbeta3 integrin. J. Cell. Biochem. 2008;105:437–446. doi: 10.1002/jcb.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL. Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J. Immunol. 2001;166:1507–1516. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- 51.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellegatta F, Bertelli AA, Staels B, Duhem C, Fulgenzi A, Ferrero ME. Different short- and long-term effects of resveratrol on nuclear factor-kappaB phosphorylation and nuclear appearance in human endothelial cells. Am. J. Clin. Nutr. 2003;77:1220–1228. doi: 10.1093/ajcn/77.5.1220. [DOI] [PubMed] [Google Scholar]
- 53.Bijli KM, Fazal F, Minhajuddin M, Rahman A. Activation of Syk by protein kinase C- delta regulates thrombin-induced intercellular adhesion molecule-1 expression in endothe lialcells via tyrosine phosphorylation of RelA/p65. J. Biol. Chem. 2008;283:14674–14684. doi: 10.1074/jbc.M802094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guttmann RP, Elce JS, Bell PD, Isbell JC, Johnson GV. Oxidation inhibits substrate proteolysis by calpain I but not autolysis. J. Biol. Chem. 1997;272:2005–2012. doi: 10.1074/jbc.272.3.2005. [DOI] [PubMed] [Google Scholar]
- 55.Guttmann RP, Johnson GV. Oxidative stress inhibits calpain activity in situ. J. Biol. Chem. 1998;273:13331–13338. doi: 10.1074/jbc.273.21.13331. [DOI] [PubMed] [Google Scholar]
- 56.Lametsch R, Lonergan S, Huff-Lonergan E. Disulfide bond within mu-calpain active e site inhibits activity and autolysis. Biochim. Biophys. Acta. 2008;1784:1215–1221. doi: 10.1016/j.bbapap.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Jang S-H, Hwang SA, Kim M, Yun S-H, Kim M-S, Karnik SS, Lee C. A protein tyrosine phosphatase inhibitor, pervanadate, inhibits angiotensin II-induced β-arrestin cleavage. Mol. Cells. 2009;28:25–30. doi: 10.1007/s10059-009-0104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release fromthe ER. J. Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong F, Harr MW, Bultynck G, Monaco G, Parys JB, De Smedt H, Rong Y-P, Molitoris JK, Lam M, Ryder C, Matsuyama S, Distelhorst CW. Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J. Cell Biol. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Averna M, de Tullio R, Passalacqua M, Slaamino F, Pontremoli S, Melloni E. Changes in intracellular calpastatin localization are mediated by reversible phosphorylation. Biochem. J. 2001;354:25–30. doi: 10.1042/0264-6021:3540025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rong Y-P, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaecher K, Goust JM, Banik NL. The effects of calpain inhibition on IkB alpha degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochem. Res. 2004;29:1443–1451. doi: 10.1023/b:nere.0000026410.56000.dd. [DOI] [PubMed] [Google Scholar]
- 64.Chen F, Demers LM, Vallyathan V, Lu Y, Castranova V, Shi X. Impairment of NF-kappaB activation and modulation of gene expression by calpastatin. Am. J. Physiol. Cell Physiol. 2000;279:C709–C716. doi: 10.1152/ajpcell.2000.279.3.C709. [DOI] [PubMed] [Google Scholar]
- 65.Han Y, Weinman S, Boldogh I, Walker RK, Brasier AR. Tumor necrosis factor-alpha-inducible IkappaBalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. J. Biol. Chem. 1999;274:787–794. doi: 10.1074/jbc.274.2.787. [DOI] [PubMed] [Google Scholar]
- 66.Lin TH, Rosales C, Mondal K, Bolen JB, Haskill S, Juliano RL. Integrin-mediated tyrosine phosphorylation and cytokine message induction in monocytic cells. A possible signaling role for the Syk tyrosine kinase. J. Biol. Chem. 1995;270:16189–16197. doi: 10.1074/jbc.270.27.16189. [DOI] [PubMed] [Google Scholar]
- 67.Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, Lowell CA, Shattil SJ. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. J. Cell. Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu F, Sells MA, Chernoff J. Protein tyrosine phosphatase 1B negatively regulates integrin signaling. Curr. Biol. 1998;8:173–176. doi: 10.1016/s0960-9822(98)70066-1. [DOI] [PubMed] [Google Scholar]
- 69.Du X, Saido TC, Tsubuki S, Indig FE, Williams MJ, Ginsberg MH. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J. Biol. Chem. 1995;270:26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- 70.Iliuk AB, Martin VA, Alicie BM, Geahlen RL, Tao WA. In-depth analyses of kinase-dependent tyrosine phosphoproteomes based on metal ion-functionalized soluble nanopolymers. Mol. Cell. Proteomics. 2010;9:2162–2172. doi: 10.1074/mcp.M110.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Storr SJ, Mohammeda RAA, Woolston CM, Green AR, Parr T, Spiteri I, Caldas C, Ball GR, Ellis IO, Martin SG. Calpastatin is associated with lymphovascular invasion in breast cancer. Breast. 2011;20:413–418. doi: 10.1016/j.breast.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Beckerle MC, Burridge K, DeMartino GN, Croall DE. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987;51:569–577. doi: 10.1016/0092-8674(87)90126-7. [DOI] [PubMed] [Google Scholar]
- 73.Cuevas BD, Abell AN, Witowsky JA, Yujiri T, Johnson NL, Kesavan K, Ware M, Jones PL, Weed SA, DeBiasi RL, Oka Y, Tyler KL, Johnson GL. MEKK1 r egulatescalpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO J. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent pr otease calpain. J. Biol. Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 75.Yuan Y, Liu H, Sahin A, Dai JL. Reactivation of SYK expression by inhibition of DNA methylation suppresses breast cancer cell invasiveness. Int. J. Cancer. 2005;113:654–659. doi: 10.1002/ijc.20628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.