Abstract
Objective Skull base metastases (SBMs) are rare lesions in close proximity to critical neural and vasculature structures. This rarity and complexity have led many to only offer nonsurgical therapies. The surgical outcomes for patients with SBM therefore remain unknown.
Design Retrospective, comparison analyses.
Setting Johns Hopkins Hospital.
Participants All patients who underwent intracranial metastatic tumor surgery.
Main Outcome Measure Survival and recurrence.
Results Of the 708 patients who underwent intracranial metastatic tumor surgery, 29 (4%) had SBM: 3 (10%) involved the anterior skull base, 7 (24%) the sella, 6 (21%) the orbit, 2 (7%) the sphenoid wing, 3 (10%) the clivus, 4 (14%) the petrous bone, and 4 (14%) the paranasal sinuses. Following surgery, 6 (50%) had improvements in vision and 14 (88%) had improvement and/or maintenance of their cranial nerve symptoms. Three (10%), 0(0%), and 1(3%) developed a new motor, language, and vision deficit, respectively. There were no differences in median survival (10.0 versus 9.2 months, p = 0.48) and local progression-free survival (PFS) (p = 0.52), but there was improved distal PFS (p = 0.04) between patients with and without SBM.
Conclusions Patients with SBM are relatively rare. These patients can tolerate surgery with minimal morbidity and mortality, and they have similar prognoses to patients without SBM.
Keywords: metastatic brain tumor, recurrence, skull base, surgery, survival
Introduction
The most common type of brain malignancy in adults is metastatic brain tumors.1,2 It is estimated that 25 to 45% of patients with cancer develop metastatic brain lesions each year.1 Further exacerbating this issue is that patients are surviving longer with cancer as a result of developments in surgical therapy, chemotherapeutic options, and radiation therapy.2,3,4,5,6 This has resulted in patients presenting with more numerous intracranial metastases and/or atypical location of their metastases.3,7 An uncommon location for metastases is the skull base.8,9 Skull base metastases (SBMs) are challenging to manage because they are rare and develop in close proximity to several critical neural and vascular structures. This rarity and complexity have precluded defining optimal treatment for patients who present with SBM.
Patients who present with SBM are rarely offered surgical therapies, unlike patients with intracranial metastases elsewhere.10,11,12 Patients with SBM are typically only offered radiation therapy in the form of stereotactic radiosurgery, whole-brain radiation, or proton beam therapy.6,10,11,12,13 Surgical interventions are withheld because the surgical outcomes for patients with SBM remain unclear. The goals of this study were to therefore (1) ascertain the proportion of patients who underwent surgery of a SBM, (2) understand the clinical differences between patients who develop SBM and non-SBM, and (3) understand the survival and recurrence outcomes for this group of patients who undergo surgical resection. A better understanding of these characteristics may better define if surgery is a viable option for this uncommon subset of patients, which are usually offered only nonsurgical therapies.10,11,12
Methods
Approval was obtained from the Institutional Review Board (36875) prior to the start of this study.
Patient Selection and Recorded Variables
All adult patients (age > 18 years) (n = 708) who underwent needle biopsy and/or surgical resection of an intracranial metastasis between 1997 and 2011 were retrospectively reviewed. This included patients undergoing resection of a single or multiple intracranial metastases. Among these patients, patients who underwent surgical resection of a SBM were identified. A SBM was defined as involvement of the bone of the skull base by pathology-proven metastasis (Figs. 1 and 2). This included metastases involving bone of the anterior cranial fossa, orbit, sella, sphenoid wing and paranasal sinuses, clivus, and petrous bone. Metastases involving the cerebellopontine angle were not included. Metastases were classified based on where the tumor was believed to primarily originate. The pathology was determined by a senior neuropathologist in all cases. Primary tumors that locally infiltrated the skull base, rather than distally metastasized, were excluded.
Fig. 1.
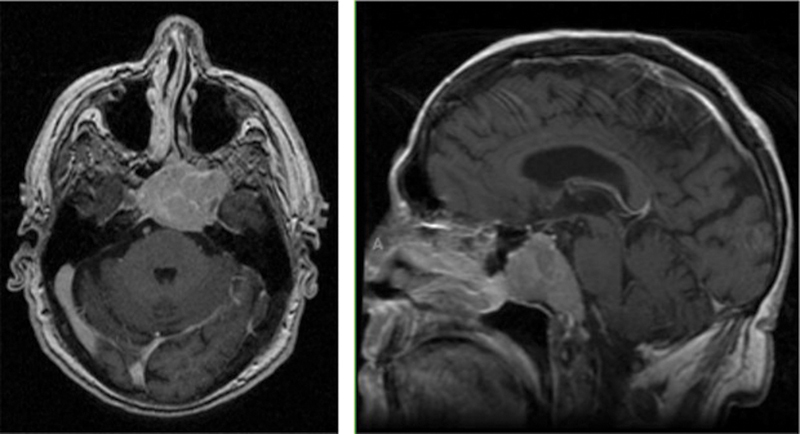
Example of a patient with a skull base metastasis. This is a 58-year-old man with a history of non-small cell lung cancer who presented with a solitary metastasis primarily involving the clivus and sphenoid sinus.
Fig. 2.
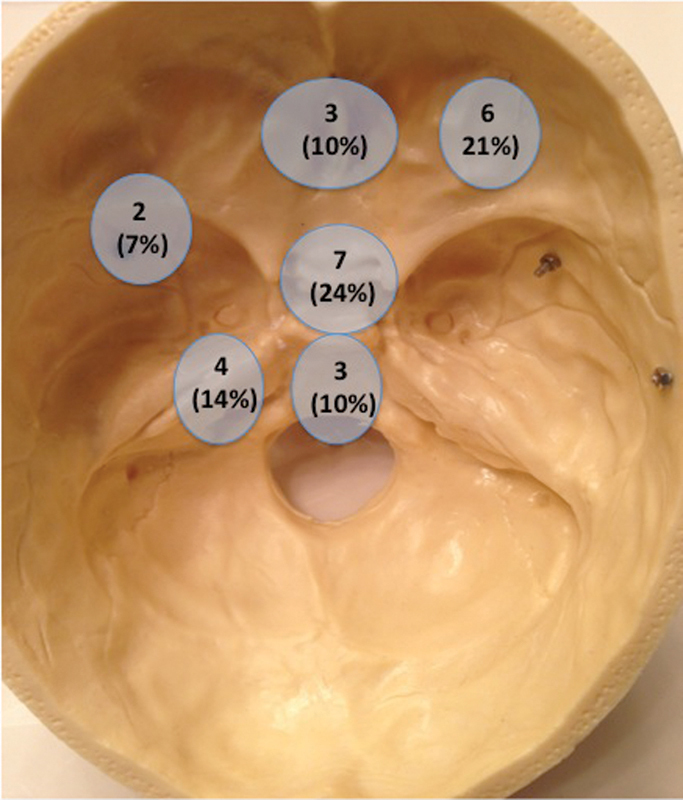
Distribution of skull base metastases in this study.
The information collected from neurosurgery and neuro-oncology clinical notes included patient demographics, comorbidities, presenting symptoms, brain and body imaging characteristics, postoperative neurological function, and adjuvant therapy. Patients were assigned a Karnofsky performance scale (KPS) index14 and recursive partitioning analysis (RPA) classification group15 by a reviewer blinded to patient outcomes at the clinic visit prior to surgery during a chart review. The presence of a motor deficit was defined as decreased strength, a language deficit as any combination of receptive and/or expressive aphasia, a cognitive deficit as any complaint of decreased mental status or ability, and a vision deficit as any decrease in visual acuity or visual field perception.
All patients underwent preoperative and postoperative magnetic resonance imaging (MRI). The characteristics that were recorded included the lesion's size (largest diameter based on gadolinium enhancement), specific lobe involvement, number of intracranial metastases, and presence of hydrocephalus. Extent of resection was determined by comparing preoperative and postoperative MRIs obtained < 48 hours after surgery as gross total resection (GTR) if no residual enhancement, near-total resection (NTR) if only a rim of enhancement is seen in the resection cavity, or subtotal resection (STR) if residual nodular enhancement was noted on postoperative MRI.16 The presence of dilated ventricles with transependymal flow was used to classify the presence of hydrocephalus. In addition to neuro-imaging, all patients underwent computed tomography (CT) scans of the chest, abdomen, pelvis, and spine with oral and intravenous contrast to identify control of primary tumor and presence of extracranial spread.
Outcome Variables
Survival data was obtained from the Social Security Death Index database.17 Survival time was calculated from time of surgery to death. Patients whose deaths were unconfirmed were classified as lost to follow-up at the time of their last clinic visit. Local recurrence was noted when there was recurrence or progression of tumor in the previous surgical cavity. Distal recurrence was noted when new tumor not present at the time of surgery appeared in the brain not adjacent to the previous surgical cavity. Local and distal progression-free survival (PFS) times were calculated from the time of surgery to MRI evidence of local and distal recurrence, respectively. Patients whose recurrence was unconfirmed were classified as lost to follow-up at the time of their last neuroimaging.
General Treatment Strategy
Surgery was generally advocated for patients with intracranial metastases without a diagnosis or for lesions causing symptoms due to location or swelling. For all surgically resectable tumors, the general aim was to achieve GTR of the tumor when possible. STR was achieved typically when the tumor involved eloquent brain or critical neurovascular structures as confirmed by intraoperative mapping and/or monitoring (awake/speech language mapping, direct cortical motor stimulation, and motor-evoked or somatosensory-evoked potentials). Surgery was pursued for multiple metastases when the metastases were easily accessible and/or causing symptoms. Motor-evoked and somatosensory-evoked potentials were used in the majority of cases, and surgical navigation (CT and/or MRI wand) was used in all cases after 2001.
Postoperative MRI with gadolinium was typically performed at 3-month intervals following surgery or when symptoms developed. In regards to adjuvant therapy, the uses of radiation therapy, including whole-brain radiation (WBRT) and/or stereotactic radiosurgery (SRS), as well as chemotherapy, were determined by a multidisciplinary team (neurosurgery, neuro-oncology, medical oncology, and radiation oncology) and the patients and their families.
Statistical Analysis
Summary data were presented as mean ± standard deviation for parametric data and as median (interquartile range [IQR]) for nonparametric data. For intergroup comparisons between patients with SBM and non-SBM, student's t-test was used for continuous data and Fisher exact test for categorical data. Survival, local recurrence, and distal recurrence as a function of time were plotted using the Kaplan-Meier method, and Log-rank analysis was used to compare plots (GraphPad Prism 5, La Jolla, California, USA). Values with p < 0.05 in these analyses were considered statistically significant. JMP 9 (SAS, Cary, North Carolina, USA) was used unless otherwise specified.
Results
Preoperative Characteristics of All Patients
The preoperative characteristics of the 708 adults patients in this study are summarized in Table 1. Out of the 708 patients who underwent surgery for an intracranial metastasis, 29 (4%) presented with a SBM. The average age of the entire population at the time of surgery was 58.4 ± 12.1 years. A total of 336 (47%) patients were male, and the major presenting symptoms were headaches in 293 (41%), motor deficits in 254 (36%), cognitive deficits in 146 (21%), vision deficits in 120 (17%), language deficits in 111 (16%), and seizures in 110 (16%). The median (IQR) preoperative KPS of these patients was 80 (70 to 80), and 152 (21%), 421 (59%), and 135 (19%) patients presented with an RPA class of 1, 2, and 3, respectively. The average size of the operated tumor was 3.2 ± 1.5 cm, and 256 (36%) involved the frontal lobe, 154 (22%) the parietal lobe, 102 (14%) the temporal lobe, 101 (14%) the occipital lobe, and 140 (20%) the cerebellum. The median (IQR) number of intracranial metastases was 1 (1 to 2).
Table 1. Preoperative Characteristics of Patients Undergoing Surgery of Skull Base and Nonskull Base Intracranial Metastases from 1997 to 2011.
| Study population (n = 708) | |||
|---|---|---|---|
| Characteristics | Skull base metastases N = 29 |
Nonskull sase metastases N = 679 |
p value |
| Demographics | |||
| Agea Male KPSb Headaches Seizures Motor deficit Language deficit Cognitive deficit Vision deficit |
58.4 ± 13.1 14 (48%) 80 (70-90) 7 (24%) 0 (0%) 2 (7%) 2 (7%) 4 (14%) 12 (41%) |
58.4 ± 12.0 322 (47%) 80 (70-80) 286 (42%) 110 (16%) 252 (37%) 109 (16%) 142 (21%) 108 (16%) |
0.99 0.99 0.49 0.06 0.01 0.0005 0.29 0.48 0.001 |
| Tumor characteristics | |||
| Control of primary Extracranial spread No. of metastatic body met sitesb RPA class RPA class 1 RPA class 2 RPA class 3 Solitary metastasis |
16 (55%) 11 (38%) 1 (1-2) 4 (14%) 18 (62%) 7 (24%) 22 (76%) |
486 (72%) 313 (46%) 1 (1-3) 148 (22%) 403 (59%) 128 (19%) 438 (65%) |
0.06 0.45 0.71 0.36 0.85 0.47 0.24 |
| Primary tumor | |||
| NSCLC SCLC Breast cancer GI cancer Melanoma Renal cell cancer Primary bone cancer Thyroid Unknown Other |
11 (38%) 2 (7%) 7 (24%) 2 (7%) 1 (3%) 0 (0)%) 4 (14%) 1 (3%) 1 (3%) 0 (0%) |
259 (38%) 31 (5%) 99 (15%) 70 (10%) 87 (13%) 51 (8%) 13 (2%) 8 (1%) 8 (1%) 53 (8%) |
0.99 0.39 0.18 0.76 0.24 0.26 0.004 0.05 0.05 0.16 |
| Radiographics | |||
| Tumor sizea No. of brain metsb Hemorrhagic |
3.1 ± 1.6 1 (1-1) 3 (10%) |
3.2 ± 1.5 1 (1-2) 135 (20%) |
0.86 0.18 0.34 |
Abbreviations: KPS, Karnofsky performance score; NSCLC, non-small cell lung cancer; RPA, recursive partitioning analysis; SCLC, small cell lung cancer.
Note: The bolded values are statistically different from one or more of the other histological groups (p < 0.05).
mean ± standard deviation
median (interquartile range).
Perioperative and Postoperative Characteristics of All Patients
The perioperative and postoperative outcomes are summarized in Table 2. 80 (11%) patients underwent resection of multiple intracranial metastases. GTR of the resected lesions was achieved in 502 (71%) patients. Perioperatively, 65 (9%), 10 (1%), and 16 (2%) developed a new motor, language, and vision deficit, respectively. Thirteen (2%) patients developed a wound infection, and 10 (1%) had an intracranial hemorrhage requiring operative evacuation. A total of 249 (35%) patients underwent postoperative chemotherapy, and 449 (63%) underwent radiation therapy. Among patients who underwent radiation therapy, 340 (76%) underwent WBRT and 222 (49%) underwent SRS. At last follow-up, 51 (7%) patients developed leptomeningeal disease.
Table 2. Perioperative and Postoperative Characteristics of Patients Undergoing Skull Base and Nonskull Base Intracranial Metastases from 1997 to 2011.
| Study population (n = 708) | |||
|---|---|---|---|
| Characteristics | Skull base metastases N = 29 |
Nonskull Base metastases N = 679 |
p value |
| Surgery | |||
| Gross total resection Near total resection Subtotal resection Biopsy Multiple mets resected |
10 (34%) 11 (31%) 6 (21%) 2 (7%) 1 (3%) |
492 (72%) 117 (17%) 54 (8%) 16 (2%) 79 (12%) |
0.0001 0.01 0.03 0.17 0.24 |
| New symptoms | |||
| Motor deficit Language deficit Vision deficit |
3 (10%) 0 (0%) 1 (3%) |
62 (9%) 20 (3%) 15 (2%) |
0.74 0.99 0.49 |
| Adjuvant therapy | |||
| Chemotherapy Radiation therapy Whole-brain XRT Stereotactic XRT |
9 (31%) 19 (66%) 10 (34%) 9 (31%) |
240 (35%) 430 (63%) 330 (49%) 213 (31%) |
0.70 0.99 0.18 0.99 |
| Complications | |||
| Wound infection Intracranial hemorrhage Leptomeningeal disease DVT/PE Pneumonia |
2 (7%) 0 (0%) 2 (7%) 2 (7%) 0 (0%) |
11 (2%) 10 (1%) 49 (7%) 27 (4%) 29 (4%) |
0.10 0.99 0.99 0.34 0.63 |
| Survival | |||
| Deaths Median survival Local recurrence 12-month local PFS rate Distal recurrence 12-month distal PFS rate |
18 (62%) 10.0 6 (21%) 75.0% 5 (17%) 82.9% |
496 (73%) 9.2 101 (15%) 76.8% 221 (33%) 53.4% |
0.20 0.48 0.42 0.52 0.10 0.04 |
Abbreviations: DVT, deep vein thrombosis; PE: pulmonary embolism; PFS: progression free survival; XRT, radiation therapy.
Note: The bolded values are statistically different from one or more of the other histological groups (p < 0.05).
In this study, 514 (73%) patients died, 107 (15%) developed local recurrence, and 226 (32%) developed distal recurrence at last follow-up. The median survival of the entire cohort was 9.3 months, with 6-, 12-, and 24-month survival rates of 61%, 42%, and 26%, respectively. The 6-, 12-, and 24-month local PFS rates were 87%, 77%, and 69%, respectively. The median distal PFS was 15.7 months, with 6-, 12-, and 24-month distal progression free survival rates of 72%, 55%, and 42%, respectively. The median (IQR) follow-up time for surviving patients was 10.2 (2.8 to 22.8) months.
Differences between Patients Undergoing Surgery for Skull Base and Nonskull Base Metastases
The differences between patients undergoing resection of SBM and non-SBM are summarized in Tables 1 and 2. Preoperatively, patients undergoing resection of a SBM more commonly presented with visual deficits (p = 0.001) and, less commonly, seizures (p = 0.01) and motor deficits (p = 0.0005). Moreover, patients with SBM more commonly had primary bone cancers (p = 0.004), thyroid cancers (p = 0.05), and unknown primary cancers (p = 0.05). There were no differences between the cohorts in regards to age, KPS, control of primary extracranial spread, and RPA classification group.
Perioperatively, patients who underwent SBM resection less commonly underwent GTR (p = 0.0001) and more commonly underwent NTR (p = 0.01) and STR (p = 0.03). There were no statistical differences in the number of metastases resected, occurrence of iatrogenic deficits, development of leptomeningeal disease, and use of adjuvant therapies including chemotherapy and radiation therapy among patients who underwent SBM and non-SBM resection.
At last follow-up, patients undergoing SBM resection had no difference in overall survival times (p = 0.20) or local PFS (p = 0.52) as compared with patients with non-SBM. However, patients with SBM had longer median distal PFS rates than patients with non-SBM (82.9% versus 53.4%, p = 0.04).
Perioperative Outcomes for Patients with Skull Base Metastases
Metastases were located along the floor of the frontal bone in three patients (10%) with SBM, in the sellar/suprasellar region in seven (24%), the orbital region in six (21%), the sphenoid wing in two (7%), the clivus in three (10%), the petrous bone in four (14%), and the paranasal sinuses in four (14%) (Figs. 1 and 2). Among patients with SBM, 16 (55%) presented with cranial nerve deficits: 6 (38%) had deficits of cranial nerve II; 4 (25%) with cranial nerve III, IV, or VI; 3 (19%) with cranial nerve V; and 6 (38%) with cranial nerve VII. Following surgery, three (50%) had improvements in vision among those with visual complaints, and seven (44%) and seven (44%) had improvements and maintenance of their cranial nerve symptoms among those with cranial nerve deficits, respectively. Three (10%) developed a new motor deficit, none (0%) had new language deficit, one (3%) had new vision deficit, and two (7%) had a new cranial nerve deficit. The patient with a new vision deficit had decreased visual acuity where their eye vision went from 20/25 to 20/200. The other patient who developed new cranial nerve deficit developed a new cranial nerve III deficit.
Survival for Patients with Skull Base Metastases
At last follow-up, 18 (62%) patients with SBM died as compared with 496 (73%) patients with non-SBM (p = 0.20) (Fig. 3). The median survival for patients with SBM was 10 months versus 9.2 months for patients with non-SBM (p = 0.48). The 6-, 12-, 24-, and 36-month survival rates for patients with SBM were 58.4%, 49.3%, 38.4%, and 23.0%, respectively. The 6-, 12-, 24-, and 36-month survival rates for patients with non-SBM were 60.8%, 41.2%, 25.6%, and 17.4%, respectively (p = 0.48).
Fig. 3.
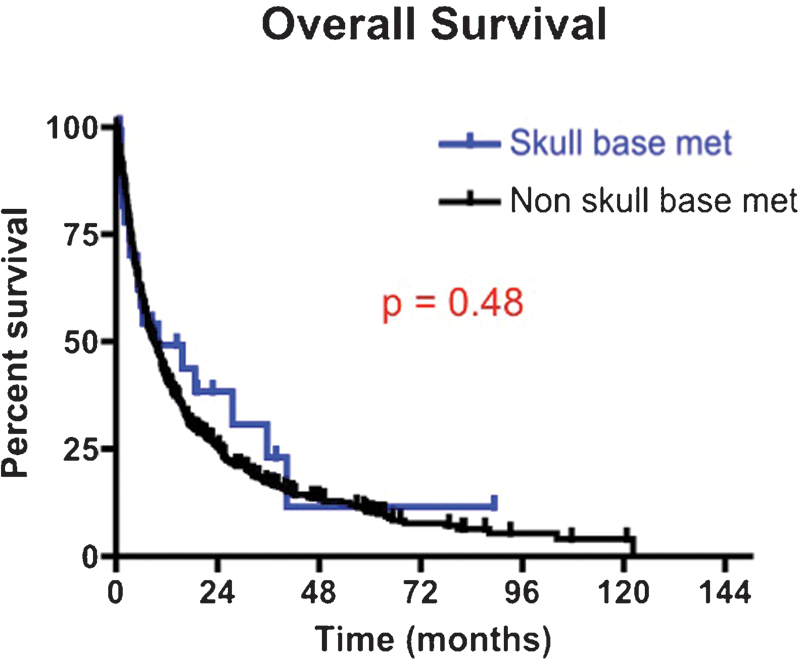
Kaplan-Meier survival curves for patients undergoing intracranial surgery of a skull base metastasis versus nonskull base metastases between 1997 and 2011. The median survival for patients with skull base metastases was 10.0 months versus 9.2 months for patients with nonskull base metastases (p = 0.48).
Local PFS for Patients with Skull Base Metastases
At last follow-up, 6 (21%) patients with SBM incurred local recurrence as compared with 101 (15%) patients with non-SBM (p = 0.42) (Fig. 4). The 6-, 12-, 24-, and 36-month local PFS rates for patients with SBM were 81.8%, 75.0%, 56.3%, and 56.3%, respectively. In comparison, the 6-, 12-, 24-, and 36-month local PFS rates for patients with non-SBM were 86.7%, 76.8%, 69.7%, and 62.0%, respectively (p = 0.52).
Fig. 4.
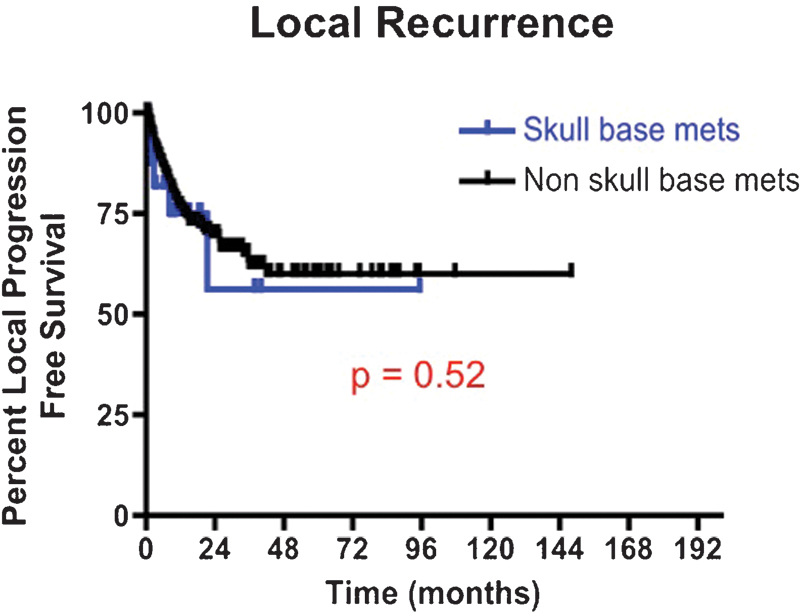
Kaplan-Meier local progression-free survival curves for patients undergoing intracranial surgery of a skull base metastasis versus nonskull base metastases between 1997 and 2011. The 12-month local progression-free survival rate for patients with skull base metastases was 75.0% versus 76.8% for patients with nonskull base metastases (p = 0.52).
Distal Progression Free Survival for Patients with Skull Base Metastases
At last follow-up, 5 (17%) patients with SBM incurred recurrence at a distal intracranial site as compared with 221 (33%) patients with non-SBM (p = 0.10) (Fig. 5). The 6-, 12-, 24-, and 36-month distal PFS rates for patients with SBM were 89.8%, 82.9%, 62.2%, and 62.2%, respectively. In comparison, the 6-, 12-, 24-, and 36-month local PFS rates for patients with non-SBM were 70.8%, 53.4%, 40.9%, and 31.6%, respectively (p = 0.04).
Fig. 5.
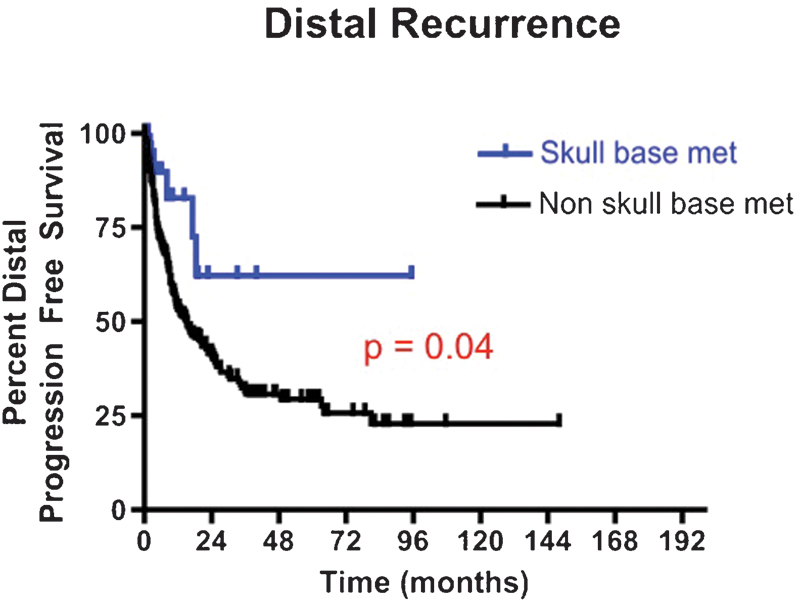
Kaplan-Meier distal, intracranial progression-free survival curves for patients undergoing intracranial surgery of a skull base metastasis versus nonskull base metastases between 1997 and 2011. The 12-month distal, intracranial progression-free survival rate for patients with skull base metastases was 82.9% versus 53.4% for patients with nonskull base metastases (p = 0.04).
Discussion
Patients with SBM represent a rare subgroup of patients with intracranial metastases.10,11,12 In this study, 29 (4%) patients underwent surgery for a SBM during the reviewed period. Patients with SBM more commonly presented with vision deficits, primary bone cancers, thyroid cancers, and unknown primary cancers, unlike patients with non-SBM. Moreover, patients with SBM less frequently had seizures and motor deficits. Perioperatively, patients with SBM less commonly underwent GTR but more commonly had NTR. Among patients with SBM, seven (44%) reported improvement in cranial nerve symptoms and four (14%) reported a new iatrogenic deficit. At last follow-up, there were no significant differences between SBM and non-SBM patients in regards to overall survival and local PFS, but SBM had improved distal PFS.
The brain is a common metastatic site for several types of primary cancers.1 The overwhelming majority of these intracranial metastases involve the brain parenchyma.1 However, a small subset may also involve the skull base.10,11,12 The skull base is in close proximity to several critical neural and vascular elements, which if damaged can cause significant morbidity and mortality. This rarity and proximity to critical structures have made many refrain from offering surgery to patients with SBM, even though patients with other intracranial metastatic locations frequently undergo surgery.10,11,12
Prior studies on SBM are few and limited (Table 3).10,11,12 Mori et al studied the effects of stereotactic radiosurgery on cranial nerve improvements for 11 patients who presented with cranial nerve deficits and SBM.10 The median survival for these patients was 16 months, and 1 (9%) had local recurrence.10 More importantly, 10 of 11 patients reported improvements in cranial nerve symptoms after radiation treatment.10 Pan et al evaluated the effects of gamma knife radiosurgery on 43 patients with skull base malignancies, of which 27 had metastases.11 Patients with SBM had shorter median survival than patients with primary malignancies of the skull base (15 months versus 47 months).11 Tokuuye and colleagues studied the role of fractionated radiation in 64 patients, of which 6 had skull base tumors.12 In this heterogeneous population, better survival was seen in patients with tumors < 5 cm in diameter and/or three or fewer intracranial metastases.12 The pathology of these skull base tumors, however, was not reported.12
Table 3. Summary of Studies on Metastases Involving the Skull Base.
Patients with SBM had different presenting symptoms than patients without SBM. Patients with SBM more commonly presented with vision deficits, and less frequently seizures and motor deficits. This makes intuitive sense as these skull base tumors are in close proximity to the optic nerve, thus more commonly causing vision deficits. Likewise, SBM are not nearby areas commonly responsible for seizures and motor deficits, including cortical regions, mesial temporal lobe, and perirolandic areas.18,19 These differences in location most likely account for the differences seen in presenting symptoms between patients with and without SBM.
It remains unclear which tumors more commonly metastasize to the skull base because studies comparing pathology of SBM to non-SBM have yet to be done. Pan et al had 27 patients with SBM who underwent radiation therapy.11 The most common SBMs were breast cancer in seven (26%), nasopharyngeal carcinoma in five (19%), lung cancer in four (15%), thyroid cancer in one (4%), and unknown cancer in one (4%).11 Mori et al had 11 patients in their study, and 5 (45%) had breast cancer, 3 (27%) had gastrointestinal cancers, and 1 (9%) each had renal cell cancer, melanoma, and prostate cancer.10 The present study primarily comprised patients with lung cancer, breast cancer, and primary bone cancer. However, the proportion of patients with thyroid cancer, primary bone malignancies, and unknown cancers had a larger frequency of SBM as compared with non-SBM.
Patients with SBM are rarely offered aggressive surgery.10,11,12,13 It is presumed that these patients do not do well as compared with primary skull base malignancies.10,11,12,13 It is also assumed that they have a poorer prognosis than patients with non-SBM.10,11,12,13 These assumptions are most likely due to their rarity. In this study, 21 (72%) patients underwent radical resection (GTR or NTR). Despite this, there was no increase in frequency of iatrogenic deficits. Moreover, many of the patients in this study experienced improvement in symptoms following surgical resection. This study thus advocates for aggressive surgical resection of SBM, as with non-SBM, when it is safe to do so.
The long-term outcomes for patients with SBM remain unclear because there is a paucity of studies.10,11,12,13 In this study, the overall survival and local PFS were not significantly different between patients with and without SBM. However, the distal PFS was significantly longer for patients with SBM. This improved distal PFS among patients with SBM may be due to their primary extra-axial location along the skull base. This location may minimize seeding of cerebrospinal fluid pathways during surgery and/or tumor growth. Additionally, this discrepancy may be due to differences in tumor pathology, where tumors that acquire the ability to metastasize to intra-axial areas have a higher propensity to metastasize to other intracranial areas than do SBM. Nonetheless, patients with SBM do not have a worse outcome following surgery than patients with non-SBM. Surgery can also result in improvement in symptoms.
Strength and Limitations
We believe this study provides several useful insights for patients with SBM. First, the outcomes for patients with SBM who undergo surgical resection are unclear. Studies on SBM are limited and only evaluate the effects of radiation therapy.10,11,12,13 Second, this study is the first to compare surgical outcomes for patients with SBM and non-SBM. This study shows that surgery can be pursued with no increase in perioperative morbidity and mortality as compared with patients with non-SBM. Third, this study compared long-term outcomes for patients with SBM and non-SBM. This study shows there are no survival and/or local recurrence differences between patients undergoing surgery for a SBM or non-SBM. Patients with SBM, however, may have improved distal PFS than non-SBM patients. Lastly, this study may provide useful information that may help guide treatment strategies aimed at prolonging survival and delaying recurrence for patients with SBM. These patients, if offered surgery, can have improvement in symptoms and survival outcomes that rival patients without SBM.
This study, however, has some limitations. One limitation is that these findings only apply to patients undergoing surgery for SBM. Patients who did not undergo surgery were excluded, and patients with primary tumors that invaded the skull base were not considered. Patients who were offered surgery for their SBM are likely self-selected to have good outcomes as compared with patients who were not offered surgery. Patients who were not offered surgery were not included in this study. This study was also underpowered to perform multivariate analyses to identify clinical factors associated with survival and recurrence. Larger studies may be necessary to identify these factors, but national databases may be required given the rarity of these lesions. Moreover, this study does not evaluate the effects of surgical approaches. This study was intended to evaluate long-term outcomes for a subset of patients with SBM, and not use of skull base approaches. Furthermore, the patients in this study underwent disparate treatment regimens. A significant number of patients in this study did not undergo WBRT, SRS, and/or chemotherapy. Mori et al found good results in terms of survival and cranial nerve symptoms for patients with SBM and SRS.10 Patients in this study were not routinely offered SRS because of close proximity and potential endangerment of cranial nerves. The results of this study may be altered in the context of patients receiving more aggressive therapies. Finally, this study is inherently limited by its retrospective design. This design means that there may be an inherent bias associated with patient selection, where patients who were offered surgery may have a propensity for better outcomes. However, we tried to create a uniform patient population by utilizing strict inclusion criteria, thus providing more relevant information for patients undergoing surgery for a SBM. Given these criteria and relatively precise outcome measures, we believe our findings offer useful insights into outcomes for patients with SBM. However, prospective studies are needed to provide better data to guide clinical decision-making.
Conclusion
Patients with SBM are a rare subset of patients with intracranial metastases. These tumors are in close proximity to critical neural and vascular structures. Their rarity and close proximity to critical structures have made many tend to refrain from offering aggressive surgical therapies. This study highlights the findings that patients with SBM can be treated aggressively without an increase in morbidity and mortality and have survival and recurrence rates that are comparable to patients with non-SBM. Patients with SBM should therefore be considered for aggressive therapies.
Acknowledgments
KLC was supported the NIH T32 Training Grant.
References
- 1.Eichler A F, Loeffler J S. Multidisciplinary management of brain metastases. Oncologist. 2007;12(7):884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 2.Ewend M G Morris D E Carey L A Ladha A M Brem S Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy J Natl Compr Canc Netw 200865505–513., quiz 514 [DOI] [PubMed] [Google Scholar]
- 3.Claus E B. Neurosurgical management of metastases in the central nervous system. Nat Rev Clin Oncol. 2012;9(2):79–86. doi: 10.1038/nrclinonc.2011.179. [DOI] [PubMed] [Google Scholar]
- 4.Ewend M G, Williams J A, Tabassi K. et al. Local delivery of chemotherapy and concurrent external beam radiotherapy prolongs survival in metastatic brain tumor models. Cancer Res. 1996;56(22):5217–5223. [PubMed] [Google Scholar]
- 5.Ewend M G, Brem S, Gilbert M. et al. Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control. Clin Cancer Res. 2007;13(12):3637–3641. doi: 10.1158/1078-0432.CCR-06-2095. [DOI] [PubMed] [Google Scholar]
- 6.Frazier J L, Batra S, Kapor S. et al. Stereotactic radiosurgery in the management of brain metastases: an institutional retrospective analysis of survival. Int J Radiat Oncol Biol Phys. 2010;76(5):1486–1492. doi: 10.1016/j.ijrobp.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Grossman R, Mukherjee D, Chang D C. et al. Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann Surg Oncol. 2011;18(2):521–528. doi: 10.1245/s10434-010-1299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barajas R F Jr, Cha S. Imaging diagnosis of brain metastasis. Prog Neurol Surg. 2012;25:55–73. doi: 10.1159/000331174. [DOI] [PubMed] [Google Scholar]
- 9.Delattre J Y, Krol G, Thaler H T, Posner J B. Distribution of brain metastases. Arch Neurol. 1988;45(7):741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 10.Mori Y, Hashizume C, Kobayashi T, Shibamoto Y, Kosaki K, Nagai A. Stereotactic radiotherapy using Novalis for skull base metastases developing with cranial nerve symptoms. J Neurooncol. 2010;98(2):213–219. doi: 10.1007/s11060-010-0179-8. [DOI] [PubMed] [Google Scholar]
- 11.Pan J, Liu A L, Wang Z C. Gamma knife radiosurgery for skull base malignancies. Clin Neurol Neurosurg. 2013;115(1):44–48. doi: 10.1016/j.clineuro.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Tokuuye K, Akine Y, Sumi M. et al. Fractionated stereotactic radiotherapy of small intracranial malignancies. Int J Radiat Oncol Biol Phys. 1998;42(5):989–994. doi: 10.1016/s0360-3016(98)00293-4. [DOI] [PubMed] [Google Scholar]
- 13.Pommier P, Liebsch N J, Deschler D G. et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132(11):1242–1249. doi: 10.1001/archotol.132.11.1242. [DOI] [PubMed] [Google Scholar]
- 14.Dutta D, Vanere P, Gupta T, Munshi A, Jalali R. Factors influencing activities of daily living using FIM-FAM scoring system before starting adjuvant treatment in patients with brain tumors: results from a prospective study. J Neurooncol. 2009;94(1):103–110. doi: 10.1007/s11060-009-9810-y. [DOI] [PubMed] [Google Scholar]
- 15.Gaspar L, Scott C, Rotman M. et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 16.McGirt M J, Chaichana K L, Gathinji M. et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 17.Social Security Death Index Database Available at: http://searches.rootsweb.ancestry.com/. Accessed March 22, 2013.
- 18.Chaichana K L, Parker S L, Olivi A, Quiñones-Hinojosa A. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009;111(2):282–292. doi: 10.3171/2009.2.JNS081132. [DOI] [PubMed] [Google Scholar]
- 19.Chaichana K L, Pendleton C, Zaidi H. et al. Seizure Control for Patients Undergoing Meningioma Surgery. World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.02.051. [DOI] [PubMed] [Google Scholar]


