Abstract
Complete tumor resection with preservation or improvement of visual function is the goal of tuberculum sellae meningioma (TSM) treatment. The authors retrospectively reviewed 51 patients treated surgically for TSM between 2003 and 2010, with special attention to surgical technique, visual outcomes, and prognostic factors for treatment outcome.
All patients were operated via the lateral subfrontal approach. The cohort mean age and Karnofsky performance status (KPS) on admission was 57.1 ± 13.6 and 84.3 ± 11.7, respectively. The most common presenting sign was visual impairment. The mean tumor size was 29.4 ± 10.7 mm. In 45 of the patients (88.2%), gross total resection was achieved. Improvement and/or preservation of visual acuity and visual field were achieved in 95.9% and 85.3%, respectively. Visual functions on admission were found to be the strongest predictors for postoperative improvement in visual outcome, followed by better KPS on admission, smaller tumor size, and young age. Postoperative neurological complications included cerebrospinal fluid (CSF) leak, meningitis, and postoperative seizures.
TSM can be safely operated on through the lateral subfrontal approach. A high percentage of complete tumor resection and excellent visual outcomes are achieved using this technique. Surgical treatment in the early stage of the disease may result in a better visual outcome.
Keywords: meningioma, optic nerve, surgical technique, visual outcome
Introduction
Tuberculum sellae meningiomas (TSMs) represent a distinct clinical entity among the intracranial meningiomas. They arise from the tuberculum sellae, planum sphenoidale, and chiasmatic sulcus and account for 5 to 10% of all intracranial meningiomas.1,2 The tumor characteristically involves the optic chiasm and optic nerves (ONs), causing a progressive visual loss that may result in blindness. Several transcranial microsurgical approaches have been traditionally used for the resection of TSMs, and the pterional-transsylvian and unilateral subfrontal approaches are the most popular among them. There have been several recent reports on an extended endoscopic endonasal transsphenoidal approach as an alternative route to the transcranial ones, but its long-term outcome has yet to be determined.
We present our experience with microsurgical treatment via a unilateral subfrontal approach on 51 patients presenting with TSM. We focus on detailed neuro-ophthalmological evaluation, surgical technique, related complications, and outcome. In addition, we identify prognostic factors associated with improved visual outcome following surgery.
Patients and Methods
Study Population
Between October 2003 and September 2010, 51 patients with TSMs were treated surgically by the senior author (NM) at the Department of Neurosurgery of the Tel Aviv Medical Center, Tel Aviv, Israel. Approval from the institutional review board (#0323-11 TLV) was obtained prior to data collection and analysis. Patients' medical records, including clinical and ophthalmological reports, imaging studies, operative reports, follow-up evaluations from the outpatient clinic, and histopathological records of the tumors were evaluated retrospectively.
Neuro-ophthalmological Evaluations
All patients underwent detailed neuro-ophthalmological evaluations before and after surgery. Evaluation of visual acuity (VA) was done using the Snellen chart; visual field (VF) evaluation was done using the Humphrey and Goldman automated perimetry, and the optic discs were examined.
Neuro-ophthalmological evaluation records were available for analysis in 50 of the 51 patients that were included in the study. Postoperative neuro-ophthalmological evaluations were partial in three patients. The preoperative and postoperative visual evaluation used the following grading systems: VA was graded using the logMAR scale modified to also represent patients with very low vision.3 This modification gives a quantitative measurement to patients with poor visual status and allows further analytical tests to be performed. The logMAR modification includes counting fingers (CF = 1.86), hand motion (HM = 2.30), light perception (LP = 2.70), and no light perception (NLP = 3.0). VFs were graded on an ordinal scale range from 0 to 6 as follow: 6 = normal, 5 = slight constriction, 4 = loss of single quadrant, 3 = loss of 2 quadrants, 2 = loss of 3 quadrants, 1 = severe constriction, and 0 = blindness. Optic disc appearance was categorized as normal, swollen disc, and optic atrophy. Preoperative neuroradiological evaluations included computed tomography (CT) and magnetic resonance imaging (MRI) studies. Shortly following surgery, all patients had a repeat CT study, and a repeat MRI was obtained at 3 months postoperative and again once yearly thereafter. Extent of tumor resection was evaluated by the surgeon's impression during surgery and validated by the first postoperative MRI. The grade of tumor resection was determined using the Simpson grading system.4
Measurement of Study Parameters
Changes in two visual indices (VA, VF) resulting from the 51operations were measured. Patient characteristics were also retrieved, including age, KPS on admission, tumor size, gender, duration of symptoms, and ophthalmological evaluation.
Surgical Technique
Our surgical technique has been described in a previous paper.5 We used a unilateral subfrontal approach in most of the cases described in the current report, with the reasoning that tumors located lateral to the optic nerve (ON, anterior clinoid and sphenoid wing) are approached through lateral approaches, whiereas the medial tumors (olfactory, planum, and tuberculum) are approached through frontal approaches.6 The unilateral subfrontal approach allows a direct view of the ipsilateral optic nerve (ON), the ipsilateral internal carotid artery (ICA), and the ipsilateral sylvian fissure. Early in the course of surgery, following the removal of the anterior dural insertion of the tumor, the approach also gives a direct visualization of the contralateral ON and ICA.
In our approach, we identify the ipsilateral ON in an early stage of the surgery, as it may be found behind or lateral to the ipsilateral margin of the tumor. Then, using bipolar cautery, we disconnect the tumor from the tuberculum base while approaching the contralateral side. At the expected location of the contralateral ON, we stop using the bipolar cautery and use either a dissector or forceps until we identify the “hidden” ON. We can then coagulate the entire tumor base.
Choosing the side of the craniotomy is a cardinal issue when using the unilateral subfrontal approach. In our decision-making process, we consider the following: lateralization of brain function, location of the tumor (median versus para-median) and documented visual deficit affecting one eye more than the other. In patients whose brain imaging studies demonstrate a midline tumor and symmetrical involvement of the ONs, we approach the tumor from the nondominant cerebral hemisphere. This approach is aimed to minimize possible damage to a dominant frontal lobe. We tend to approach the tumor from the side contralateral to where it predominantly affects, such as in the following cases:
When the tumor's location is predominantly paramedian or further lateral on the tuberculum
Where preoperative imaging demonstrates a tumor that is closely associated with one ON or located under and lateral to that ON
In patients with a documented visual deficit affecting one side more than the other
In general, we find that looking at the contralateral ON provides us with a wider visual field of the contralateral tumor border, especially the parts located inferiorly to the ON and the contralateral ICA. It is true that the contralateral ON may not be visible immediately following the craniotomy; however, following the demonstration of the contralateral ON, as described previously, the operative field is wider. This is unlike the situation with the ipsilateral ON that requires its manipulation while removing tumor that is located posterior to or under it. We think that any further manipulation to an ON, which is already compromised by tumor abutment, may worsen the patient's visual status. For that reason, our surgical aim is to minimize the manipulation of the most affected ON. Importantly, identifying the position of the contralateral ICA coursing below the ON in this segment is crucial to avoid injury to the artery.
We perform a relatively small craniotomy over the lateral half of the orbit and down to the upper part of the lateral wall of the orbit. The orbital rim is left in place. Of note, we usually use a neuronavigation system to identify the frontal sinus and to plan the frontal aspect of the craniotomy. In the frontal portion of the craniotomy, we aim as low and medial as possible without violating the frontal sinus. An important step in our approach is the drilling of the prominences of the orbital roof without the rim. This allows us a flat and low trajectory to the tuberculum and tumor base. Before opening the dura, we orient ourselves toward the area of the tumor base and make sure that all the bony protuberances are drilled (Fig. 1). We usually do not use a retractor for the frontal lobe. The head positioning and CSF drainage at the beginning of the intradural part allows the frontal lobe to “fall” from the frontal base, preventing injury to the frontal lobe.
Fig. 1.
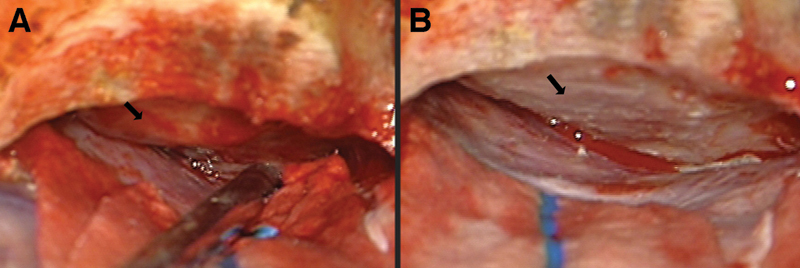
Flattening the bony orbital roof; depiction of a right orbital roof following dural elevation of the right frontal lobe. The arrow demonstrates the bony protuberance that is on the trajectory to the tuberculum area (A) before and (B) after drilling the protuberance of the bony orbital roof to gain a better view of the tuberculum.
The ON can be identified either extradurally or intradurally. In most cases, we elevate the frontal lobe extradurally and identify the dural sleeve of the ON entering the optic canal. Intradurally, we follow the nerve toward the orbit and localize the optic canal. We section the falciform ligament to free the nerve and to enable manipulation of the nerve laterally or medially using an arachnoid knife or sharp hook. This is followed by the drilling of the optic canal7,8 using a diamond drill (3 or 4 mm) under continuous irrigation. The drilling has the advantage of unroofing the ON while leaving the anterior clinoid process intact. This is different from clinoid meningioma cases where drilling the clinoid process is done extradurally and where removing the clinoid tip is crucial for complete removal of the tumor. Hyperostosis on the tuberculum or planum area in these cases is drilled during this stage, as well. When we drill the optic canal or the tuberculum area, we are very cautious and observant for any entry into air cell. If an air cell is opened, we enlarge the opening to accommodate a plug of fat or a piece of pericranium. This will be covered with Surgicel (Ethicon, Somerville, New Jersey, USA) and biological glue.
The microsurgical removal of the tumor begins with coagulation of the tumor base on the planum and tuberculum. This is done in the midline area first, taking care to avoid the ONs. At this stage, only the ipsilateral ON can be located because the contralateral ON is hidden behind the tumor. After the tumor has been disconnected from its base, debulking is done either with an ultrasonic aspirator or with forceps in smaller tumors. Bleeding from the base of the tumor, from the dura or from the bone under is coagulated with bipolar or monopolar set to low power and with the use of bone wax. The debulked tumor is then removed from the ipsilateral ON. An arachnoid plane can be found between the ON and the tumor in most of our cases, even in the larger tumors. At this stage, the contralateral ON is identified either from the bony tuberculum and coursing posteriorly or from the cistern of the chiasm coursing anteriorly. After the tumor base has been coagulated in the area of the contralateral nerve, we make sure that the contralateral ICA is identified and preserve the arachnoid between it and the tumor.
The last part of the procedure involves the tumor interface with the frontal lobes, the A1, ACOM, and the A2 of the anterior cerebral arteries, and the optic chiasm. It is important to bear in mind that there are no “tumor vessels” in this area since the blood supply to the tumor comes from the dural attachment. Therefore, all the arterial perforators found on the tumor surface should be preserved and carefully dissected from the tumor. The pituitary stalk is also located posterior to the tumor with a protective arachnoid membrane in front of it. The exceptions here are true diaphragm sella tumors, where the tumor base is posterior and inferior to the tuberculum and on the diaphragm itself. In those cases, the stalk may not possess the protecting arachnoid membrane between it and the tumor, so protecting it may be more difficult (Fig. 2).
Fig. 2.
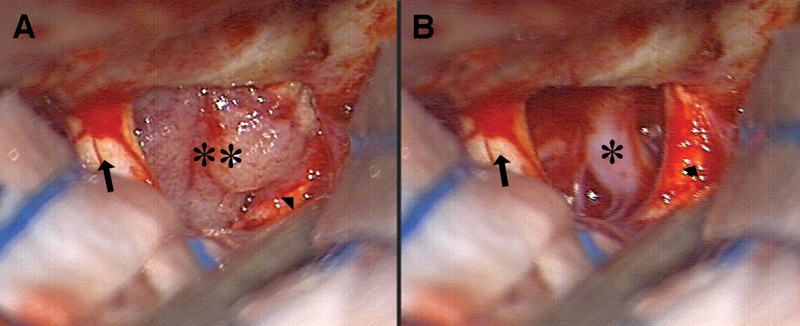
Surgical view before and after tumor removal. (A) Surgical view seen at the beginning of tumor removal. Note the left optic nerve (arrow), the tumor (two asterisks), and the right optic nerve (arrow head), which can be difficult to identify at this early stage of tumor removal. (B) Surgical view after tumor removal. Note the released right optic nerve (arrow head) and the visible right carotid artery (asterisk) with its perforators. The tumor attachment reached the area of the right carotid artery and coagulation of this area is done after identifying the artery.
Following the resection of tumor and the release of both ONs and chiasm, we focus our attention on completing a gross total tumor removal (Simpson I). For the removal of the dural attachments, we initially coagulate the dura with bipolar or low-current Bovie cautery and then remove it using microscissors. Any drilling of the tuberculum area bone is done with a 3-mm diamond drill in small steps to prevent violation of the sphenoid or ethmoidal air cells.
Statistics Analysis
The Wilcoxon signed rank nonparametric tests were used to evaluate the postoperative changes in visual indices given on an ordinal scale and for the logMAR given as a continuous variable using paired sample t-test. The association between patient characteristics on admission and ophthalmological evaluation on admission were evaluated by the Spearman or Pearson correlation coefficient as needed. A multiple linear regression analysis was performed to evaluate the difference between preoperative and postoperative visual indices as a function of patient characteristics on admission. A multivariate ordered logistic regression and linear regression were used to examine the association between visual outcome scores and the patients' ophthalmological status on admission adjusted to demographic characteristics on admission. An ordinal logistic regression based on the proportional odds model was employed where the dependent variable consisted of a scaled variable (VFs). To determine which variables predict improvement in the logMAR we used the logMAR as a dichotomous variable and applied logarithmic regression model. Statistical analysis was performed by the SAS for Windows version 9.2 and by the SPSS V19.1 (SPSS Inc., Chicago, Illinois, USA).
Results
The study group comprised of 38 women and 13 men with an age range of 28 to 83 years (mean + standard deviation [SD] 57.1 ± 13.6). The mean KPS on admission was 84.3 ± 11.7 (range 50 to 100). The most common presenting sign was visual impairment, followed by headache and dizziness. The tumor was found incidentally in 11 patients (11/51, 21.6%). The mean duration of symptoms for the entire study group was 13.9 ± 17.61 months (range 1 month to 5 years). The tumor size, as measured from the preoperative imaging studies, ranged from 12 mm to 76.6 mm (mean 29.4 ± 10.7). The patients' demographic and pathology data are summarized in Tables 1 and 2a, 2b.
Table 1. Summary of Demographics and Pathology Data in 51 Patients.
| Age, y (min-max) (Mean ± SD) |
28 - 83 57.1 ± 13.6 |
| Sex | Female: 38 (74.5%) Male: 13 (25.5%) |
| KPS (min-max) (Mean ± SD) |
50 - 100 84.3 ± 11.7 |
| Visual impairment | Both eyes: 37 (72.5%) Rt eye: 3 (5.9%) Lt eye: 3 (5.9%) None: 7 (13.7%) |
| Presenting signs | Visual impairment: 34 (66.7%) Headache: 10 (19.6%) Dizziness: 1(2%) Incidental: 11 (21.6%) |
| Tumor size, mm (Mean ± SD) | 29.4 ± 10.7 |
| Duration of symptoms, month (Mean ± SD) | 13.9 ± 17.6 |
Abbreviations: KPS, Karnofsky performance scale; Lt, left; Rt, right; SD, standard deviation.
Table 2a. Summary of Patient Characteristics (Patients 1-25).
| Pt | Age, y | Sex | KPS | Tumor size, mm | Presenting signs | Simpson grade | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 75 | F | 80 | 29 | Hallucinations | 3 | Apnea and death 16 days postoperative |
| 2 | 69 | F | 100 | 23 | Incidental finding | 1 | None |
| 3 | 28 | F | 90 | 40 | Headaches | 1 | None |
| 4 | 57 | F | 80 | 29 | Visual disturbances | 2 | None |
| 5 | 66 | M | 70 | 26 | Visual disturbances | 4 | Rt frontal contusion |
| 6 | 35 | F | 80 | 40 | Incidental finding | 2 | Rt frontal contusion |
| 7 | 52 | F | 90 | 18 | Headaches | 2 | Rt frontal contusion |
| 8 | 60 | F | 80 | 28 | Visual disturbances | 1 | None |
| 9 | 46 | F | 70 | 42 | Visual disturbances | 3 | None |
| 10 | 56 | F | 100 | 17 | Incidental finding | 1 | None |
| 11 | 80 | M | 80 | 23 | Incidental finding | 1 | None |
| 12 | 51 | F | 90 | 41 | Visual disturbances | 1 | None |
| 13 | 40 | F | 80 | 30 | Visual disturbances and headaches | 2 | None |
| 14 | 83 | F | 80 | 30 | Visual disturbances and headaches | 2 | None |
| 15 | 62 | M | 90 | 20 | Visual disturbances | 1 | None |
| 16 | 65 | F | 70 | 30 | Incidental finding | 2 | None |
| 17 | 52 | F | 90 | 30 | Visual disturbances | 2 | None |
| 18 | 67 | F | 80 | 13 | Visual disturbances | 2 | None |
| 19 | 74 | F | 90 | 25 | Visual disturbances | 3 | None |
| 20 | 63 | M | 90 | 35 | Visual disturbances | 2 | None |
| 21 | 53 | F | 90 | 12 | Headaches | 1 | None |
| 22 | 66 | F | 90 | 28 | Visual disturbances | 1 | None |
| 23 | 38 | F | 90 | 28.8 | Visual disturbances | 3 | None |
| 24 | 70 | M | 80 | 28.9 | Visual disturbances | 2 | None |
| 25 | 68 | F | 70 | 28.5 | Incidental finding | 1 | None |
Abbreviations: F, female; KPS, Karnofsky performance scale; M, male; Rt, right; Pt, patient; SD, standard deviation.
Table 2b. Summary of Patient Characteristics (Patients 26-51).
| Pt | Age,y | Sex | KPS | Tumor size, mm | Presenting signs | Simpson grade | Complications |
|---|---|---|---|---|---|---|---|
| 26 | 71 | F | 50 | 35.2 | Visual disturbances | 1 | Pneumocephalus; CSF leak 1 month postop. |
| 27 | 71 | F | 90 | 30 | Visual disturbances | 1 | None |
| 28 | 66 | F | 90 | 24 | Visual disturbances | 1 | None |
| 29 | 36 | F | 90 | 33 | Visual disturbances | 1 | CSF leak |
| 30 | 68 | M | 80 | 18 | Visual disturbances | 1 | None |
| 31 | 38 | F | 60 | 40.2 | Visual disturbances | 1 | Seizures |
| 32 | 58 | M | 90 | 15 | Visual disturbances and headaches | 1 | None |
| 33 | 63 | F | 100 | 15.5 | Visual disturbances, headaches, dizziness | 2 | None |
| 34 | 67 | M | 80 | 76.6 | 2 | Aseptic meningitis | |
| 35 | 57 | M | 100 | 13.25 | Incidental finding | 1 | Seizures |
| 36 | 48 | F | 90 | 28.9 | Visual disturbances | 1 | None |
| 37 | 57 | F | 90 | 33.2 | Headaches | 1 | None |
| 38 | 79 | F | 90 | 26.1 | Visual disturbances | 2 | None |
| 38 | 39 | F | 90 | 25 | Visual disturbances | 1 | None |
| 40 | 72 | M | 80 | 35.8 | Incidental finding | 1 | None |
| 41 | 60 | F | 50 | 41.2 | Visual disturbances | 2 | Pulmonary embolism; meningitis |
| 42 | 52 | F | 70 | 27 | Visual disturbances | 2 | Seizures |
| 43 | 51 | F | 90 | 42.3 | Visual disturbances | 2 | None |
| 44 | 44 | M | 80 | 32 | Visual disturbances | 1 | None |
| 45 | 47 | M | 100 | 28.1 | Incidental finding | 1 | None |
| 46 | 70 | M | 90 | 28.2 | Visual disturbances | 2 | None |
| 47 | 73 | F | 70 | 44.9 | Incidental finding | 1 | None |
| 48 | 42 | F | 100 | 30 | Visual disturbances | 2 | None |
| 49 | 35 | F | 90 | 15.4 | Visual disturbances and headaches | 1 | None |
| 50 | 39 | F | 90 | 25 | Visual disturbances and headaches | 1 | None |
| 51 | 64 | F | 100 | 41.2 | Incidental finding | 3 | CSF leak |
Abbreviations: CSF, cerebrospinal fluid; F, female; KPS, Karnofsky performance scale; M, male; Pt, patient; SD, standard deviation.
Extent of Resection and Follow-up
Gross total resection was achieved in 45 patients (45/51, 88.2%), and graded Simpson 1 in 26 patients (51.0%) and Simpson 2 in 19 patients (37.2%). A small residual piece of tumor was left (Simpson 3) either on the ON or on the carotid artery in five patients. Only partial removal (Simpson 4) was possible in one patient (#5 in Tables 1 and 2a, 2b): his preoperative vision was very poor and characterized by blindness in the right eye and visual impairment in the left eye. The tumor of Patient #5 had infiltrated into the chiasm and the decision was made intraoperatively to stop resection to prevent further damage to the left ON and chiasm function. See Fig. 3 and Fig. 4 for preoperative and postoperative MRI images of patients with small and big TSM, respectively.
Fig. 3.
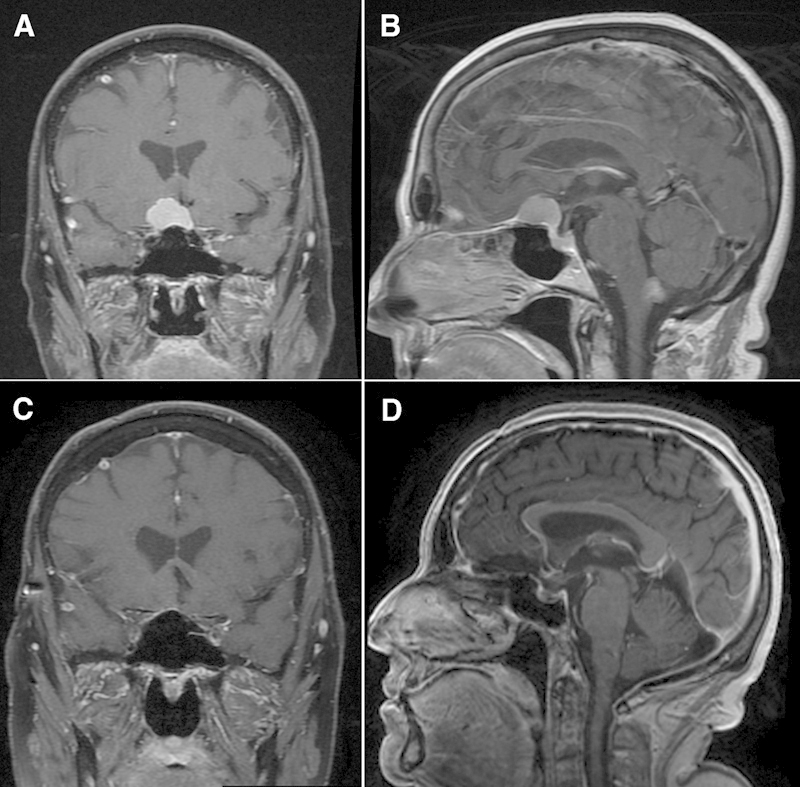
Preoperative and postoperative magnetic resonance imaging (MRI) studies of representative small tuberculum sellae meningioma case. (A, B) Preoperative coronal and sagittal view, respectively, of a brain T1-weighted MRI study with contrast material. (C, D) Postoperative coronal and sagittal view, respectively, of a brain T1-weighted MRI studies with contrast material.
Fig. 4.
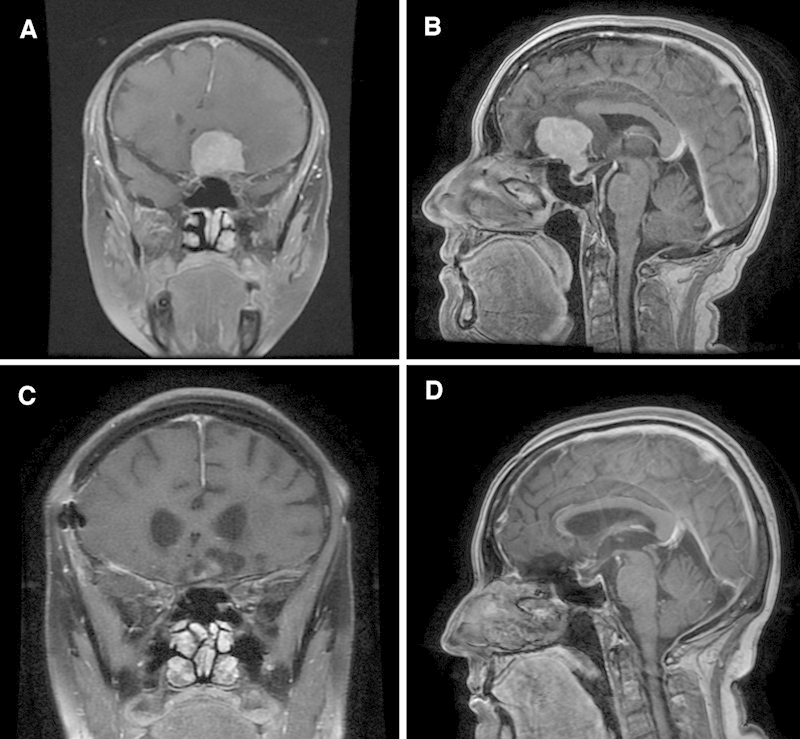
Preoperative and postoperative magnetic resonance imaging (MRI) studies of representative large tuberculum sellae meningioma case. (A, B) Preoperative coronal and sagittal view, respectively, of a brain T1-weighted MRI studies with contrast material. (C, D) Postoperative coronal and sagittal view, respectively, of a brain T1-weighted MRI studies with contrast material.
The postoperative follow-up ranged from 7 years for the first patient of our series to 2 months for the last patient undergoing surgery before study closure. The median postoperative follow-up period was 42.1 months. Three patients were lost to follow-up. None of the patients underwent reoperation or radiation for residual tumors or for recurrences.
Neuro-ophthalmological Evaluations
On Admission
Preoperative VA deficits were present in 41 (41/50, 82%) patients, ranging from very poor VA state (2.30 [HM] to 3.0 [NLP] on LogMAR scale) in 6 eyes (6/99, 6.1%) to normal VA (0.1 to 0 on LogMAR scale) in 38 eyes (38/99, 38.4%). The mean preoperative VA for the entire cohort was 0.54 ± 0.8. A VF defect was detected in 44 patients (44/50, 88%), and it consisted mainly of a 2/4 VF loss (stage 3 on the VF scale) in 23 eyes (23/99, 23.5%). The next most common defect was single quadrantanopsia (stage 4 on the VF scale) involving 15 eyes (15/99, 15.3%). The mean pre-operative VF status of the entire cohort was 3.36 ± 1.97.
Optic discs examinations on admission revealed 50 eyes with normal appearance and 14 eyes with optic disc atrophy. Three patients presented with swollen discs. The VA and VF scores obtained at presentation and at the postoperative follow-up evaluation are summarized in Table 3a, 3b.
Table 3a. Summary of Visual Acuity and Visual Fields in 51 Patients Preoperative and Postoperative (Patients 1-25).
| Pt | Age, y | Sex | Preop VA, Lt eye | Postop VA, Lt eye | Preop VA, Rt eye | Postop VA, Rt eye | Preop VF, Lt eye | Postop VF, Lt eye | Preop VF, Rt eye | Postop VF, Rt eye |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | F | 0.2 | 0.2 | 0.2 | 0.2 | 1 | 1 | 1 | 2 |
| 2 | 69 | F | 0.2 | 0.2 | 0.4 | 0.4 | 3 | 3 | 3 | 3 |
| 3 | 28 | F | 0 | 0 | 0 | 0 | 6 | 6 | 6 | 6 |
| 4 | 57 | F | 0.4 | 0.4 | 0.4 | 0.4 | 3 | 3 | 2 | 2 |
| 5 | 66 | M | 0.4 | 0 | 3 | 1.86 | 3 | 3 | 0 | 0 |
| 6 | 35 | F | 3 | 3 | 0.2 | 0.2 | 0 | 0 | 3 | 3 |
| 7 | 52 | F | 0 | 0 | 0 | 0 | 6 | 6 | 6 | 6 |
| 8 | 60 | F | 0 | 0 | 0.4 | 0 | 6 | 6 | 2 | 5 |
| 9 | 46 | F | 0.2 | 0.2 | 3 | 3 | 3 | 3 | 0 | 0 |
| 10 | 56 | F | 0 | 0.2 | 0 | 0 | 6 | 3 | 6 | 5 |
| 11 | 80 | M | 0.4 | 0.2 | 1 | 0.5 | 6 | 3 | 5 | 3 |
| 12 | 51 | F | 0.8 | 0 | 1.86 | 0 | 4 | 5 | 1 | 5 |
| 13 | 40 | F | 0.1 | 0.1 | 0.2 | 0.2 | 4 | 6 | 0 | 4 |
| 14 | 83 | F | 1.86 | 1.86 | 0.4 | 0.3 | 1 | UK | 4 | UK |
| 15 | 62 | M | 0 | 0 | 0.4 | 0 | 6 | 6 | 1 | 3 |
| 16 | 65 | F | 0.4 | 0.2 | 0 | 0 | 4 | 4 | 4 | 4 |
| 17 | 52 | F | 0.2 | 0.1 | 0.3 | 0.2 | 1 | 2 | 1 | 1 |
| 18 | 67 | F | 0 | 0.1 | 1.3 | 1.3 | 5 | 5 | 3 | 2 |
| 19 | 74 | F | 0.7 | 1.86 | 0.2 | 0.1 | 3 | 0 | 5 | 3 |
| 20 | 63 | M | 0.2 | 0.2 | 0.1 | 0.1 | 4 | 4 | 5 | 5 |
| 21 | 53 | F | 0 | 0 | 0 | 0 | 6 | 6 | 6 | 6 |
| 22 | 66 | F | 0 | 0 | 0.1 | 0.1 | 4 | 5 | 3 | 5 |
| 23 | 38 | F | 0.1 | 0 | 0.5 | 0 | 5 | 6 | 4 | 5 |
| 24 | 70 | M | 0.2 | 0.1 | 0.2 | 0.2 | 1 | 2 | 5 | 4 |
| 25 | 68 | F | 1.86 | 0.2 | 0.1 | 0 | 1 | 3 | 5 | 5 |
Abbreviations: Lt, left; Postop, postoperative; Preop, preoperative; Pt, patient; Rt, right; UK, unknown; VA, visual acuity; VF, visual field.
A total of 101 eyes were evaluated in the 51 patients.
Visual acuity was graded using the logMAR scale modified to represent also patients with very low vision: counting fingers = 1.86, hand motion = 2.30, light perception = 2.70, and no light perception = 3.0.
Visual fields were graded using a scale of 7 stages: 6 = normal visual fields; 5 = mild, constriction; 4 = ¼ of visual field defect; 3 = ½ of visual field defect; 2 = ¾ of visual field defect; 1 = severe constriction of visual fields; 0 = total scotoma.
Table 3b. Summary of Visual Acuity and Visual Fields in 51 Patients Preoperative and Postoperative (Patients 26-51).
| Pt | Age,y | Sex | Preop VA, Lt eye | Postop VA, Lt eye | Preop VA, Rt eye | Postop VA, Rt eye | Preop VF, Lt eye | Postop VF, Lt eye | Preop VF, Rt eye | Postop VF, Rt eye |
|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 71 | F | 0.2 | 0.2 | 3 | 1.86 | 3 | 3 | 0 | 0 |
| 27 | 71 | F | 0.8 | 0.2 | 0 | 0 | 3 | 5 | 6 | 5 |
| 28 | 66 | F | 0.1 | 0.1 | 0.2 | 0.2 | 4 | 5 | 0 | 5 |
| 29 | 36 | F | 0.1 | 0 | 0 | 0 | 2 | 6 | 3 | 6 |
| 30 | 68 | M | UK | UK | UK | UK | UK | UK | UK | UK |
| 31 | 38 | F | 0 | 0 | 0.2 | 0 | 4 | 5 | 3 | 5 |
| 32 | 58 | M | 1.86 | 0.3 | 2.28 | 0.3 | 0 | 3 | 0 | 3 |
| 33 | 63 | F | 0 | 0 | 1 | 0.2 | 5 | 5 | 4 | 2 |
| 34 | 67 | M | 0 | 0 | NE | NE | 3 | 5 | NE | NE |
| 35 | 57 | M | 0 | 0 | 0 | 0 | 6 | 5 | 6 | 6 |
| 36 | 48 | F | 0 | 0 | 0 | 0.2 | 5 | 6 | 3 | 4 |
| 37 | 57 | F | 0 | 0 | 0 | 0 | 6 | 6 | 6 | 6 |
| 38 | 79 | F | 0.8 | 0.3 | 1.86 | 1.86 | 4 | 5 | 1 | 1 |
| 39 | 39 | F | 0.2 | 0 | 0 | 0 | 4 | 6 | 6 | 6 |
| 40 | 72 | M | 0.1 | 0.1 | 1.86 | 1.3 | 5 | 3 | 1 | 1 |
| 41 | 60 | F | 1.86 | 1.3 | 3 | 3 | 1 | 1 | 0 | 0 |
| 42 | 52 | F | 0.8 | 0.2 | 0.8 | 0 | 3 | 2 | 3 | 2 |
| 43 | 51 | F | 0.2 | 0.2 | 0.3 | 0.3 | 3 | 3 | 3 | 3 |
| 44 | 44 | M | 0.2 | 0 | 0 | 0 | 3 | 5 | 3 | 3 |
| 45 | 47 | M | 0.1 | 0 | 0 | 0 | 6 | 6 | 6 | 6 |
| 46 | 70 | M | 0.2 | 0.1 | 0.2 | 0.1 | 3 | 5 | 2 | 5 |
| 47 | 73 | F | 1.3 | 1.3 | 1 | 1 | 2 | 2 | 1 | 1 |
| 48 | 42 | F | 0.4 | 0.2 | 0 | 0 | 1 | 5 | 5 | 4 |
| 49 | 39 | F | 0.3 | 0.1 | 0 | 0 | 3 | 6 | 6 | 6 |
| 50 | 35 | F | 1.86 | 1 | 0.1 | UK | 0 | 5 | 4 | 5 |
| 51 | 64 | F | 0.2 | 0.2 | 0.2 | 0.2 | 4 | 5 | 5 | 5 |
Abbreviations: Lt, left; NE, no eye; Postop, postoperative; Preop, preoperative; Pt, patient; Rt, right; UK, unknown; VA, visual acuity; VF, visual field.
A total of 101 eyes were evaluated in the 51 patients.
Visual acuity was graded using the logMAR scale modified to represent also patients with very low vision: counting fingers = 1.86, hand motion = 2.30, light perception = 2.70, and no light perception = 3.0.
Visual fields were graded using a scale of 7 stages: 6 = normal visual fields; 5 = mild, constriction; 4 = ¼ of visual field defect; 3 = ½ of visual field defect; 2 = ¾ of visual field defect; 1 = severe constriction of visual fields; 0 = total scotoma.
Postoperative Neuro-ophthalmological Evaluations
The overall assessment of postoperative ophthalmological status for the entire cohort revealed a significant improvement in visual function tests. Postoperative follow-up evaluation of VA and VF status was 0.34 ± 0.7 and 3.93 ± 1.83, respectively. This represents a mean improvement of 35.2% in VA (p < 0.0001) and a 16.4% improvement in VF (p = 0.001) scores. There was a postoperative improvement in the VA of 40 eyes (40/98, 40.8%); deterioration was documented in 4 eyes (4/98, 4.1%). Assessment of the postoperative VFs revealed an improvement in 36 eyes (36/97, 37.1%) and constriction of the VF in 15 eyes (15/97, 15.5%).
Looking into a possible correlation between the patients' characteristics on admission (age, KPS, gender, and tumor size) and VA scores on admission, we found that high KPS on admission (rp = 0.72; p < 0.0001) and small tumor size (rp = 0.34; p = 0.014) were significantly correlated with better VA LogMAR scores on admission. Similarly, high KPS on admission (p < 0.0001) and a small tumor size (p = 0.0002) were associated with better VF scores on admission.
Multivariate analysis adjusted for age, gender, KPS on admission, and tumor size revealed that KPS on admission and small tumor size were the most significant prognostic factors for postoperative change in VA status. A good performance score on admission and smaller tumor size were associated with improvement of VA at the postsurgical follow-up (p = 0.015 and p = 0.056, respectively). In addition, good performance score on admission (p = 0.0001), smaller tumor size (p = 0.03), and young age (p = 0.012) were all predictors for significantly higher VF scores at the postsurgical follow-up ophthalmological evaluation. The patient's gender was not a significant covariate for improvement in visual function following TSM resection surgery.
An additional regression model was applied to model postoperative visual indices as a function of the patient's preoperative visual indices, as well as all the other above-mentioned predictors: VA and VF scores on admission were the only significant predictors for visual status outcome. Higher VA scores on admission were associated with greater improvement in VA on postoperative evaluations (p < 0.0001). Similarly, better VF scores on admission were associated with greater improvement in VF scores on postoperative evaluations (p < 0.0001; odds ratio (OR) 2.26 [95% confidence interval 1.72 to 2.96]).
Complications
Postoperative neurological complications were as follows: three patients had a right frontal contusion that did not require surgical intervention, and they recovered without further neurological sequelae. Two patients sustained a transient CSF leak that was successfully sealed by transnasal endoscopic approach. Two patients had meningitis and recovered completely after appropriate antibiotic treatment. Three patients experienced postoperative seizures that were controlled with anticonvulsant therapy. Two patients had systemic complications. One of them had acute respiratory failure, suspected as being a massive pulmonary embolism, that presented 2 weeks postoperatively, and he died shortly thereafter. Another patient was diagnosed as having a pulmonary embolism that was successfully treated with anticoagulation therapy.
Multivariate analysis adjusted for age, gender, KPS on admission, tumor size, and the patient's preoperative ophthalmological covariates revealed a borderline association of KPS on admission with postoperative complications (p = 0.08). No other covariate predicted the occurrence of postoperative complications.
Discussion
TSMs comprise ∼ 5 to 10% of all intracranial meningiomas. Many reports on their presentation and treatment have been published over the years.7,9,10,11,12 We report a large series of patients treated surgically over a relatively short period of time using a distinctive surgical technique. We had reported our first 20 cases a few years ago with a relatively short follow-up.5 The follow-up period of the current report is significantly extended, a factor that is especially important in terms of tumor recurrence. The definition of tuberculum meningioma and its borders is described in detail by Jallo et al.13
Most of our patients presented with visual deterioration, consistent with other reports.7,8,14,15 Comparisons of our detailed preoperative and postoperative visual examinations yielded a significant improvement in the mean VA and VF. The VA of 40 eyes improved, and the VA of 4 eyes deteriorated. The VFs of 36 eyes improved, and the VFs of 15 eyes deteriorated. We found that the patient's visual status on admission (VA and VF) is the strongest predictor for postoperative improvement in visual outcome. Young age, better KPS on admission, and smaller tumor size were also found to be associated with improved postoperative visual function tests. We believe that our results suggest that early surgical intervention, when the patients are more likely to have good visual function, will provide the best visual function outcome for patients with TSM.
Surgical Technique
Multiple surgical approaches have been described for the removal of tuberculum sella tumors. Ganna et al14 recently summarized several papers by leading surgeons. Different craniotomies were used, all of them with relatively good results. These included unilateral and bilateral frontal approaches, as well as pterional and frontotemporal approaches. We used the unilateral subfrontal approach and achieved good surgical resection with good visual outcome. We find that approaching the ONs from the tumor side (i.e., medial to it) is easier and safer for the ON on both sides, and the side of the approach can be decided upon for individual patients. Drilling of the optic canal is an important part of this kind of surgery, both for complete tumor removal and for easier and safer manipulation of the ON.
Craniotomy Compared with Endoscopic Techniques
A new alternative for the removal of tuberculum sella tumors is the extended transsphenoidal endoscopic technique. De Divitiis et al16 compared a group of 44 patients on whom they operated over a period of 21 years to 7 patients operated on through a transsphenoidal route. They reported that smaller midline tumors without lateral extension and without encasement of vascular or neural structures can be approached from below. The larger tumors with lateral or optic canal extension and/or encasement should be approached via a craniotomy. Laufer et al17 reported a group of 10 patients with suprasellar supradiaphragmatic tumors without sellar extension. Those authors were able to use an extended transsphenoidal approach to resect various tumors that included anterior base meningiomas, craniopharyngiomas, and one Rathke cleft cyst. Comparing this approach to the microscopic transsphenoidal approach as well as to craniotomy approaches led them to the conclusion that the endoscopic approach is a feasible alternative for excising these tumors but not without morbidity, and that a larger series is needed to show its advantages.17 Another report on 35 patients with different anterior skull base meningiomas was recently published.18 The patients in that series comprised a heterogeneous group: 15 had olfactory meningiomas, 13 had tuberculum tumors, 5 had parasellar lesions, and 2 had petroclival tumors. This heterogeneity makes it difficult to compare visual results, degree of resection, and complications between tuberculum meningiomas and other anterior skull base meningiomas, each group having different presenting symptoms, complications, and surgical difficulty. Those authors reported a 40% rate of CSF leak and noted that the percentage dropped after they improved their reconstruction technique by using vascularized flaps.
We do not find an advantage of the transsphenoidal route over the open craniotomy techniques. Indeed, the open technique has no limitations in terms of tumor size, lateral location, involvement of the optic canal, or involvement of major vessels. The incidence of CSF leak in the endoscopic technique is a disadvantage that continues to be problematic in spite of the improvement afforded by newer sealing techniques. This complication is far less frequent in the craniotomy approach: we had two cases in our current series (3.9%), a similar rate to other published reports.7,10,12,15
The idea of incomplete resection and adding radiation for patients who, for the most part, could have had a complete resection if they had been operated on through a craniotomy is another disadvantage. We agree that partial tumor removal for optic apparatus decompression is acceptable in older sicker patients. However, in the majority of cases, we aim for complete resection to achieve a cure. We performed complete resection in 45 patients (88.2%) with a relatively low complication rate and good visual outcome. In the few cases where the tumor is firmly adhered to the ON or chiasm, there will be residual tumor whatever the surgical approach. Future reports on radiation to the optic apparatus area for the residual tumor using fractionated techniques will show if the visual outcome and the long-term tumor control rate are acceptable.
In terms of brain retraction and frontal lobe damage, we encountered no clinically apparent neurological deterioration as a result of frontal lobe injury associated with the surgery. We are now in the process of carrying out detailed neuropsychological evaluations to check for any minor frontal lobe damage in all the patients that underwent surgery in this series.
In summary, we find the extended endoscopic approach an extremely useful technique for extradural tumors and for selected tumors that involve intracranial and extracranial compartments. For TSMs, however, the craniotomy route is effective and safe, and we do not think that an endoscopic technique will provide any special benefit.
Conclusion
TSMs are a specific group of tumors with special considerations regarding their removal and preservation of the optic apparatus. A unilateral frontal craniotomy achieved good surgical resection with preservation or improvement of visual function in most cases. Young age, KPS on admission, and tumor size were important factors predicting visual outcome and likelihood of complications. The visual function before surgery was the most important predictor for postsurgical visual outcome. These findings support the choice of early surgery when the patient may have a better KPS, the tumor is smaller, and the visual function is good.
Disclosure of Funding
None.
Acknowledgment
This work was performed in partial fulfillment of the MD thesis requirements of the Sackler Faculty of Medicine, Tel Aviv University.
Note
Nevo Margalit and Tal Shahar authors are contributed equally to this work.
References
- 1.Yaşargil M G. New York: Thieme; 1996. Microsurgery of CNS Tumors. Vol IVB. [Google Scholar]
- 2.Gökalp H Z, Arasil E, Kanpolat Y, Balim T. Meningiomas of the tuberculum sella. Neurosurg Rev. 1993;16(2):111–114. doi: 10.1007/BF00258241. [DOI] [PubMed] [Google Scholar]
- 3.Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis. Sci Mar. 2006;47(3):1236–1240. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 4.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margalit N Kesler A Ezer H Freedman S Ram Z Tuberculum and diaphragma sella meningioma—surgical technique and visual outcome in a series of 20 cases operated over a 2.5-year period Acta Neurochir (Wien) 2007149121199–1204., discussion 204 [DOI] [PubMed] [Google Scholar]
- 6.Margalit N S Lesser J B Moche J Sen C Meningiomas involving the optic nerve: technical aspects and outcomes for a series of 50 patients Neurosurgery 2003533523–532., discussion 532-533 [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud M Nader R Al-Mefty O Optic canal involvement in tuberculum sellae meningiomas: influence on approach, recurrence, and visual recovery Neurosurgery 201067(3, Suppl Operative):ons108–118.; discussion ons118-119 [DOI] [PubMed] [Google Scholar]
- 8.Sade B Lee J H High incidence of optic canal involvement in clinoidal meningiomas: rationale for aggressive skull base approach Acta Neurochir (Wien) 2008150111127–1132., discussion 1132 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M Roser F Struck M Vorkapic P Samii M Tuberculum sellae meningiomas: clinical outcome considering different surgical approaches Neurosurgery 20065951019–1028., discussion 1028-1029 [DOI] [PubMed] [Google Scholar]
- 10.Goel A Muzumdar D Desai K I Tuberculum sellae meningioma: a report on management on the basis of a surgical experience with 70 patients Neurosurgery 20025161358–1363., discussion 1363-1364 [PubMed] [Google Scholar]
- 11.Schick U, Hassler W. Surgical management of tuberculum sellae meningiomas: involvement of the optic canal and visual outcome. J Neurol Neurosurg Psychiatry. 2005;76(7):977–983. doi: 10.1136/jnnp.2004.039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathiesen T Kihlström L Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression Neurosurgery 2006593570–576., discussion 570-576 [DOI] [PubMed] [Google Scholar]
- 13.Jallo G I Benjamin V Tuberculum sellae meningiomas: microsurgical anatomy and surgical technique Neurosurgery 20025161432–1439., discussion 1439-1440 [PubMed] [Google Scholar]
- 14.Ganna A, Dehdashti A R, Karabatsou K, Gentili F. Fronto-basal interhemispheric approach for tuberculum sellae meningiomas; long-term visual outcome. Br J Neurosurg. 2009;23(4):422–430. doi: 10.1080/02688690902968836. [DOI] [PubMed] [Google Scholar]
- 15.Fahlbusch R, Schott W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96(2):235–243. doi: 10.3171/jns.2002.96.2.0235. [DOI] [PubMed] [Google Scholar]
- 16.de Divitiis E Esposito F Cappabianca P Cavallo L M de Divitiis O Tuberculum sellae meningiomas: high route or low route? A series of 51 consecutive cases Neurosurgery 2008623556–563., discussion 556-563 [DOI] [PubMed] [Google Scholar]
- 17.Laufer I, Anand V K, Schwartz T H. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106(3):400–406. doi: 10.3171/jns.2007.106.3.400. [DOI] [PubMed] [Google Scholar]
- 18.Gardner P A Kassam A B Thomas A et al. Endoscopic endonasal resection of anterior cranial base meningiomas Neurosurgery 200863136–52., discussion 52-54 [DOI] [PubMed] [Google Scholar]


