Abstract
Multiple myeloma (MM) is a very radiosensitive tumor. Fractionated external beam radiation, which takes approximately 2 weeks of therapy, is typically used to irradiate myelomatous bone lesions with the goal of palliation. However, traditional radiotherapeutic techniques are not only lengthy but they also involve a considerable amount of healthy bone marrow in the treatment ports, which may undermine the total marrow reserve of a patient. Because of the limited survival time of patients with metastatic cancer, novel treatment concepts shortening the overall treatment time is desirable. We present an innovative approach of delivering targeted intra-operative radiotherapy to a solitary osteolytic metastasis in one application, while sparing healthy bone marrow from radiation toxicity and substantially reducing the overall treatment time. A 78-year-old Caucasian male with MM, previously treated with chemotherapy, who was off chemotherapy for 2 years due to bone marrow suppression, presented with a solitary recurrence at the left anterior superior iliac spine of the left iliac wing as diagnosed by PET-CT scan. This lesion was treated with a minimally invasive osteoplasty and intra-operative brachytherapy with to a dose of 8 Gy delivered to the surgical cavity only, followed by injection of the bone cement into the cavity. Three months after the procedure, the area of treatment demonstrated no uptake on a follow-up PET-CT scan. At 1.5 years after this procedure, 100% local control continues to persist in the treated area, as evidenced on nuclear imaging. To our knowledge, this is the first case of using focal intra-operative brachytherapy confined to the area of the pelvis in a patient treated for a solitary metastasis from MM. The purpose of the article is to present a novel approach as a more convenient and focal treatment of bony lesions of MM.
Keywords: multiple myeloma, radiotherapy, conformal radiation, brachytherapy
Introduction
Although MM has no cure, the course of the disease can be controlled with a number of regimens of systemic therapy, stem cell transportation and palliative radiotherapy. Palliative radiation therapy (RT) is used for osteolytic lesions of MM. It typically entails external beam approach of delivery of RT in a fractionated manner over a period of 2 weeks [4, 8, 17]. A number of reports have emerged regarding a single dose of RT in the treatment of bony metastases [3, 5, 9, 10, 12, 14, 18, 26, 28]. Additionally, various minimally invasive techniques have been employed in the treatment of bony lesions, such as kyphoplasty, vertebraplasty, and percutaneous osteoplasty [6, 7, 9, 13, 15, 16, 20, 24, 25, 27, 28]. To our knowledge, this is the first report of a combination approach of osteoplasty and a single dose of intraoperative RT in a patient with an osteolytic metastasis in a setting of MM.
We report a case of a 78-year-old Caucasian male, who had been off chemotherapy for his MM due to bone marrow suppression and who presented with a left anterior superior iliac spine (ASIS) metastasis on positron emission tomography-computerized tomography (PET-CT) scan. The affected area was focally resected and intraoperative radiation therapy (RT) was delivered only to the surgical cavity. This focal radiotherapeutic approach rendered the patient complete long-term local control while sparing radiation toxicity to the unaffected bone marrow. The purpose of the article is to set the stage for an innovative method of treating osteolytic lesions of MM with a combined approach of a minimally invasive osteoplasty and intraoperative brachytherapy.
Case Report
This is a 78-year-old Caucasian male with a history of IgA kappa multiple myeloma (MM), after a 9-year history of monoclonal gammopathy of undetermined Significance (MGUS) turning smoldering myeloma and then indolent myeloma. He completed Biaxin, Revlimid (Celgene Corporation, Summit, NJ), Dexamethasone (BiRD) induction therapy for a total of 21 Cycles. The patient was then on Revlimid (Celgene Corporation, Summit, NJ) 25 mg but was taken off this drug due to neutropenia and thrombocytopenia. A whole body PET-CT in February of 2009 demonstrated a 2.5 × 4.0-cm lytic lesion in the left iliac wing at the ASIS with increased metabolic activity as compared to the prior study in April 2008 (SUVmax = 9.0, previously 3.9). No other findings on imaging were felt to be reflective of active disease. On the brief pain inventory (BPI), which evaluates pain intensity on a scale 0–10 (0 = no pain and 10 = worst possible pain), the patient’s score was 4. The patient underwent a CT-guided biopsy of this mass confirming plasma cell neoplasm, consistent with MM. Medical history is significant for a low risk adenocarcinoma of the prostate (Gleason score 3 + 3) treated with definitive radiotherapy in November 2008, squamous cell carcinoma of the left ear treated with Mohs surgery, and hypertension. The patient was seen in our clinic for a consideration of radiotherapy and was offered a standard 2-week course of palliative radiotherapy with external beam irradiation to a total of 20.0 Gy. He rejected this treatment option due to the fear of radiation toxicity to the unaffected bone marrow. He was also evaluated by an orthopedic surgeon for this osteolytic myelomatous lesion with a rising maximum standardized uptake value (SUVmax). We proposed to treat this mass with a combined approach of minimally invasive osteoplasty and intraoperative brachytherapy, followed by an injection of the bone cement. The patient was informed of the potential benefits and risks of this novel approach. A written informed consent was obtained from the patient.
We prepared a careful preoperative dosimetry plan of radiation delivery which was to be executed in the operating room (Fig. 1). An X-ray tube source from the Axxent® Electronic Brachytherapy System, eBx™ (Xoft, Inc., Sunnyvale, CA) was utilized to apply a therapeutic radiation dose directly to resected tumor cavity as a target. The calculations were made based on the dimensions of the lesion. A 3.0-cm-diameter applicator (a deflated balloon of the type 3–4 cm spherical fit in the cavity) was planned to be used based on the width of the tumor. An 8.0-Gy dose extrapolated from the single dose irradiation of the bone metastasis data [3, 14, 18, 19, 23] was used for the calculations to be prescribed at a distance of 1.5 cm from the centerline of the source. The treatment length was determined to be 4.5 cm, based on the total length of the tumor of 4.0- and 0.25-cm margins on the proximal and distal ends. The pre-calculated time for the radiation treatment delivery was 4 min and 2 s.
Fig. 1.
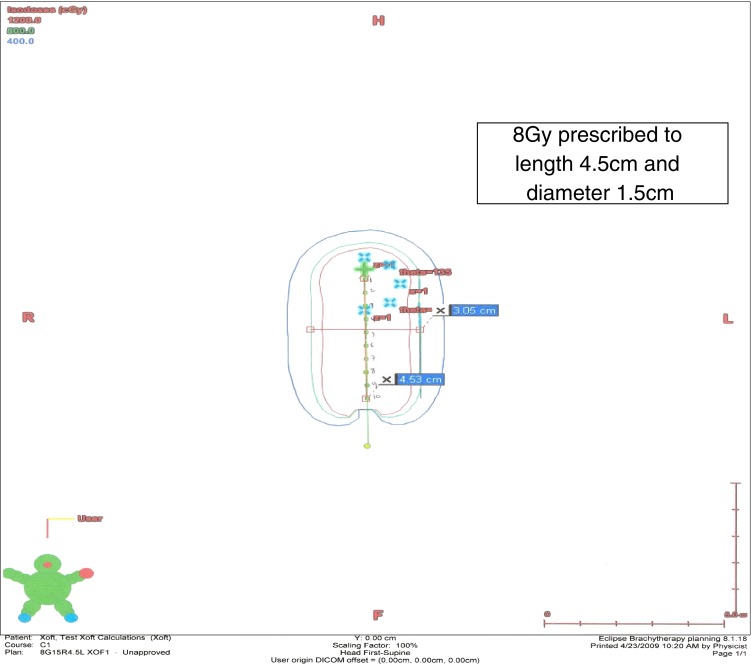
The pretreatment dosimetric plan of delivery of a single 8 Gy dose to the surgical cavity
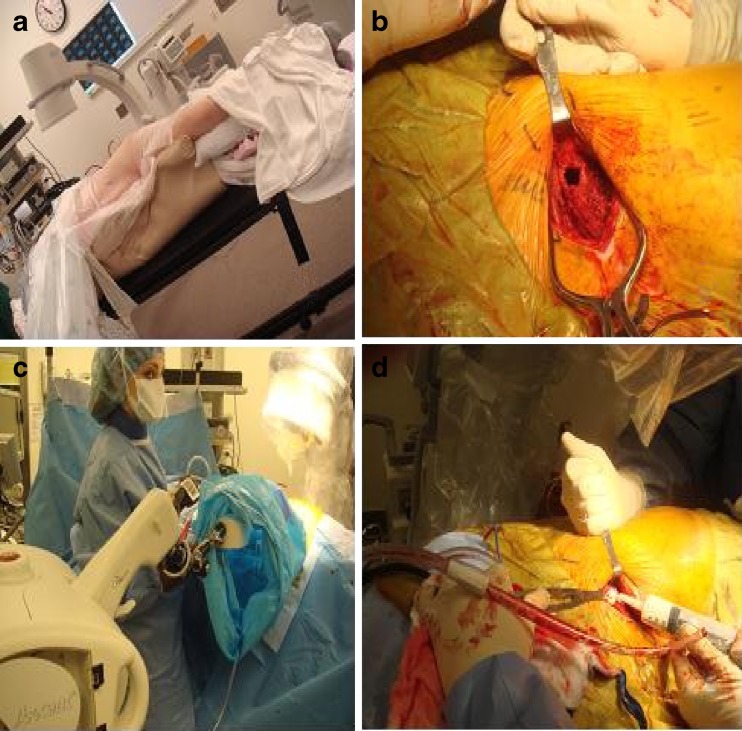
In order to perform the osteoplasty, the patient was positioned in the right lateral decubitus position, and palpation was used to locate the ASIS (Fig. 2a). After draping and prepping of the area, a 3.0-cm skin incision was made to expose the ASIS. Subsequently, a 1.0-cm aperture was drilled into the lesion (Fig. 2b) so as to accommodate the deflated balloon of the applicator of intraoperative brachytherapy. The tumor was excised by intraluminal curettage, and a frozen section confirmed the diagnosis of MM.
Fig. 2.
Four images obtained from the operating room. a A patient is in the right decubitus position, exposing the left hemi-pelvis. b An aperture in the left ASIS after the tumor is evacuated. c Radiation delivery with Xoft applicator which is draped in sterile fashion and secured in the aperture in the left ASIS. d Injection of the cement in to the tumor cavity
In order to perform the intraoperative radiotherapy the X-ray tube source from the Axxent® Electronic Brachytherapy System, eBx™ (Xoft, Inc.) was draped with sterile sleeve, and a 3.0-cm diameter deflated balloon applicator was inserted and secured into the cavity. Its alignment was checked and the balloon was inflated to anchor it into place. The patient was connected to the high dose rate (HDR) unit (Fig. 2c). The number of dwell positions was 10 and they were spaced 0.5 cm apart. The dwell times were optimized to deliver a uniform dose of 8.0 Gy at 1.5 cm radius. A dose of 8.0 Gy, prescribed at a distance of 1.5 cm from the centerline of the source, to a total treatment length of 4.5 cm, was delivered using a computerized dosimetry plan (Brachyvision Brachytherapy System, Varian Inc.). The overall treatment time was 4 min and 2 s. Upon withdrawal of the radiation applicator, the surgical cavity was subsequently injected with bone cement (Simplex Stryker Howmedich Osteonics, Mahwah, NJ, USA) (Fig. 2d). The skin was closed. The patient’s blood pressure, electrocardiogram, and pulse oximetry were continuously monitored and were steady during the procedure. The patient tolerated the procedure well and was monitored in the hospital overnight. No clinical complications occurred.
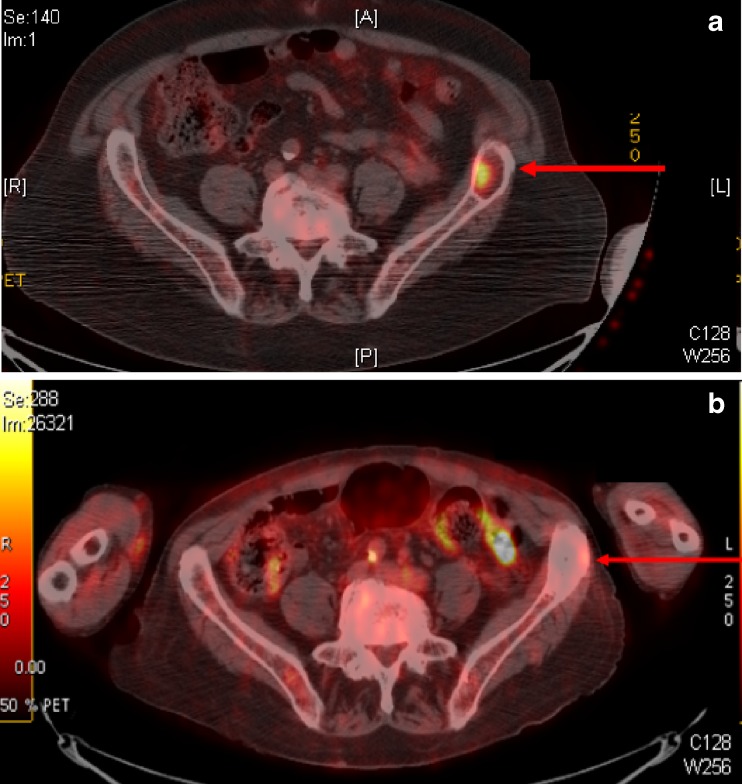
The follow-up of this patient consisted of physical examination, rigorous blood work and nuclear imaging. As compared to the findings of the pre-procedure PET-CT scan (February 24, 2009) where the SUVmax in the iliac crest was 9.0 (Fig. 3a), the first PET-CT scan after the afore-mentioned procedure obtained at 5 months (October 18, 2009) revealed complete resolution of the SUV of the treated area and formation of the non-metabolic sclerotic appearance. Subsequent PET-CT scans to place at 11- and 17-month intervals (April 26, 2010 and October 08, 2010), with the last obtained 1.5 years after the intraoperative radiation, again demonstrating non-metabolic sclerotic lesion (Fig. 3b).
Fig. 3.
Nuclear medicine positron emission tomography-computerized tomography (PET-CT) scans. a A pre-procedure scan revealing a lesion in the left ASIS with an SUV that rose from 3.9 on the prior study to 9.0 on the current one (2/24/2009). b The last PET-CT scan obtained at 1.5-year follow-up (10/08/2010) continues to demonstrate complete resolution of the treated lesion which is now non-metabolic but stable sclerotic non-metabolic lesion in the left iliac crest
Discussion
Radiotherapy in the management of MM is typically reserved for palliation of painful osteolytic lesions. Traditional RT is delivered by means of external beam in a fractionated manner over a period of approximately 2 weeks [4, 8, 17]. A margin of 2.0–2.5 cm to the gross tumor burden is given to account for microscopic spread of disease, organ motion and set up uncertainty during radiotherapy. Such a treatment field may cause detriment to the neighboring bone marrow, thereby damaging and potentially depleting the bone marrow reserve.
A single dose of focused RT, rather than the traditionally accepted 10–15 treatments, has been demonstrated as safe and effective in the treatment of bony metastases [3, 5, 9, 10, 12, 14, 18, 26, 28]. Kaasa et al. [14] reported in the prospective randomized multicenter trial that 8Gyx1 single fraction RT is equivalent to the 3 Gy × 10 multiple fractions in the treatment of painful bone metastases [14]. Other prospective trials corroborated the same finding and have adopted an 8 Gy × 1 therapy in their practice [3, 18, 23].
A number of minimally invasive techniques have been employed in the treatment of painful metastatic bony lesions in the weight and non-weight-bearing bones and pathological vertebral fractures such as percutaneous osteoplasty, kyphoplasty, and vertebraplasty [6, 9, 13, 16, 20, 24, 25, 27]. Since the first report of percutaneous vertebralplasty appeared in 1987 [9], such techniques gained wide application in the treatment of osteoporotic compression, osteolytic metastases, and hemangioma of vertebrae. In recent years, percutaneouos osteoplasty as a technical extension of the vertebroplasty has been included in the treatment of osteolytic lesions in weight-bearing besides the vertebrae, including acetabulum, sacrum, pubis, pelvis, ischium, femur and sternum and has demonstrated a high efficacy for pain relief and functional improvement [6, 13, 16, 20, 24, 25, 27, 28]. Percutaneous injection of cement can stabilize and strengthen bones in the body, which has been postulated as the major mechanism of pain relief [7, 15].
A combination of such techniques with focused RT has found its application in using kyphoplasty and spinal radiosurgery for the pathological fractures [11]. Other forms of radiotherapeutic delivery have been explored in the treatment of metastatic bony lesions, such as a radioactive samarium 153 [2, 22]. A phase I trial of vertebral intracavitary cement and samarium proved to be a novel and promising technique for treatment of painful vertebral metastases [1].
To our knowledge, this is the first report of a combination approach of osteoplasty and a single dose RT in a patient with an osteolytic metastasis in a setting of MM. We developed this combination therapy approach so as to achieve three goals: (1) to maximize local control, (2) to treat the minimum amount bone marrow possible, and (3) to shorten the overall treatment time. After evacuation of tumor from ASIS, a dose of 8 Gy was used to treat the tumor bed in our patient with MM based on prior reports of prospective data which demonstrated excellent local control in patients treated with external beam radiotherapy for bony metastases [3, 5, 9, 10, 12, 14, 18, 26, 28]. The application of the X-ray tube source from the Axxent® Electronic Brachytherapy System, eBx™ (Xoft, Inc.) intraoperative brachytherapy provided the most conformal delivery of the prescribed dose with maximum normal tissue sparing. Thus, such a combination approach allows us to treat the patient’s osteolytic lesion from MM in 1 day and provides the patient tremendous satisfaction by reducing the overall treatment time of fractionated radiotherapy (2 weeks), while rendering excellent local control. To date, at a nearly 2-year follow-up since the procedure, the patient’s treated lesion remains 100% cancer free, based on nuclear and functional imaging. Furthermore, the patient has never developed any chronic toxicity.
With the emerging image-guided radiotherapy and stereotactic body radiotherapy techniques for the treatment of bony lesions [9, 10, 12, 26], the treatment paradigm has shifted to a more focally directed (smaller margins) radiotherapeutic delivery of the dose. A recent publication by Schneider F et al addressed the utility of intraoperative radiotherapy using the INTRABEAM system during kypohoplasty for spinal metastases [21]. Though most treatments still occur in the setting of spinal or paraspinal metastases, there is a strong possibility that such treatment techniques will become applicable to lesions in other areas of the body. The treatment of an osseous lesion in a patient with MM should consider such an approach, as it may deliver the dose to a very limited area burdened by myeloma, while sparing the neighboring uninvolved area of bone marrow. In patients with pathologic fractures or pending fractures, our approach of osteoplasty and intraoperative radiotherapy provides a an excellent local control, with a very convenient and patient-friendly combination treatment modality which spares the normal bone marrow reserve while decreasing the overall treatment time.
In contrast to the fractionated 2-week approach, such an accelerated and targeted method as presented in this study, achieves excellent local control and no toxicity. Thus, in centers where intraoperative radiation is not available, targeted radiation by external means delivered as one dose only achieves the same outcome. Image guidance and stereotactic body frame radiotherapy techniques are tools that can be used instead of intraoperative means for improved accuracy. In weight bearing bones cases where fixation is necessary, in-field cancerization (i.e., microscopic tumor spread) is a concern.
Hence, in such cases focused RT would have limitations of missing the treatment of a wider microscopic field. Instead, traditional larger field RT technique should be employed.
The technique described in this paper is meant only for the superficially accessible lesions. For the deep seated lesions, targeted RT with the use of precision techniques of image guidance and stereotactic radiotherapy can be accomplished by noninvasive external radiotherapeutic approach.
Disclosures
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participating in the study was obtained.
References
- 1.Ashamalla H, Cardoso E, Macedon M, et al. Phase I trial of vertebral intracavitary Cement and samarium (VICS): novel technique for treatment of painful vertebral metastasis. Int J Radiat Oncol Biol Phys. 2009;75:836–842. doi: 10.1016/j.ijrobp.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 2.Bayouth JE, Macey DJ, Kasi LP, Fossella FV. Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med. 1994;35:63–69. [PubMed] [Google Scholar]
- 3.Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol 1999;52:111–121. [PubMed]
- 4.Bosch A, Frias Z. Radiotherapy in treatment of multiple myeloma. Int J Radiat Oncol Biol Phys. 1998;15:1363–1369. doi: 10.1016/0360-3016(88)90232-5. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 6.Cotten A, Deprez X, Migaud H, Chabanne B, Duquesnoy B, Chastanet P. Malignant acetabularosteolyses: percutaneous injection of acrylic bone cement. Radiology. 1995;197:307–310. doi: 10.1148/radiology.197.1.7568843. [DOI] [PubMed] [Google Scholar]
- 7.Dean JR, Ison KT, Gishen P. The strengthening effect of percutaneous vertebroplasty. Clin Radiol. 2000;55:471–476. doi: 10.1053/crad.2000.0478. [DOI] [PubMed] [Google Scholar]
- 8.Farhangi M, Osserman E. The treatment of multiple myeloma. Semin Hematol. 1973;10:149–161. [PubMed] [Google Scholar]
- 9.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 10.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 11.Gerszten PC, Germanwala A, Burton SA, et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine. 2005;3:296–301. doi: 10.3171/spi.2005.3.4.0296. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs IC. Spinal and paraspinal lesions: the role of stereotactic body radiotherapy. Front Radiat Ther Oncol. 2007;40:407–414. doi: 10.1159/000106050. [DOI] [PubMed] [Google Scholar]
- 13.Hierholzer J, Anselmetti G, Fuchs H, Depriester C, Koch K, Pappert D. Percutaneous osteoplasty as a treatment for painful malignant bone lesions of the pelvis and femur. J Vasc Interv Radiol. 2003;14:773–777. doi: 10.1097/01.RVI.0000079987.80153.85. [DOI] [PubMed] [Google Scholar]
- 14.Kaasa S, Brenne E, Lund JA, et al. Prospective randomised multicenter trial on single fraction radiotherapy (8 Gy °× 1) versus multiple fractions (3 Gy °× 10) in the treatment of painful bone metastases. Radiother Oncol. 2006;79:278–284. doi: 10.1016/j.radonc.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Kawai N, Sato M, Iwamoto T, Tanihata H, Minamiguti H, Nakata K. Percutaneous osteoplasty with use of a cement-filled catheter for a pathologic fracture of the humerus. J Vasc Interv Radiol. 2007;18:805–809. doi: 10.1016/j.jvir.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Kelekis A, Lovblad KO, Mehdizade A, et al. Pelvic osteoplasty in osteolytic metastases: technical approach under fluoroscopic guidance and early clinical results. J Vasc Interv Radiol. 2005;16:81–88. doi: 10.1097/01.RVI.0000141717.84515.92. [DOI] [PubMed] [Google Scholar]
- 17.Leigh B, Kurtts, Mack C, et al. Radiation therapy for palliation of multiple myeloma. Int J Radiat Oncol Biol Physic. 1993;25:801–804. doi: 10.1016/0360-3016(93)90308-I. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen OS, Bentzen SM, Sandberg E, et al. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol. 1998;47:233–240. doi: 10.1016/S0167-8140(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 19.Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35:292–298. doi: 10.1016/j.jpainsymman.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Pommersheim W, Huang-Hellinger F, Baker M, Morris P. Sacroplasty: a treatment for sacral insufficiency fractures. Am J Neuroradiol. 2003;24:1003–1007. [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider F, Greineck F, Clausen S, et al. Development of a novel method for Intraoperative radiotherapy during kyphoplasty for spinal metastases (kypho-iort). Int J Rad Oncol Biol Phys 2010; Ahead of print. Oct 7 [DOI] [PubMed]
- 22.Singh A, Holmes RA, Farhangi M, et al. Human pharmacokinetics of samarium-153 EDTMP in metastatic cancer. J Nucl Med. 1989;30:1814–1818. [PubMed] [Google Scholar]
- 23.Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/S0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 24.Uemura A, Matsusako M, Numaguchi Y, et al. Percutaneous sacroplasty for hemorrhagic metastases from hepatocellular carcinoma. AJNR Am J Neuroradiol. 2005;26:493–495. [PMC free article] [PubMed] [Google Scholar]
- 25.Weill A, Kobaiter H, Chiras J. Acetabulum malignancies: technique and impact on pain of percutaneous injection of acrylic surgical cement. Eur Radiol. 1998;8:123–129. doi: 10.1007/s003300050351. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Matsumoto Y, Kita M, et al. Clinical outcome of percutaneous osteoplasty for pain caused by metastatic bone tumors in the pelvis and femur. J Anesth. 2007;21:277–281. doi: 10.1007/s00540-007-0498-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou B, Wu CG, Li MH, et al. Percutaneous osteoplasty for painful sternal lesion From multiple myeloma. Skelet Radiol. 2009;38:281–285. doi: 10.1007/s00256-008-0620-7. [DOI] [PubMed] [Google Scholar]