Abstract
Background
Long-term bisphosphonate use has often been associated with atypical femoral fractures. These fractures evolve from incomplete femoral fractures. A previous study demonstrated that the presence of a radiolucent line in an incomplete fracture can indicate a high risk of progression to complete fracture.
Questions/Purposes
The aim of this study is to present a management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. Specific study questions include the following: (1) Is there a difference in the prognosis of these fractures based on the presence or absence of a radiolucent fracture line? (2) Can treatment with teriparatide assist in clinical/radiographic healing of these incomplete fractures? (3) Is there a characteristic biochemical profile in these patients?
Patients and Methods
We retrospectively examined all femur radiographs ordered by the metabolic bone disease service at our hospital between July 1, 2006 and July 1, 2011 and identified 10 patients with a total of 14 incomplete fractures. Nine patients received bisphosphonates for a mean duration of 10 ± 5 years (range, 4–17). The mean follow-up since the time of diagnosis was 20 ± 11 months (range, 6–36 months).
Results
Five fractures did not have a radiolucent fracture line and were treated conservatively with partial weight-bearing restrictions and pharmacologic therapy. All five of these fractures healed with conservative management. Nine fractures had a radiolucent fracture line, and only two of these were treated successfully with conservative management including teriparatide. Six of the eight patients with a radiolucent line elected for surgical prophylaxis after 3 months of conservative management, whereas one patient underwent surgical prophylaxis without a trial of conservative management. Regarding the biochemical profiles, bone turnover markers for our patient cohort were in the lower quartile.
Conclusions
Fractures without a radiolucent line appear to respond to conservative management and not require surgical prophylaxis. Teriparatide treatment may hold promise in promoting healing of these fractures.
Keywords: bisphosphonates, atypical femoral fractures, incomplete fractures, teriparatide, radiolucent fracture line
Introduction
Bisphosphonates have been widely and successfully used to treat osteoporosis by inhibiting osteoclast-mediated bone resorption. They are highly effective in reducing the risk of vertebral and hip fractures, increasing bone mineral density, reducing markers of bone turnover, and even reducing the risk of new fractures in the setting of recent fractures [4, 6, 24, 25]. However, several reports have linked the prolonged use of bisphosphonates, more specifically alendronate, to atypical subtrochanteric or diaphyseal femoral fractures [15, 21, 22, 27, 29]. Although a causative role for bisphosphonates in the pathogenesis of these fractures has not been established [32], it is thought that alendronate induces long-term suppression of bone remodeling that can lead to microdamage accumulation and subsequent stress fracture formation [7, 26, 31]. These femoral stress fractures first appear as focal or diffuse cortical thickening in the lateral cortex of the subtrochanteric or femoral shaft region. As the fracture propagates, a radiolucent fracture line may appear and extend medially, ultimately displacing and becoming a complete fracture [21, 22, 27]
Several reports have focused on the initial symptomatic incomplete fracture and its likelihood of progression to a completely displaced atypical femoral fracture [3, 8, 9, 11, 17, 20, 21]. These reports are mainly descriptive analyses of 4–14 incomplete fractures, and only two of them mentioned management options and fracture healing outcomes [3, 17]. In one report, it was observed that the presence of thigh discomfort and a radiolucent fracture line (dreaded black line) were reliable predictors for fracture propagation [20].
Due to the lack of evidence, the management of bisphosphonate-associated incomplete fractures remains an orthopedic dilemma. In addition to stopping bisphosphonates and supplementing calcium and vitamin D, an anabolic agent such as teriparatide may promote the healing of these fractures. To date, no studies have investigated the role of teriparatide in this context.
In this retrospective study, our aim is to propose a management approach to symptomatic bisphosphonate-associated incomplete atypical femoral fractures. Specific study questions include the following: (1) Is there a difference in the prognosis of these fractures based on the presence or absence of a radiolucent fracture line? (2) Can treatment with teriparatide assist in clinical/radiographic healing of these incomplete fractures? (3) Is there a characteristic biochemical profile in these patients?
Patients and Methods
Patients
Following approval from the institutional review board, the attending radiologist retrospectively examined all femur radiographs ordered by the metabolic bone disease service at our hospital between July 1, 2006 and July 1, 2011. Bisphosphonate-associated incomplete atypical femoral fractures were identified according to the radiographic features defined in the report of the Task Force of the American Society for Bone and Mineral Research, including (1) location from anywhere distal to the lesser trochanter to proximal to the supracondylar flare of the distal femoral metaphysis, (2) focal or diffuse thickening of the lateral cortex, and (3) an occasional discrete transverse lateral cortical translucency with periosteal callus formation [32] (Fig. 1). A subtrochanteric fracture was defined as one located distal to the lesser trochanter and proximal to the middle one third of the diaphysis. A femoral shaft fracture was defined as one located in the middle one third of the diaphysis [5, 15]. Radiographs of 12 patients were identified. Two patients were excluded from our report because of inadequate follow-up. We conducted a retrospective chart review on the remaining 10 patients. Baseline demographics and all relevant clinical information were collected from patient charts, and no information was recalled specifically for this study (Table 1).
Fig. 1.
Patient 2: An 85-year-old woman with a 15-year history of alendronate use. Plain radiograph shows multiple foci of cortical thickening along the lateral shaft of both femora without discrete radiolucencies, consistent with incomplete atypical femoral fractures; one on the right and three on the left (white arrows)
Table 1.
Patient’s characteristics and relevant clinical information
| Patient | Sex/age (years) | BMI (kg/m2) | Prodromal pain duration (months) | BP (duration in years) | Indication | Comorbidities | Other medications | Fracture history | Fracture location | Radiolucent line | Overalla follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/65 | 26.4 | 6 | Fosamax (5) | Osteoporosis | Scoliosis, uveitis, breast tumor (radiation) | HRT (30 years) | Ribs | Right shaft | − | 12 |
| 2 | F/85 | 31.2 | 24 | Fosamax (15) | Osteoporosis | History of breast cancer, enchondroma, hypertension | Angiotensin converting enzyme inhibitor | Rib, humerus | Bilateral shaft | − Bilaterally | 14 |
| 3 | F/63 | 37.3 | Not available | Fosamax (4) | Osteoporosis | Hypertension | Aspirin, Thiazide | – | Bilateral shaft | Right: −; left: + | 26 |
| 4 | F/50 | 18.3 | 0.5 | Actonel (10) | Fractures | Ehlers–Danlos syndrome | Corticosteroid, birth control | Ankle, hand, foot, rib | Left ST | + | 6 |
| 5 | F/65 | 29.2 | 5 | Fosamax (10) | Osteopenia | Phlebitis | Warfarin | Right femur (atypical) | Bilateral shaft | + Bilaterally | 12 |
| 6 | F/71 | 21.3 | 7 | Fosamax (17) | Osteopenia | – | HRT | – | Left ST | + | 26 |
| 7 | F/82 | 22.5 | 9 | Fosamax (7) | Osteoporosis | Hypertension, Reflux, history of radiation | Omeprazole, nifedipine | – | Left shaft | + | 10 |
| 8 | F/59 | 37.5 | 8 | Fosamax (7) | Osteoporosis | Breast cancer, hypothyroidism | Synthroid, omeprazole | Right femur (atypical) | Left ST | + | 36 |
| 9 | F/63 | 24.8 | 12 | Fosamax (15) | Osteoporosis | Hypothyroidism, osteochondroma | HRT (14 years), omeprazole | – | Bilateral shaft | Right: −; left: + | 36 |
| 10 | F/65 | 22.7 | 24 | Fosamax (14) | Osteoporosis | Hypertension | Simvastatin, amlodipine | Left femur (atypical) | Right shaft | Yes (anteriorly) | 24 |
aOverall follow-up is the follow-up time since the time of diagnosis of the stress fracture
F female, BMI body mass index, BP bisphosphonate, HRT hormone replacement therapy, ST subtrochanteric
The 10 patients in this series were all women with a mean age of 67 ± 10 (range, 50–85). The mean follow-up since the time of diagnosis was 20 ± 11 months (range, 6–36 months). All cases of incomplete fractures occurred spontaneously with no history of trauma in patients who had received bisphosphonate therapy for osteoporosis/osteopenia. A prodromal pain period ranging from 2 weeks to 2 years in duration (mean, 11 months) was reported by all patients. Nine patients received alendronate for a mean duration of 10 ± 5 years (range, 4–17), and one patient received risedronate for 10 years. Concomitant drug history included hormone replacement therapy (three patients), omeprazole (three patients), and glucocorticoids (one patient). Of the 10 patients, four had bilateral involvement, and we therefore evaluated 14 fractures. Three fractures were located in the subtrochanteric area, and 11 were located in the femoral shaft.
Treatment Algorithm
Bilateral full femur plain radiographs were obtained for all patients complaining of thigh pain and a prolonged history of bisphosphonate use (>5 years). If an incomplete fracture was identified, the presence of a transverse radiolucent line across the cortical thickening was noted. Fractures were then classified into two groups depending on whether this radiolucent line was present or not (Fig. 2).
Fig. 2.
A flowchart showing the different management options and outcomes of symptomatic bisphosphonate-associated incomplete atypical femoral fractures
In the first group, the X-rays showed lateral cortical thickening without a radiolucent fracture line and magnetic resonance imaging (MRI) showed bone marrow edema. Bone marrow edema on MRI confirms the presence of an impending stress fracture [12, 34] that warrants active treatment, as opposed to an old, healed stress fracture, for which patients would only receive routine calcium and vitamin D supplementation. Patients with bone edema on MRI received conservative therapy, which consisted of rest, partial weight-bearing, calcium and vitamin D supplementation, and a 2-year course of teriparatide (recombinant parathyroid hormone 1–34). Miacalcin, or salmon calcitonin, was given instead of teriparatide for one patient in whom teriparatide treatment was contraindicated due to a history of radiation (patient 1) [10, 14]. Bisphosphonates were stopped in all patients at the time of diagnosis. Patients returned to clinic at 3, 6, 12, and 24 months for clinical evaluation. MRIs were obtained at the 3-month follow-up visit to monitor healing. Fracture healing was determined by clinical resolution of pain and complete resolution of bone marrow edema [35].
In the second group, a radiolucent fracture line was identified on the plain radiographs. Patients in this group were first given a trial of 3 months of conservative therapy, as described for the first group. All patients in this group received teriparatide except for one patient that underwent prophylactic fixation without a trial of conservative therapy due to persistent pain and choice of early weight-bearing (patient 7). Patients were then revaluated for clinical and radiographic healing. Clinical healing was defined as complete resolution of pain. Radiographic healing was defined as partial to complete disappearance of the radiolucent line [13]. Those who showed signs of both clinical and radiographic healing continued their conservative therapy. Patients with persistent pain and radiolucent fracture line were given the option to either continue conservative management or proceed to prophylactic fixation. Patients that elected for prophylactic surgical fixation were considered to have failed a 3-month trial of conservative management, and therefore follow-up data beyond surgery are not included in this series (Fig. 2).
Biochemical Studies and Bone Mineral Density
All biochemical studies were performed on samples obtained a few weeks before or after the initial visit and diagnosis, but before starting teriparatide therapy. Serum samples were assayed for creatinine, calcium, albumin, parathyroid hormone (PTH), and 25-hydroxyvitamin D. Bone turnover markers including bone-specific alkaline phosphatase (BSAP) and spot urine N-telopeptide crosslinks (NTX) were obtained. Spot urine NTX and BSAP were also measured frequently at follow-up visits to monitor the response to teriparatide therapy. The bone mineral densities of the L1–L4 vertebrae and femoral neck were measured using dual-energy X-ray absorptiometry in the same year as diagnosis.
Results
Out of 14 symptomatic bisphosphonate-associated incomplete atypical femoral fractures, five did not show a radiolucent fracture line and were treated conservatively. All five patients reported progressive improvement in pain and function and none required surgery. None of these incomplete fractures progressed to complete displaced fractures. The cortical thickening was a persistent feature in all follow-up X-rays; however, MRIs showed complete resolution of periosteal and bone marrow edema.
Nine fractures showed a radiolucent fracture line across a thickened cortex. Two of these fractures (patients 3 and 10) responded to 3 months of conservative therapy with teriparatide and had complete clinical and radiographic healing (Fig. 3). Both fractures were located in the femoral shaft. These patients continued teriparatide therapy for 2 years and did not progress to complete fracture. Six fractures did not show signs of radiographic or clinical healing after 3 months of conservative therapy with teriparatide. All six patients subsequently elected to undergo surgery for an early weight-bearing status. In one fracture, the patient did not receive teriparatide as she had history of radiation and instead underwent surgical fixation without a trial of conservative therapy due to significant pain and choice of early weight-bearing (patient 7).
Fig. 3.
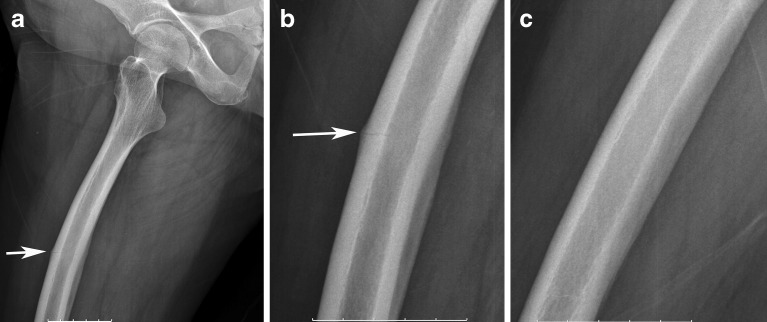
Patient 10: A 65-year-old woman on alendronate for 14 years. a A frog-leg lateral view of the right femur showing a periosteal reaction in the anterior shaft (white arrow). b Higher magnification shows a transverse radiolucent line (white arrow). c Shows disappearance of the fracture line with resolution of symptoms after 1 year of teriparatide treatment and calcium and vitamin D supplementation
For all patients, the initial serum calcium, albumin, creatinine, 25-hydroxyvitamin D, and PTH were within the reference range, except for one patient with a PTH of 6 pg/mL (Table 2). Bone turnover markers were not available for patient 10. The mean NTX level was 21.1 ± 6.9 nmol BCE/mmol creatinine. When calculating the mean, two patients (patients 5 and 8) were excluded with elevated NTX levels that can be attributed to having contralateral complete fractures. When looking at NTX levels of patients that were treated with teriparatide and did not have a contralateral complete fracture, four out of five patients demonstrated an initial increase in the 3–8-month window after starting teriparatide, followed by a slight decrease after 1–2 years (Table 3). Three patients had suppressed BSAP, whereas the mean in all nine patients was 9.3 ± 3.3 mcg/L. Although less data were available for BSAP, there was also an increase in BSAP levels after teriparatide treatment (Table 3).
Table 2.
Baseline biochemical and BMD findings
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cr (mg/dL) | 1.0 | 1.4 | 0.9 | 0.8 | 0.8 | 0.9 | 0.6 | 0.9 | 0.7 | – |
| Ca (mg/dL) | 9.9 | 10.3 | 10.0 | 9.1 | 9.7 | 9.7 | 10.6 | 9.7 | 10.4 | 9.9 |
| Albumin (g/dL) | 5.0 | 4.5 | – | 4.4 | 1.4 | 4.4 | 3.5 | 4.2 | 4.7 | – |
| PTH (pg/mL) | 23.5 | 28.0 | 50.0 | 53.6 | 18.0 | 6.0 | 20.8 | 30.3 | 33.0 | – |
| 25-hydroxyvitamin D (ng/mL) | 37.0 | 37.5 | 27.0 | 83.5 | 31.4 | 41.0 | 55.0 | 54.0 | 36.3 | 59 |
| BSAP (mcg/L) | 5.4 | 10.7 | 11.1 | 3.9 | 11.4 | 5.2 | 8.1 | 12.9 | 14.1 | – |
| NTX (nmol BCE/mmol Cr) | 11 | 15 | 23 | 19 | 46a | 15 | 22 | 52a | 28 | –a |
| DXA (T-score) | −2.9 | −2.2 | −2.0 | −1.9 | −2.9 | −1.3 | −4.1 | −1.5 | −2.8 | −2.4 |
aThese patients had contralateral complete atypical femoral fracture, which can increase the level of bone turnover markers
Cr creatinine, Ca calcium, PTH parathyroid hormone, BSAP bone-specific alkaline phosphatase, NTX N-telopeptide crosslinks, DXA dual-energy X-ray absorptiometry
Table 3.
The progression of bone turnover markers over the course of 2 years
| Patient | Bone-specific alkaline phosphatase (mcg/L) | N-Telopeptide crosslinks (nmol BCE/mmol Cr) | Overall follow-up (months) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 3–8 months | 1–2 years | Baseline | 3–8 months | 1–2 years | ||
| 1a | 6.1 | – | N/A | 11 | 16 | N/A | 12 |
| 2 | 10.7 | 12.1 | 11.5 | 15 | 48 | 45 | 14 |
| 3 | 11.1 | – | 16 | 23 | 49 | 25 | 26 |
| 4 | 3.9 | 12.6 | N/A | 19 | 17 | N/A | 6 |
| 5b | 11.4 | 12.1 | 12.5 | 46 | 102 | 75 | 12 |
| 6 | 5.2 | 10 | 10 | 19 | 36 | 30 | 26 |
| 7a | 8.1 | – | N/A | 22 | 18 | N/A | 10 |
| 8b | 12.9 | – | 15.7 | 52 | 26 | 25 | 36 |
| 9 | 14.1 | – | – | 28 | 70 | 49 | 36 |
| 10b | – | – | 12.1 | – | 29 | 38 | 24 |
aPatients did not receive teriparatide treatment
bThese patients had contralateral complete atypical femoral fracture, which can increase the level of bone turnover markers
N/A not applicable
Discussion
Our study lends further evidence to the importance of the radiolucent line in differentiating between symptomatic bisphosphonate-associated incomplete atypical femoral fractures that do and do not respond to an initial course of conservative therapy. Teriparatide may have an effect in healing fractures without a radiolucent line, but 3 months may not be sufficient to heal fractures with a radiolucent line. In addition, the biochemical profile of patients with these fractures demonstrates a specific rise and fall pattern throughout the course of teriparatide treatment. As awareness of atypical femoral fractures increases, radiologists and orthopedic surgeons will identify more incomplete fractures before they progress to complete fractures. The difficult question of how to best manage such early incomplete fractures seems to lie with the identification of a radiolucent line. The existence of this line can assist in determining if the patient can be treated conservatively or should undergo surgical prophylaxis.
There are some limitations to this study. First, the sample size is small. Unfortunately, bisphosphonate-associated incomplete atypical femoral fractures are not common and have only recently been described. A retrospective case series is currently the most feasible way to characterize these fractures and their treatments. It would be difficult to achieve a sufficient number of patients to power a prospective randomized study to make any inferences regarding the efficacy of following a non-surgical approach and teriparatide versus surgical management. Second, it is not clear what the minimum necessary follow-up time is to confidently assess healing in those patients with a persistent radiolucent line and eliminate the risk of progression to complete fracture. The mean follow-up in our study was 20 months, whereas a review of recent reports showed that the mean time for progression to complete fracture was 5.7 months (range, 0.1–19 months) [3, 11, 17]. Third, due to the retrospective nature of this study, when reviewing the chart some of the information was not available
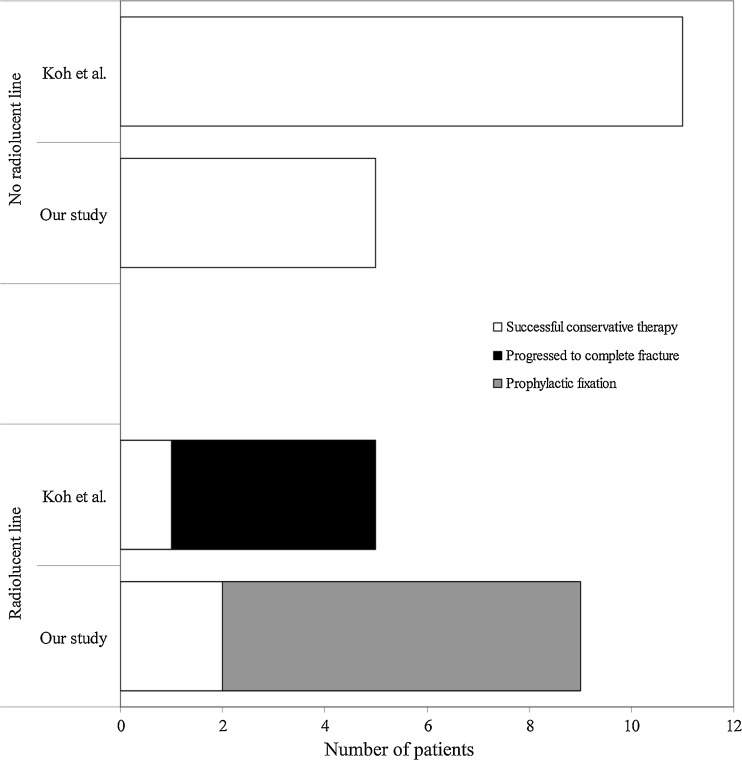
In our management approach, we divided treatment options based on the presence or absence of a radiolucent fracture line. The only other study to describe this finding in the context of bisphosphonate-associated incomplete femoral fractures, by Koh et al., showed that four out of five patients who had a radiolucent fracture line progressed to complete fracture, whereas all 11 patients who did not have this fracture line did not progress to complete fracture [20]. It was observed that the presence of thigh discomfort and the “dreaded black line” were reliable predictors for fracture propagation. Our study showed similar results to Koh et al. in that all five patients without a radiolucent fracture line healed completely without surgical intervention, and six of eight patients with a radiolucent fracture line failed to heal after a 3-month trial of conservative therapy (Fig. 4). Taken together, it is clear that surgical prophylaxis may only be required in stress fractures with a radiolucent line, while those without a radiolucent line seem to consistently heal using conservative management alone. This strategy also applies to patients who had suffered a contralateral complete fracture (patients 5, 8, and 10). Asymptomatic incomplete fractures should also be treated conservatively, and weight-bearing may be continued but should be limited until there is no bone edema on MRI [32]. Since most asymptomatic fractures are incidentally discovered after contralateral complete fracture, the operated side usually dictates the weight-bearing status.
Fig. 4.
A comparison between this study and the study by Koh et al. [20] according to the presence or absence of a radiolucent fracture line
Of note, when considering the location of fractures, all three subtrochanteric fractures had a radiolucent line and failed a 3-month trial of conservative management, while only 4 of 11 shaft fractures did not heal. This may indicate that the location of the atypical fracture could impact its progression.
Teriparatide is the only anabolic agent that has been FDA-approved for the treatment of postmenopausal osteoporosis [19]. Two recent randomized clinical trials suggested a role for PTH in accelerating fracture healing in postmenopausal women [2, 30]. In a rat stress fracture model, PTH was found to counter bisphosphonates by increasing bone resorption along the fracture line and initiating intracortical bone formation. In addition to significantly accelerating the repair of the stress fracture, PTH also enhances the mechanical properties of bone following fatigue loading [33]. These data correspond to a prior case report, by Gomberg et al. [16], where teriparatide was used to heal a bisphosphonate-associated incomplete atypical femoral fracture. Our series suggests that treatment with teriparatide can be successful in fractures without a radiolucent line, but may not achieve complete clinical and radiographic healing after 3 months in those stress fractures with a dreaded black line. Only two of the eight patients with a radiolucent line that were treated with teriparatide demonstrated clinical and radiographic healing and did not require surgery. It is possible that these fractures require a longer duration of therapy with teriparatide than 3 months to achieve complete healing. Our series only extends to 3 months of teriparatide therapy due to patients’ choice of early weight-bearing status and concerns about progression to complete fracture.
In this study, as in a prior case report [16], the baseline NTX level in patients without a concurrent complete fracture is in the lower quartile of the reference range. This may reflect a state of severely suppressed bone turnover compatible with the histomorophometric analysis of Odvina et al. Relatively few reports have included bone turnover markers, and in the majority of cases, they did not suggest oversuppression of bone turnover, as they were measured after complete atypical femoral fractures had occurred [1, 23, 28, 29, 36]. Complete fractures have been shown to significantly increase bone turnover markers, so patients in our study with concurrent complete fractures would have a misleadingly elevated NTX value [18]. The change in the NTX levels during treatment in our series of patients initially increases and then decreases, indicating an initial phase of bone resorption when teriparatide treatment is initiated. The stimulation of osteoclast resorption along existing fracture lines is an important step in the process of stress fracture healing [16]. The pre-existing suppression in some patients and mild increase in BSAP after teriparatide treatment is indicative of a blunted bone formation response. With the limited number of patients in our study, we were not able to identify differences in the behavior of bone markers between patients that did and did not heal.
The literature is still lacking evidence to identify an optimum management strategy for bisphosphonate-associated incomplete atypical femoral fractures. Our study provides further evidence that fractures with a radiolucent line appear to be more likely to fail a short course of conservative management and require surgical prophylaxis. In addition, teriparatide treatment may hold promise in promoting healing in fractures without a radiolucent line. Unfortunately, fractures with a radiolucent line may require longer duration of treatment than the 3 months given in our series. The management approach described in this study has the potential to both prevent progression to complete fracture and help define when surgical intervention is required.
Acknowledgments
Disclosures
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case, that all investigations were conducted in conformity with ethical principles of research.
Footnotes
Level of evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description levels of evidence.
References
- 1.Armamento-Villareal R, Napoli N, Diemer K, et al. Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: A case series. Calcif Tissue Int. 2009;85(1):37–44. doi: 10.1007/s00223-009-9263-5. [DOI] [PubMed] [Google Scholar]
- 2.Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: A prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25(2):404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 3.Banffy MB, Vrahas MS, Ready JE, Abraham JA. Nonoperative versus prophylactic treatment of bisphosphonate-associated femoral stress fractures. Clin Orthop Relat Res. 2011;469(7):2028–2034. doi: 10.1007/s11999-011-1828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: The fracture intervention trial long-term extension (FLEX): A randomized trial. JAMA. 2006;296(24):2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 5.Boden BP, Osbahr DC, Jimenez C. Low-risk stress fractures. Am J Sports Med. 2001;29(1):100–111. doi: 10.1177/03635465010290010201. [DOI] [PubMed] [Google Scholar]
- 6.Bone HG, Hosking D, Devogelaer JP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 7.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12(1):6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am. 2009;91(11):2556–2561. doi: 10.2106/JBJS.H.01774. [DOI] [PubMed] [Google Scholar]
- 9.Cermak K, Shumelinsky F, Alexiou J, Gebhart MJ. Case reports: Subtrochanteric femoral stress fractures after prolonged alendronate therapy. Clin Orthop Relat Res. 2010;468(7):1991–1996. doi: 10.1007/s11999-009-1192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesnut CH, 3rd, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: The prevent recurrence of osteoporotic fractures study. PROOF study group. Am J Med. 2000;109(4):267–276. doi: 10.1016/S0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 11.De Das S, Setiobudi T, Shen L, Das De S. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg Br. 2010;92(5):679–686. doi: 10.1302/0301-620X.92B5.22941. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch AL, Coel MN, Mink JH. Imaging of stress injuries to bone. radiography, scintigraphy, and MR imaging. Clin Sports Med. 1997;16(2):275–290. doi: 10.1016/S0278-5919(05)70022-3. [DOI] [PubMed] [Google Scholar]
- 13.Dijkman BG, Sprague S, Schemitsch EH, Bhandari M. When is a fracture healed? radiographic and clinical criteria revisited. J Orthop Trauma. 2010;24(Suppl 1):S76–S80. doi: 10.1097/BOT.0b013e3181ca3f97. [DOI] [PubMed] [Google Scholar]
- 14.Ellerington MC, Hillard TC, Whitcroft SI, et al. Intranasal salmon calcitonin for the prevention and treatment of postmenopausal osteoporosis. Calcif Tissue Int. 1996;59(1):6–11. doi: 10.1007/s002239900076. [DOI] [PubMed] [Google Scholar]
- 15.Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: A caution. J Bone Joint Surg Br. 2007;89(3):349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 16.Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96(6):1627–1632. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- 17.Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res. 2010;468(12):3393–3398. doi: 10.1007/s11999-010-1583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingle BM, Hay SM, Bottjer HM, Eastell R. Changes in bone mass and bone turnover following distal forearm fracture. Osteoporos Int. 1999;10(5):399–407. doi: 10.1007/s001980050246. [DOI] [PubMed] [Google Scholar]
- 19.Jobke B, Pfeifer M, Minne HW. Teriparatide following bisphosphonates: Initial and long-term effects on microarchitecture and bone remodeling at the human iliac crest. Connect Tissue Res. 2009;50(1):46–54. doi: 10.1080/03008200802412462. [DOI] [PubMed] [Google Scholar]
- 20.Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: A herald of impending fracture? J Orthop Trauma. 2010;24(2):75–81. doi: 10.1097/BOT.0b013e3181b6499b. [DOI] [PubMed] [Google Scholar]
- 21.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: A long-term complication of alendronate therapy? Injury. 2008;39(2):224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Lenart BA, Neviaser AS, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: A case control study. Osteoporos Int. 2009;20(8):1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung F, Lau TW, To M, Luk KD, Kung AW. Atypical femoral diaphyseal and subtrochanteric fractures and their association with bisphosphonates. BMJ Case Rep. 2012; in press. [DOI] [PMC free article] [PubMed]
- 24.Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N Engl J Med. 1995;333(22):1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 25.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357:nihpa40967. [DOI] [PMC free article] [PubMed]
- 26.Martin BR. The role of bone remodeling in preventing or promoting stress fractures. In: Burr DB, Milgrom C, editors. Musculoskeletal fatigue and stress fractures. Boca Raton: CRC; 2001. pp. 183–202. [Google Scholar]
- 27.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22(5):346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 28.Odvina CV, Levy S, Rao S, Zerwekh JE, Rao DS. Unusual mid-shaft fractures during long-term bisphosphonate therapy. Clin Endocrinol (Oxf). 2010;72(2):161–168. doi: 10.1111/j.1365-2265.2009.03581.x. [DOI] [PubMed] [Google Scholar]
- 29.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: A potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90(3):1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 30.Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1–84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am. 2011;93(17):1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 31.Schaffler MB. Bone fatigue and remodeling in the development of stress fractures. In: Burr DB, Milgrom C, editors. Musculoskeletal fatigue and stress fractures. Boca Raton: CRC; 2001. pp. 161–182. [Google Scholar]
- 32.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the american society for bone and mineral research. J Bone Miner Res. 2010;25(11):2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 33.Sloan AV, Martin JR, Li S, Li J. Parathyroid hormone and bisphosphonate have opposite effects on stress fracture repair. Bone. 2010;47(2):235–240. doi: 10.1016/j.bone.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Spitz DJ, Newberg AH. Imaging of stress fractures in the athlete. Radiol Clin North Am. 2002;40(2):313–331. doi: 10.1016/S0033-8389(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 35.Tervonen O, Junila J, Ojala R. MR imaging in tibial shaft fractures. A potential method for early visualization of delayed union. Acta Radiol. 1999;40(4):410–414. doi: 10.3109/02841859909177755. [DOI] [PubMed] [Google Scholar]
- 36.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93(8):2948–2952. doi: 10.1210/jc.2007-2803. [DOI] [PubMed] [Google Scholar]