Abstract
Background
Patellar Clunk Syndrome is a painful condition associated with a mechanical catching or clunking during active extension following total knee arthroplasty (TKA). The syndrome is caused by growth of interposing soft tissue usually at the superior pole of the patella. This interposed soft tissue cannot be visualized on plain radiographs.
Questions
The aim was to ascertain if magnetic resonance imaging (MRI) would prove helpful in confirming the clinical diagnosis of patellar clunk by visualizing the interposed soft tissues adjacent to the patella and that the recognition of this tissue would be highly reproducible.
Methods
MRI scans of 12 patients with clinical suspicion or related symptoms of a patellar clunk syndrome following primary TKA were retrospectively evaluated. Size of soft tissue masses proximal to the patella were determined in sagittal and axial MRI views. Largest diameters were recorded in two dimensions by two independent observers, and interobserver reliability was determined by intra-class correlation coefficients (ICC).
Results
Nine patients (75%) showed obvious MRI findings consistent with a patellar clunk lesion with high interobserver reliability (ICC values >0.75). In eight patients, this lead to operative treatment with arthroscopic debridement.
Discussion
MRI helps confirm the clinical diagnosis of patellar clunk. The data indicate that MRI is effective in defining the soft tissue lesion that is implicated in clinically evident patellar clunk syndrome after TKA.
Keywords: patellar clunk, TKA, MRI, total knee arthroplasty, magnet resonance imaging
Introduction
A diverse set of complications is attributed to the patella following total knee arthroplasty (TKA). These complications may include malposition of the patellar component and dynamic problems such as recurrent subluxation or disruption of the extensor mechanism [11]. Focal scarring at the upper patella pole may cause a locking sensation or impaired motion during movement of the knee [4].
The term patellar clunk syndrome was introduced 1989 in a case report by Hozack et al. [4]. A prominent fibrous nodule was described to be found at the junction of the proximal patellar pole and the quadriceps tendon in some cases after posterior stabilized TKA. This nodule enters the intercondylar notch with flexion and displaces with an audible and often painful clunk with extension. Cases identifying the phenomenon of suprapatellar fibrous tissue getting caught on extension were reported earlier by Insall and Simison without giving causation of the possible mechanism [3, 5, 6, 12]. Beight et al. [1] described the patellar clunk syndrome in a number of cases following the first generation of posterior stabilized TKA, which possessed a sharp edge at the superior edge of the intercondylar notch. A possible explanation was postsurgical scar formation leading to development of a clunk at a mean of 10.6 months after surgery. Arthroscopic resection of this hypertrophic nodule was described to be highly successful when compared to an open approach [14].
While the diagnosis is largely based on clinical examination, radiographic abnormities associated with patellar clunk syndrome include patella baja, a proximally placed patellar button or a subluxing or frankly dislocated patellar component. Conventional radiographs are limited in defining the soft tissue lesion. Due to its multiplanar capabilities and superior soft tissue contrast, MRI is the method of choice for the evaluation of native joints. Traditional MRI of TKA is limited due to artifact generated by the metallic components. Recent modifications in protocols have shown MRI to be helpful in the evaluation of painful joints, including total hip and knee arthroplasty [9]. As such, MRI could be a key diagnostic tool to evaluate patellar clunk syndrome after total knee replacement closing the large gap between clinical findings and conventional radiographic assessment.
It was hypothesized that MRI would prove helpful and reproducible in confirming the clinical diagnosis of patellar clunk by visualizing the soft tissues proximal to the patella. The specific aims for this study were to (1) demonstrate that MRI can identify and characterize the lesion causing the patellar clunk syndrome following TKR; (2) to show that the dimensions of the lesion can be reproducibly measured, and that (3) the information obtained from the MRI can support the decision to proceed with arthroscopic debridement as treatment for the patellar clunk syndrome.
Material and Methods
In this retrospective case series, MRI scans performed in 12 patients with the clinical suspect or related symptoms of a patellar clunk syndrome after primary TKA were evaluated followed by a chart review. Descriptive variables were recorded including patient demographics, clinical signs of patellar clunk, and revision or arthroscopic surgery, if performed.
The cohort included five women and seven men with a clinically suspected diagnosis of patellar clunk with an average age of 63.4 ± 8.8 years at index TKA (four left, eight right knees). All patients underwent TKA for degenerative joint disease. MRI was done at a mean interval of 16.2 ± 8.0 months after index procedure.
All TKAs were posterior stabilized with a Zirconium femoral component (Oxinium, Smith and Nephew, Memphis, TN, USA) in one case, and conventional components of cobalt/chrome/molybdenum alloy (CoCrMo) in 11 cases. The corresponding tibial components were modular with a PE insert in a titanium/aluminium/vanadium alloy base plate (TiAlV).
To reduce artifacts caused by the metallic implant, it is necessary to modify the pulse sequence parameters. Magnetic resonance imaging scans were acquired with a 1.5-Tesla superconducting magnet (HDx; GE Medical Systems, Milwaukee, WI, USA). The patients were imaged using a standard phased-array extremity coil (phased-array extremity coil, Med Rad, Indianola, PA, USA). Sagittal inversion recovery images were obtained with an inversion time of 150 ms, a repetition time (TR) of 4,000 to 5000 ms, an echo time (TE) of 17 ms (4,000–5000/17), a 256–224 matrix, a 20- to 22-cm field of view, 4-mm thick sections with no intersection gap, and two excitations. The receiver bandwidth was 32 kHz. Sagittal fast spin echo images were obtained with a TR/TE of 4,000 to 5,000/34, 12- to 18-echo train length, 3.5-mm slice thickness with no interslice gap, 62.5–83-kHz bandwidth (over the entire frequency domain), 512–288 matrix, 18-cm field of view, 4 to 5 excitations. Axial and coronal fast spin echo images were obtained with similar parameters but with a field of view of 16 cm and slice thickness of 3 mm with no interslice gap.
The size of the abnormal soft tissue lesion proximal to the patella was determined in sagittal and axial MRI views. The nodule tissue was characteristically of intermediate signal intensity on the moderate echo time pulse sequence, characteristic of synovial scarring. There were no MR features to suggest a hemorrhagic content. Largest diameters were recorded in two dimensions by two independent observers who had not been involved in treatment of patients.
Continuous variables were shown as mean and SD. Categorical data were given in absolute figures. For analysis of data, p < 0.05 was considered as statistically significant. After verifying equal distribution, values were analyzed by Student's t test. The mean values of the results by two independent investigators (L.C. and T. H.) were used as basis for assessment of interobserver reliability by intra-class correlation coefficients (ICC). They were calculated by two-way random effects analysis of variance with random observers (ICC (2,1)). Values >0.75 are considered as significant for a reproducible interobserver reliability. Statistical analysis was supported by using Microsoft Excel (Microsoft Corporation, Seattle, USA) and IBM SPSS Statistics 18 (PASW 18, SPSS Inc., Chicago, IL, USA).
Results
MRI can predictably identify the soft tissue lesion associated with patellar clunk syndrome. Nine patients showed obvious MRI findings attributable to a patellar clunk lesion (75%). In all cases, these were intra-articular soft tissue masses proximal to the patella. In two patients, MRI did not show any signs of abnormal soft tissue. In one patient, artifact caused by the femoral CoCrMo component precluded evaluation of the extensor mechanism.
The dimensions of the lesion causing the soft tissue nodule can be reproducibly measured from MRI scans. In the sagittal plane (Fig. 1), mean diameters of the nodule were 6.7 ± 2.1 mm (observer 1) and 7.4 ± 1.7 (observer 2, p = 0.22, ICC 0.76) for the anteroposterior dimension and 17.3 ± 7.1 (observer 1) and 17.6 ± 8.6 (observer 2, p = 0.77, ICC 0.892) for the proximo-distal dimension. Artifact interfered with evaluation in two patients on sagittal images. In the axial plane (Fig. 2), mean diameters of the nodule were 9.5 ± 1.7 (observer 1) and 10.0 ± 2.4 mm (observer 2, p = 0.39, ICC = 0.755) for the antero-posterior dimension and 33.1 ± 7.9 (observer 1) and 32.2 ± 9.5 mm (observer 2, p = 0.53, ICC = 0.929) for the transverse dimension. In one patient, artifact generated by the femoral component prevented evaluation of the extensor mechanism in the axial view. MRI provides supportive evidence helpful in making the decision to treat the patellar clunk symptoms by arthroscopic debridement. Eight of the nine patients with an MRI confirmed clinical diagnosis had an arthroscopic debridement. One patient accepted the diagnosis and refused surgery. In the three patients without MRI findings, no surgery was performed, and the patients were managed conservatively.
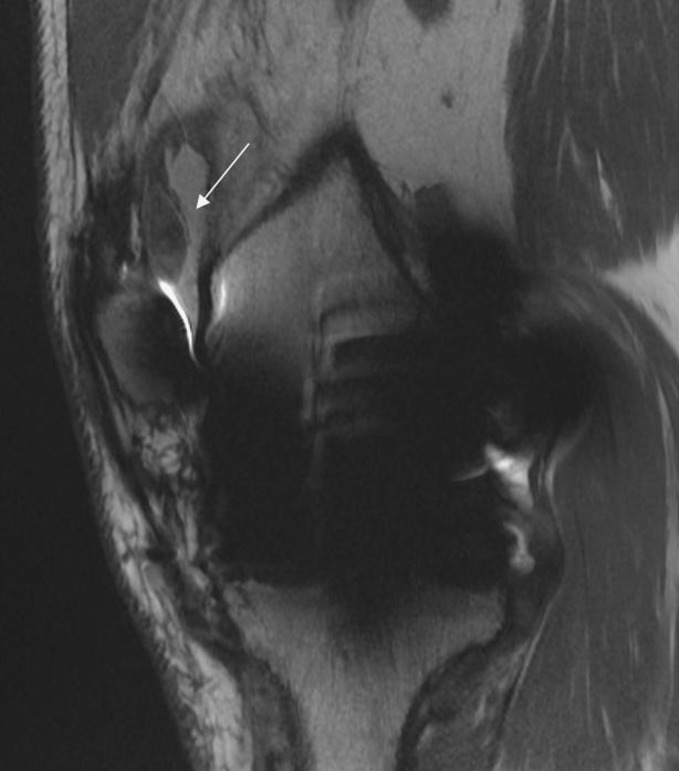
Fig. 1.
The soft tissue nodule causing patellar clunk in the sagittal view after TKA with a conventional CoCrMo femoral component is shown. There is significant artifact, but the intra-articular soft tissue mass proximal to the patella (24.8 × 8.8 mm) can clearly be visualized
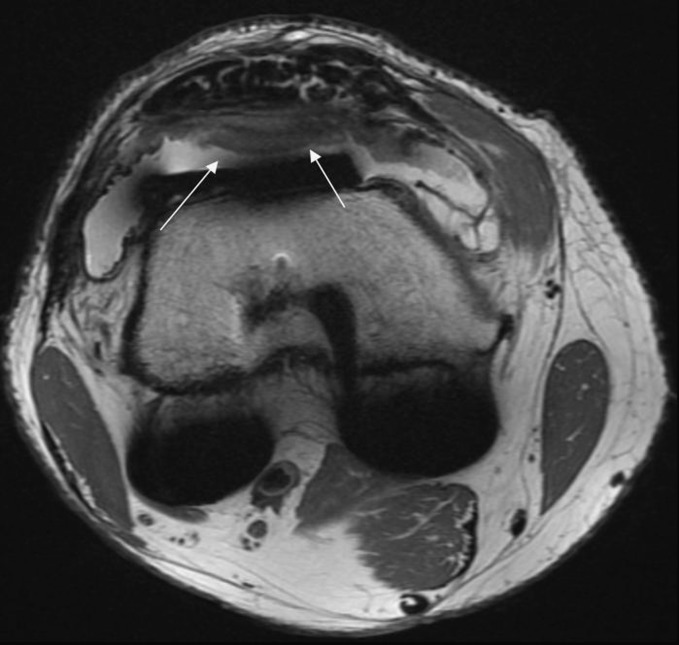
Fig. 2.
This illustrates the nodule in the same patient in the axial view (50.4 × 11.3 mm)
Discussion
MRI is helpful and highly reproducible in confirming the clinical diagnosis of patellar clunk. The reproducibility of the MRI findings and determination of the size of patellar clunk leison could be detected and measured with high interobserver reliability. The information obtained from the MRI supported the decision to proceed with arthroscopic debridement in eight of the twelve patients in this series.
This study has some limitations mainly due to its retrospective design and to the small group size of this rare entity following posterior stabilized TKA. Raphael et al. [10] demonstrated improved visualization and higher interobserver agreement in MRI evaluation of zirconium knee implants compared to CoCrMo components. In the presented data, more artifacts were seen around CoCrMo femoral components. In one case, artifact precluded evaluation of the extensor mechanism. In ten cases, the soft tissues proximal to the patella could be successfully visualized despite a CoCrMo component (91%). MRI confirmed patellar clunk at a mean interval of 16 months after TKA. Patellar clunk has been described to occur relatively early after TKA in another series, at a mean of 10.6 months [1].
Modified MRI protocols reduce metal artifact, allowing for visualization of ligaments, tendons, and the implant–bone interface, and these protocols are available using most 1.5 T commercial MRI units [13]. A number of applications for MRI after TKA have been described [2, 7–9, 15].
In conclusion, the presented cases indicate that MRI is effective in the confirmation of clinically evident patellar clunk after TKA by directly visualizing the intra-articular soft tissue proliferation proximal to the patella, thereby directing appropriate management. MRI is a powerful tool for assessment of a painful TKA. Indications for MRI following TKA are still being established and the advent of new pulse sequences and technical refinements will further our understanding of the utility of advanced imaging following arthroplasty. Future works will have to focus on establishing diagnostic evaluation algorithms.
Acknowledgements
This work was initiated and inspired by Dr. Richard S. Laskin who passed away in 2008. The authors dedicate this work to his remembrance.
The authors would like to thank Dr. Nina Timmesfeld from the Institute for Medical Biometry and Epidemiology, Marburg, Germany for her support with statistical analysis. The authors also thank Ed O'Connell and Tina Miller (Office Managers, HSS) for their warm, efficient, and rapid support.
Disclosures
The study was performed at the Hospital for Special Surgery, New York, NY, USA. Steven B. Haas, MD receives royalties from and is a consultant to Smith & Nephew. Thomas J. Heyse, MD is a consultant to Smith & Nephew. Institutional research support is granted by GE Healthcare and NIH. Hollis Potter, MD is a consultant to Biomet.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participating in the study was obtained.
Footnotes
Level of Evidence: Level IV: Retrospective Case Series
References
- 1.Beight JL, Yao B, Hozack WJ, Hearn SL, Booth RE., Jr The patellar "clunk" syndrome after posterior stabilized total knee arthroplasty. Clin Orthop Relat Res. 1994;299:139–42. [PubMed] [Google Scholar]
- 2.Carpenter RD, Brilhault J, Majumdar S, Ries MD. Magnetic resonance imaging of in vivo patellofemoral kinematics after total knee arthroplasty. Knee. 2009;16(5):332–6. doi: 10.1016/j.knee.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Figgie HE, 3rd, Goldberg VM, Heiple KG, Moller HS, Gordon NH., 3rd The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68(7):1035–40. [PubMed] [Google Scholar]
- 4.Hozack WJ, Rothman RH, Booth RE, Jr., Balderston RA: The patellar clunk syndrome. A complication of posterior stabilized total knee arthroplasty. Clin Orthop Relat Res 1989(241): 203–8. [PubMed]
- 5.Insall JN, Binazzi R, Soudry M, Mestriner LA. Total knee arthroplasty. Clin Orthop Relat Res. 1985;192:13–22. [PubMed] [Google Scholar]
- 6.Insall JN, Lachiewicz PF, Burstein AH. The posterior stabilized condylar prosthesis: a modification of the total condylar design. Two to four-year clinical experience. J Bone Joint Surg Am. 1982;64(9):1317–23. [PubMed] [Google Scholar]
- 7.Lee KY, Slavinsky JP, Ries MD, Blumenkrantz G, Majumdar S. Magnetic resonance imaging of in vivo kinematics after total knee arthroplasty. J Magn Reson Imaging. 2005;21(2):172–8. doi: 10.1002/jmri.20233. [DOI] [PubMed] [Google Scholar]
- 8.Mosher TJ, Davis CM., 3rd Magnetic resonance imaging to evaluate osteolysis around total knee arthroplasty. J Arthroplasty. 2006;21(3):460–3. doi: 10.1016/j.arth.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Potter HG, Foo LF. Magnetic resonance imaging of joint arthroplasty. Orthop Clin North Am. 2006;37(3):361–73. doi: 10.1016/j.ocl.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Raphael B, Haims AH, Wu JS, Katz LD, White LM, Lynch K. MRI comparison of periprosthetic structures around zirconium knee prostheses and cobalt chrome prostheses. AJR Am J Roentgenol. 2006;186(6):1771–7. doi: 10.2214/AJR.05.1077. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM: Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res 2002(404): 7–13. [DOI] [PubMed]
- 12.Simison AJ, Noble J, Hardinge K. Complications of the Attenborough knee replacement. J Bone Joint Surg Br. 1986;68(1):100–5. doi: 10.1302/0301-620X.68B1.3941125. [DOI] [PubMed] [Google Scholar]
- 13.Sofka CM, Potter HG, Figgie M, Laskin R. Magnetic resonance imaging of total knee arthroplasty. Clin Orthop Relat Res. 2003;406:129–35. doi: 10.1097/00003086-200301000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Vernace JV, Rothman RH, Booth RE, Jr, Balderston RA. Arthroscopic management of the patellar clunk syndrome following posterior stabilized total knee arthroplasty. J Arthroplasty. 1989;4(2):179–82. doi: 10.1016/S0883-5403(89)80072-5. [DOI] [PubMed] [Google Scholar]
- 15.Vessely MB, Frick MA, Oakes D, Wenger DE, Berry DJ. Magnetic resonance imaging with metal suppression for evaluation of periprosthetic osteolysis after total knee arthroplasty. J Arthroplasty. 2006;21(6):826–31. doi: 10.1016/j.arth.2005.10.017. [DOI] [PubMed] [Google Scholar]