Abstract
Background
Degenerative and iatrogenic conditions may lead to flat back or even to kyphotic deformity, and sagittal imbalance can cause significant clinical impairment. Minor imbalance cases are usually treated with conservative care. Among currently popular surgical techniques for the correction of sagittal imbalance are posterior-based procedures, which are associated with access-related risks (mostly neurological) and postoperative morbidity risks.
Purpose
This study aims to report a minimally invasive lateral approach using hyperlordotic cages in the treatment of mild sagittal imbalance. Radiological correction, clinical improvement, and safety will be analyzed.
Methods
Eight patients (mean age 71.8 years, SD 7.8; mean BMI 27.5, SD 2.3) with symptomatic sagittal imbalance were retrospectively reviewed. Eight cases were treated by anterior interbody fusion with lordotic cages. A minimally invasive lateral retroperitoneal approach was used in the surgical procedures, with or without percutaneous pedicle screw supplementation.
Results
No major complications occurred and just one case needed revision for direct decompression. Clinical outcomes Visual Analog Scale score changed from 88 at preoperative visit to 51 at 1-week visit, and Oswestry Disability Index score decreased from 82 at preoperative visit to 44 at 6-week visit. The 6-month radiological assessment revealed improvement in spinopelvic parameters: Focal lordosis improved from 2.3° ± 7.7 to 27.1° ± 6.7. Sagittal vertical alignment improved from 11.7 ± 5.3 to 6.2 ± 4.0 cm. Preoperative sacral slope improved from 20.1° ± 5.8 to 29.4° ± 10.3 and preoperative pelvic tilt improved from 35.2° ± 5.2 to 23.8° ± 4.3. Short-term results indicate that the minimally invasive lateral approach can be applied to the treatment of mild sagittal imbalance, with special advantage in elderly patients or those in which posterior approaches are relatively contraindicated.
Keywords: spine, lumbar spine, spine fusion, sagittal balance, minimally invasive surgery, interbody fusion
Introduction
Life expectancy is increasing worldwide, and in the population >60 years of age, degenerative arthritis of the spine is increasingly prevalent. The degeneration of the intervertebral discs with collapse of disc height leads to a loss of the normal sagittal curves and a straighter profile which is not biomechanically efficient [31]. Lower lumbar lordosis plays important clinical and radiological roles in sagittal alignment and balance [14]. The main cause of sagittal imbalance in the degenerative lumbar spine is the loss of lumbar lordosis. Also, there is strong correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion [9, 37].
Posterior-based procedures have been described in the literature for the surgical management of primary sagittal plane deformity [4–6, 8, 16, 31, 34]. These osteotomies that aim to correct kyphosis by posterior spine shortening carry a high risk for complications including permanent neurologic damage, massive bleeding, deep infection, and even fatality [4, 12, 31, 38].
Minimally invasive lateral interbody fusion (LIF) has been reported to provide satisfactory results for the treatment of disc-based conditions such as discogenic pain [2, 3, 24, 32], stenosis [3, 11, 17, 25], adjacent-level disease [2, 3], degenerative scoliosis [1–3, 10, 13, 23], spondylolisthesis [17, 27, 32], and corpectomy [3, 33], even for elderly [15, 30] or obese patients [29]. It has been demonstrated that minimally invasive LIF can provide correction in the sagittal plane [3, 28, 33, 36, 39].
The primary goal of this study was to determine the power of lordotic intervertebral lateral cages to correct sagittal plane alignment and spinopelvic parameters. Secondly, we aimed to determine if the lateral access is clinically effective and safe for elderly sagittal imbalanced patients. Finally, we aimed to determine limitations and points that may be addressed in future practice in this field. This information can be used to introduce a controversy and further discuss and apply anterior elongation as an alternative for correcting thoracic and lumbar curves.
Patients and Methods
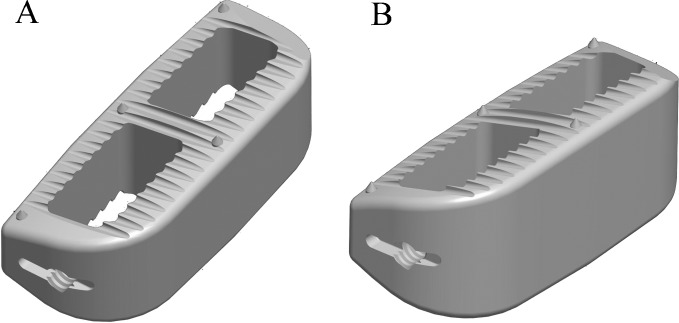
This retrospective case series presents the short-term results of nine consecutive patients treated by LIF for disability due to sagittal imbalance with or without disc disease. Relative contraindications to the posterior approach were previous posterior spine surgery, >65 years of age, and presence of comorbidities (coronary heart disease, renal failure, diabetes, and infection history). Eight patients were enrolled between August 2009 and December 2010 (mean age 71.8 years, range 69–80 years; mean BMI 27.5, SD 2.4; all female; Table 1), with treatment for 17 spine levels (4–1, L12–L5, range). Four of the cases developed sagittal imbalance due to degenerative disc disease and the other four due to the development adjacent segment disease following limited spinal fusion or failed back syndrome. Surgical goals were to correct the kyphotic lumbar condition and also to open foraminal spaces, aiming to prevent and treat foraminal stenosis. Minimally invasive lateral access was performed as previously reported [26] with dilators and split-blade retractor (MaXcess, NuVasive, Inc., San Diego, CA, USA) and stimulated and continuous EMG (NeuroVision, NuVasive, Inc., San Diego, CA, USA). Wide discectomies were performed, maintaining anterior longitudinal ligament (ALL) and posterior longitudinal ligament for insertion of the lordotic interbody cages. In these cases, 22-mm-wide (anteroposterior) polyetheretherketone cages, which lay on the vertebral body lateral apophyseal ring, were utilized. The cages had 20° or 30° of lordotic angulation (Fig. 1; CoRoent XL, NuVasive, Inc., San Diego, CA, USA). Interbody device sizing was performed intraoperatively for each patient and each level, and implant angulation was chosen based on the surgical plan of sagittal correction. If severe osteoporosis and/or segmental instability at the index level were proven, posterior supplementation was carried out with percutaneous pedicle screws system (MIP®; MDT®, Rio Claro, Brazil). Four cases were supplemented posteriorly with percutaneous pedicle screws. Procedures did not include direct posterior decompression. All surgeries were performed by the same senior surgeon (LP).
Table 1.
Case series
| No. | Age | Sex | BMI | PreOp lordosis (°) | PreOp SVA (cm) | Level | Cages |
|---|---|---|---|---|---|---|---|
| 1 | 55 | F | 23.8 | 9 | 5 | L3L4; L4L5 | 20°; 20° |
| 2 | 76 | F | 27.5 | 15 | 6 | L1L2; L3L4 | 20°; 20° |
| 3 | 80 | F | 25.6 | 18 | 13 | L1L2; L2L3 | 20°; 20° |
| 4 | 74 | F | 29.4 | 21 | 9 | L3L4; L4L5 | 20°; 20° |
| 5 | 76 | F | 26.8 | 25 | 9 | L2L3; L3L4 | 20°; 20° |
| 6 | 69 | F | 31.2 | 15 | 19 | L2L3; L4L5 | 30°; 20° |
| 7 | 76 | F | 26.2 | 20 | 18 | L2L3; L3L4; L4L5 | 20°; 20°; 30° |
| 8 | 68 | F | 29.6 | 19 | 15 | L3L4; L4L5 | 20°; 20° |
F female, BMI body mass index, PreOp SVA preoperative sagittal vertical alignment
Fig. 1.
Lordotic intervertebral cages. a 20° and b 30° lordotic polyetheretherketone cages (CoRoent XL, NuVasive, Inc., San Diego, CA, USA)
Clinical evaluations included a physical exam for lower extremity motor and sensory function by a senior spine surgeon (LP), Visual Analog Scale (VAS) for back and leg pain, reported in millimeters, and Oswestry Disability Index (ODI) at the preoperative visit and after 1 and 6 weeks and 3 and 6 months. Follow-up was a minimum of 6 months to a maximum of 24 months.
Student’s t test and ANOVA (Analyse-It Software, Ltd., Leeds, England) were used to determine statistically significant changes from preoperative visit to follow-up as appropriate, with a level of significance of 0.05.
Results
Overall, sagittal plane alignment and spinopelvic parameters were significantly changed with anterior elongation with lordotic interbody cages (Table 2; Figs. 2, 3, and 4). Mean lordosis “built in” to the cages per patient was 45.0° (SD 3.2) per patient, and surgical results showed an average gain of 22.2° (SD 3.2) on lumbar lordosis, an average of 50% regarding cage angulation to real lordosis change. Although normal values were not achieved, substantial reduction (50.0%) on SVA was achieved in the studied group (Figs. 3 and 4). Additionally, spinopelvic parameters were also significantly changed.
Table 2.
Spinopelvic parameters
| Preoperative | 6 months | p value | |
|---|---|---|---|
| Focal lordosis (°) | 2.3 (7.7) | 27.1 (6.7) | <0.001a |
| Global lumbar lordosis (°) | 14.9 (7.4) | 40.0 (8.2) | <0.001a |
| SVA (cm) | 24.1 (7.3) | 9.2 (3.3) | 0.006a |
| Sacral slope (°) | 20.1 (5.8) | 29.4 (10.3) | 0.004a |
| Pelvic tilt (°) | 35.2 (5.2) | 23.8 (4.3) | 0.009a |
Results are presented as the mean (standard deviation)
aStatistically significant
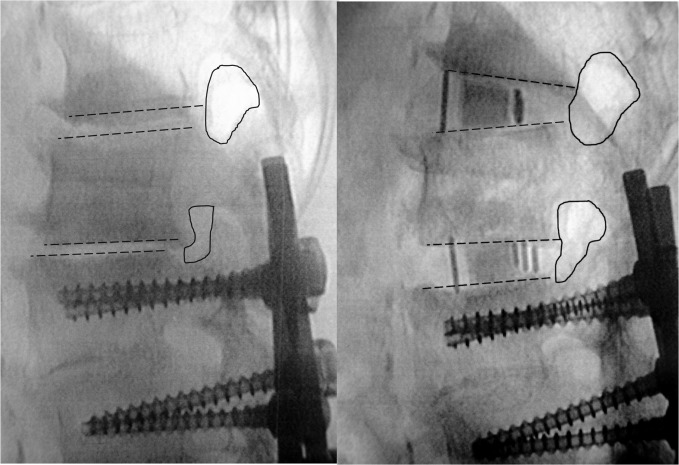
Fig. 2.
Disc and foraminal space distraction with lordosis gain (case no. 2). Preoperative and postoperative fluoroscopic images with surgical goals at L1–L2 and L2–L3. Preoperative L1–L4 kyphosis 18° and postoperative L1–L4 lordosis 10°
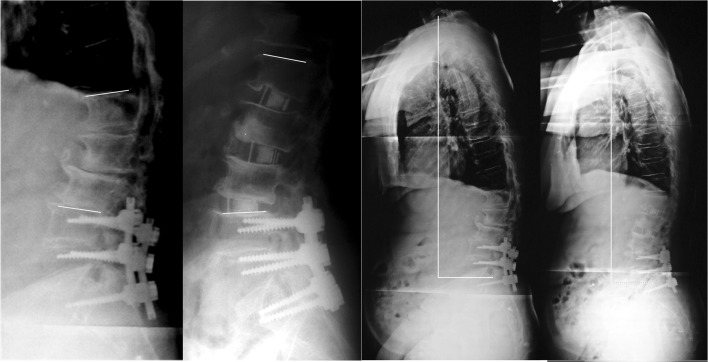
Fig. 3.
Adjacent segment disease (case no. 3). Preoperative and 6 months lateral X-ray images. Lines evidence lordosis gain and reduction in sagittal vertical alignment value
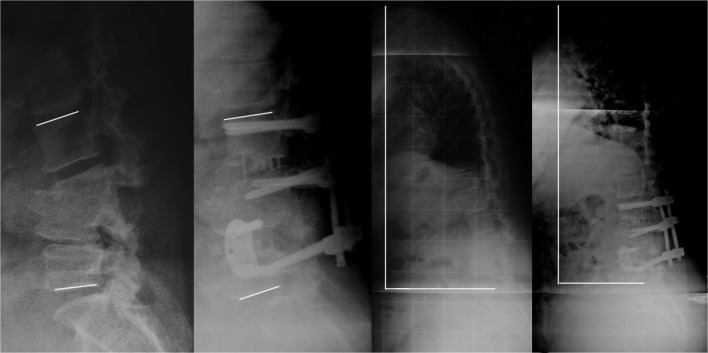
Fig. 4.
Iatrogenic kyphotic lumbar spine (case no. 6). Preoperative and 6 months lateral X-ray images. Lines evidence lordosis gain and reduction in sagittal vertical alignment value
Surgical procedures were completed in only one stage and no intraoperative complication occurred. The mean operative time was 3.5 h, SD 2.12 and mean blood loss was 131.3, SD 92.3. There were no major complications during follow-up. One stand-alone case needed revision at 3 months due to restenosis following severe subsidence. The revision was carried out as a minimally invasive over-the-top decompression. The VAS assessing pain and the ODI scores were improved by the procedure (p < 0.01; Table 3). An early (1 week) and significant pain relief was further maintained and physical disability gradually decreased.
Table 3.
Clinical questionnaire data
| Preoperative | 1 week | 6 months | p value | |
|---|---|---|---|---|
| VAS back | 88 (12) | 51 (10) | 37 (20) | <0.001a |
| VAS legs | 67 (26) | 38 (18) | 32 (23) | 0.006a |
| ODI | 82 (13) | 57 (11) | 49 (19) | <0.001a |
Results are presented as the mean (standard deviation)
VAS Visual Analog Scale for pain (in millimeters), ODI Oswestry Disability Index
aStatistically significant
Intraoperative anterior endplate violation by the cage led to diminished lordosis gain (p = 0.024). In 35.3% of treated levels, it was possible to observe that the anterior endplate was slightly damaged during lordotic cage insertion. For spine levels in which this damage did not occur, focal lordosis gain achieved 58% (SD 35%) of cage angulation, while for the levels with anterior endplate violation, only 23% (SD 12%) of cage angulation was realized.
Discussion
Normal sagittal balance of the spine can be altered by naturally occurring changes associated with age [18], occurs also due to iatrogenic changes [14, 19–21], and is more prevalent in elderly population. Traditional surgical correction is based on posterior osteotomies [7], an option which exposes patients to important risks and morbidity. In this report, we presented results on a minimally invasive and low-morbidity treatment for mild sagittal imbalance in elderly population. Using lateral access, hyperlordotic intervertebral cages were able to correct global sagittal alignment of the spine and spinopelvic parameters and provide clinical improvement for patients.
One limitation of the present study is that it was done as a retrospective analysis of consecutive patients who had sagittal imbalance. Also, as a preliminary report on this novel indication for lateral access, it is reported based on a single-center experience with a small group after a short follow-up. It should also be emphasized that these patients presented with concurrent degenerative conditions in conjunction to sagittal imbalance, so it can be difficult to distinguish between the direct clinical benefit of arthrodesis and sagittal correction.
In order to compensate for decreased lumbar lordosis, the thoracic curve decreases kyphosis and the pelvis creates a retroversion with decrease of sacral slope [14, 22, 31, 35]. As was shown in the present work and in other studies [14], spontaneous changes in sacral slope can be achieved following the surgical correction of lumbar lordosis.
In this series, interbody fusion was not performed in combination with the described ALL resection technique. With the ALL intact, it was more difficult to achieve anterior release and disc space distraction. We believe that this increases the risk of intervertebral cage subsidence into the endplate during cage insertion, leading to a loss of potential sagittal correction. Preliminary data from our group on recent cases have shown that ALL rupture may provide better distraction of anterior disc height with better correction of the lordosis. As previously shown in a radiological study [25], the disc height distraction and indirect decompression can be partially lost due to cage subsidence. In that study, nonlordotic cages were analyzed and they seemed to subside equally in their anterior and posterior portions. In the present study, it was observed in some cases that lordotic cages seemed to subside anteriorly, resulting in a parallel, instead of a lordotic, distraction of the index level, with posterior disc height being maintained.
Regarding the surgical correction, it was possible to observe a frequent pattern in the procedures reported here: lordosis gain was 50% of total cage angle. So, by accessing three lumbar levels with a total cage angle of 60°, without ALL resection, we typically achieved an average of 30° correction. In the case series reported here, lordotic cages (mean 45°, range 40–70°) resulted in an average lordosis gain of 22.2° in a mean of 2.2 treated lumbar levels, 10.3° per level, higher than the 2.8° per level previously reported with nonlordotic cages [32]. Polysegmental wedge osteotomies usually obtain 10–15° [8, 34] and Smith-Petersen osteotomy adds 10° of lordosis per level, but if a substantial correction is achieved, it is necessary to extend the procedure anteriorly to obtain stable constructs [6].
As reviewed by Roussouly and Nnadi [31], posterior shortening through spinal osteotomies remains a complex procedure, but it cannot be substituted for by anterior-only approach in major deformities with fixed posterior construction in disease such as ankylosing spondylitis.
Patients who have sagittal imbalance usually are elderly, have surgical limitations, possess comorbidities, have previous surgeries, and/or may present major risks at the perioperative period. It must be remembered that even the lateral approach has its related risks as anterior thigh pain, dysesthesias, motor weakness (potentially permanent), vascular injury, segmental artery injury, and subsidence [27]. These procedures must be advocated, keeping in mind risk-effectiveness. To improve risk, existing comorbidities must be optimized prior to surgery. In addition to posing the question “How much correction is needed to relieve disability,” the surgeon must ask “How much correction can be tolerated by the patient.” We believe that minimally invasive anterior construction may provide an answer to these challenges. This preliminary report suggests that our technique of minimally invasive lateral fusion may be a safe and risk-effective technique for treating sagittal imbalance.
Acknowledgements
Portions of this work, including early and interim reports, were presented in abstract/poster/oral presentation form at the Society of Lateral Access Surgery (SOLAS) in Mar 31, 2011; San Diego, California; the Spine Arthroplasty Society (SAS) in April 28, 2010; New Orleans, Louisiana; North America Spine Society (NASS) in October 6, 2010; Orlando, Florida; and Congress of Neurological Surgeons (CNS) in October 20, 2010; San Francisco, California; as well at the Honorary and Distinguished Lecture at the Hospital for Special Surgery (HSS) June 3 2011; New York, New York.
Disclosures
One of the authors (L.P.) certifies that he or she has received or may receive payments or benefits from NuVasive, Inc. Other authors have nothing to disclose.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participating in the study was obtained.
Work was performed at Instituto de Patologia da Coluna, São Paulo, Brazil.
Footnotes
Level of Evidence: Level IV retrospective case series.
References
- 1.Benglis DM, Elhammady MS, Levi AD, Vanni S. Minimally invasive anterolateral approaches for the treatment of back pain and adult degenerative deformity. Neurosurgery. 2008;63(3 Suppl):191–196. doi: 10.1227/01.NEU.0000325487.49020.91. [DOI] [PubMed] [Google Scholar]
- 2.Bergey DL, Villavicencio AT, Goldstein T, Regan JJ. Endoscopic lateral transpsoas approach to the lumbar spine. Spine. 2004;29(15):1681–1688. doi: 10.1097/01.BRS.0000133643.75795.EF. [DOI] [PubMed] [Google Scholar]
- 3.Billinghurst J, Akbarnia BA. Extreme lateral interbody fusion - XLIF. Curr Orthopaedic Pract. 2009;20(3):238–251. doi: 10.1097/BCO.0b013e3181a32ead. [DOI] [Google Scholar]
- 4.Bridwell KH, Lewis SJ, Edwards C, Lenke LG, Iffrig TM, Berra A, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine. 2003;28(18):2093–2101. doi: 10.1097/01.BRS.0000090891.60232.70. [DOI] [PubMed] [Google Scholar]
- 5.Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85-A(3):454–463. doi: 10.2106/00004623-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bridwell KH. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine. 2006;31(19 Suppl):S171–S178. doi: 10.1097/01.brs.0000231963.72810.38. [DOI] [PubMed] [Google Scholar]
- 7.Bridwell KH. Causes of sagittal spinal imbalance and assessment of the extent of needed correction. Instr Course Lect. 2006;55:567–575. [PubMed] [Google Scholar]
- 8.Cho K-J, Bridwell KH, Lenke LG, Berra A, Baldus C. Comparison of Smith-Petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance. Spine. 2005;30(18):2030–2037. doi: 10.1097/01.brs.0000179085.92998.ee. [DOI] [PubMed] [Google Scholar]
- 9.Chow DH, Luk KD, Evans JH, Leong JC. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine. 1996;21(5):549–555. doi: 10.1097/00007632-199603010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurgical FOCUS. 2010 mar;28(3):E8. [DOI] [PubMed]
- 11.Dezawa A, Yamane T, Mikami H, Miki H. Retroperitoneal laparoscopic lateral approach to the lumbar spine: a new approach, technique, and clinical trial. J Spinal Disord. 2000;13(2):138–143. doi: 10.1097/00002517-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Hyun S-J, Rhim S-C. Clinical outcomes and complications after pedicle subtraction osteotomy for fixed sagittal imbalance patients : a long-term follow-up data. J Korean Neurosurg Soc. 2010;47(2):95–101. doi: 10.3340/jkns.2010.47.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine. 2010;35(26 Suppl):S322–S330. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 14.Jang J-S, Lee S-H, Min J-H, Maeng DH. Changes in sagittal alignment after restoration of lower lumbar lordosis in patients with degenerative flat back syndrome. J Neurosurg Spine. 2007;7(4):387–392. doi: 10.3171/SPI-07/10/387. [DOI] [PubMed] [Google Scholar]
- 15.Karikari IO, Grossi PM, Nimjee SM, Hardin C, Hodges TR, Hughes BD, et al. Minimally Invasive Lumbar Interbody Fusion in Patients Over Seventy Years of Age: analysis of peri- and post-operative complications. Neurosurgery. 2011;68(4):897–902. doi: 10.1227/NEU.0b013e3182098bfa. [DOI] [PubMed] [Google Scholar]
- 16.Kim K-T, Park K-J, Lee J-H. Osteotomy of the spine to correct the spinal deformity. Asian Spine J. 2009;3(2):113–123. doi: 10.4184/asj.2009.3.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klopfenstein JD, Kim LJ, Feiz-Erfan I, Dickman CA. Retroperitoneal approach for lumbar interbody fusion with anterolateral instrumentation for treatment of spondylolisthesis and degenerative foraminal stenosis. Surg Neurol. 2006;65(2):111–116. doi: 10.1016/j.surneu.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Knight R, Jackson R, Killian J, Stanley E. White paper on sagittal alignment [Internet]. Available from: http://www.srs.org/professionals/resources/sagittal_plane_white_paper.pdf.
- 19.Kostuik JP, Maurais GR, Richardson WJ, Okajima Y. Combined single stage anterior and posterior osteotomy for correction of iatrogenic lumbar kyphosis. Spine. 1988;13(3):257–266. doi: 10.1097/00007632-198803000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10(4):314–319. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazennec JY, Ramaré S, Arafati N, Laudet CG, Gorin M, Roger B, et al. Sagittal alignment in lumbosacral fusion: relations between radiological parameters and pain. Eur Spine J. 2000;9(1):47–55. doi: 10.1007/s005860050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7(2):99–103. doi: 10.1007/s005860050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundis GM, Akbarnia BA, Phillips FM. Adult Deformity Correction Through Minimally Invasive Lateral Approach Techniques. Spine. 2010;35(Supplement):S312–S321. doi: 10.1097/BRS.0b013e318202495f. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira L, Marchi L, Coutinho E, Abdala N, Pimenta L. The use of rh-BMP2 in Standalone eXtreme Lateral Interbody Fusion (XLIF®): Clinical and Radiological Results After 24 Months Follow-up. WSCJ. 2010;1(1):19–25. [Google Scholar]
- 25.Oliveira L, Marchi L, Coutinho E, Pimenta L. A Radiographic Assessment of the Ability of the Extreme Lateral Interbody Fusion Procedure to Indirectly Decompress the Neural Elements. Spine. 2010;35(Supplement):S331–S337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 26.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine. 2011;36(1):26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers WB, Cox C, Gerber E. Experience and Early Results with a Minimally Invasive Technique for Anterior Column Support Through eXtreme Lateral Interbody Fusion (XLIF®) US Musculoskelet Rev. 2007;2:28–32. [Google Scholar]
- 29.Rodgers WB, Cox CS, Gerber EJ. Early Complications of Extreme Lateral Interbody Fusion in the Obese. J Spinal Disord Tech [Internet]. 2010 jan 15 [citado 2010 ago 2];Available from: http://www.ncbi.nlm.nih.gov/pubmed/20084027. [DOI] [PubMed]
- 30.Rodgers WB, Gerber EJ, Rodgers JA. Lumbar Fusion in Octogenarians. Spine. 2010;35(Supplement):S355–S360. doi: 10.1097/BRS.0b013e3182023796. [DOI] [PubMed] [Google Scholar]
- 31.Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J. 2010;19(11):1824–1836. doi: 10.1007/s00586-010-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral Lumbar Interbody Fusion: Clinical and Radiographic Outcomes at 1 Year: A Preliminary Report. J Spinal Disord Tech [Internet]. 2010 set 14 [citado 2010 nov 17];Available from: http://www.ncbi.nlm.nih.gov/pubmed/20844451. [DOI] [PubMed]
- 33.Smith WD, Dakwar E, Le TV, Christian G, Serrano S, Uribe JS. Minimally Invasive Surgery for Traumatic Spinal Pathologies. Spine. 2010;35(Supplement):S338–S346. doi: 10.1097/BRS.0b013e3182023113. [DOI] [PubMed] [Google Scholar]
- 34.Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6–9. [PubMed] [Google Scholar]
- 35.Vaz G, Roussouly P, Berthonnaud E, Dimnet J. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11(1):80–87. doi: 10.1007/s005860000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: Initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28(3):1–8. doi: 10.3171/2010.1.FOCUS09286. [DOI] [PubMed] [Google Scholar]
- 37.Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine. 1995;20(5):526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 38.Yang BP, Ondra SL, Chen LA, Jung HS, Koski TR, Salehi SA. Clinical and radiographic outcomes of thoracic and lumbar pedicle subtraction osteotomy for fixed sagittal imbalance. J Neurosurg Spine. 2006;5(1):9–17. doi: 10.3171/spi.2006.5.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Youssef JA, McAfee PC, Patty CA, Raley E, DeBauche S, Shucosky E, et al. Minimally Invasive Surgery: Lateral Approach Interbody Fusion. Spine. 2010;35(Supplement):S302–S311. doi: 10.1097/BRS.0b013e3182023438. [DOI] [PubMed] [Google Scholar]