Overview:
Because pain is a common and debilitating symptom of osteoarthritis in older adults, the authors reviewed data on the efficacy and safety of commonly used oral, topical, and intraarticular drug therapies in this population. A search of several databases found that most studies have focused on knee osteoarthritis and reported only short-term outcomes. Also, treatment efficacy was found to vary by drug class; the smallest effect was observed with acetaminophen and the largest with opioids and viscosupplements. Acetaminophen and topical agents had the best safety profiles, whereas oral nonsteroidal antiinflammatory drugs and opioids had the worst. Little data were available on patients ages 75 years old and older and on patients from diverse racial and ethnic groups. Most drug therapies gave mild-to-moderate pain relief; their long-term safety and efficacy and their effects in diverse populations (particularly older adults) remain undetermined.
Keywords: Analgesia, older adults, osteoarthritis, pain
Osteoarthritis (OA) constitutes a significant public health problem.1, 2 About 50 million adults in the United States have arthritis (with OA being the most common type), including half of all people over the age of 65.1 The prevalence of OA is greater in women than men, and risk increases with age; it affects all racial and ethnic groups.3 Because no disease-modifying therapies are available (several are under development4, 5), treatment is directed at managing symptoms such as pain and swelling, minimizing functional impairment, and preserving quality of life. Nonpharmacologic treatments—patient education, exercise, weight loss, and physical therapy— can be of substantial benefit,6 but many older adults are reluctant to use them to manage their pain.7 Pharmacologic interventions are the treatments most often prescribed8 and employed9 for OA. Joint replacement is routinely considered when severe symptoms don’t respond to other therapies. Although such surgery can be effective,10 many patients (particularly older adults and racial or ethnic minorities) forgo it.11
This article synthesizes what we know about the pharmacologic management of OA-related pain in older adults—those ages 65 and older. A focus on older adults is appropriate for several reasons. In this age group, undertreated pain leads to poor self-rated health and decreased cognition and mobility.12, 13 Pain is by far the most frequently cited symptom causing disability in later life, threatening independence.14 Older adults’ rate of chronic analgesic use is the highest of all age groups, and they’re the most susceptible to the adverse effects of analgesics.15 Finally, several factors challenge even seasoned clinicians in managing OA-related pain in older adults: the presence of multiple chronic conditions or causes of pain; a high rate of polypharmacy; patients’ concerns about analgesic use, such as fear of addiction; and the availability of too few age-relevant studies to guide decision making.15
Methods
We searched MEDLINE, PubMed, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane reviews for studies published from January 1995 to June 2011, using the search terms acetaminophen, nonsteroidal antiinflammatory agents, opioids, intraarticular injections, corticosteroids, hyaluronic acid, capsaicin, lidocaine, glucosamine, and chondroitin. We linked these terms with osteoarthritis, degenerative joint disease, randomized controlled trials, systematic review, and meta-analysis. (We did not include duloxetine [Cymbalta], which the Food and Drug Administration (FDA) approved for the treatment of OA pain in 2010, because it is only one medication in a class of drugs not generally used for OA.)
All abstracts produced from the searches were reviewed in detail and selected for analysis when they met the following criteria: the article was published in English, the study enrolled adults with OA, the study design was a randomized controlled trial (RCT) or a systematic review, and the reported outcomes included relevant end points such as symptom relief or improved function. The reference lists of retained articles were also reviewed to identify additional studies for review. Effect sizes (to include standardized mean differences, which are summary statistics that provide an estimate of the overall treatment effect) are presented below and allow for comparison across analgesic classes. An effect size of less than 0.2 is thought to be negligible, 0.2 to 0.49 is considered small, 0.5 to 0.79 is moderate, and 0.8 or above is thought to be large.16 Two of us (MCR, RS) reviewed the study abstracts and then independently abstracted information (such as estimates of treatment effect and safety data) from all relevant articles.
Fig. 1.
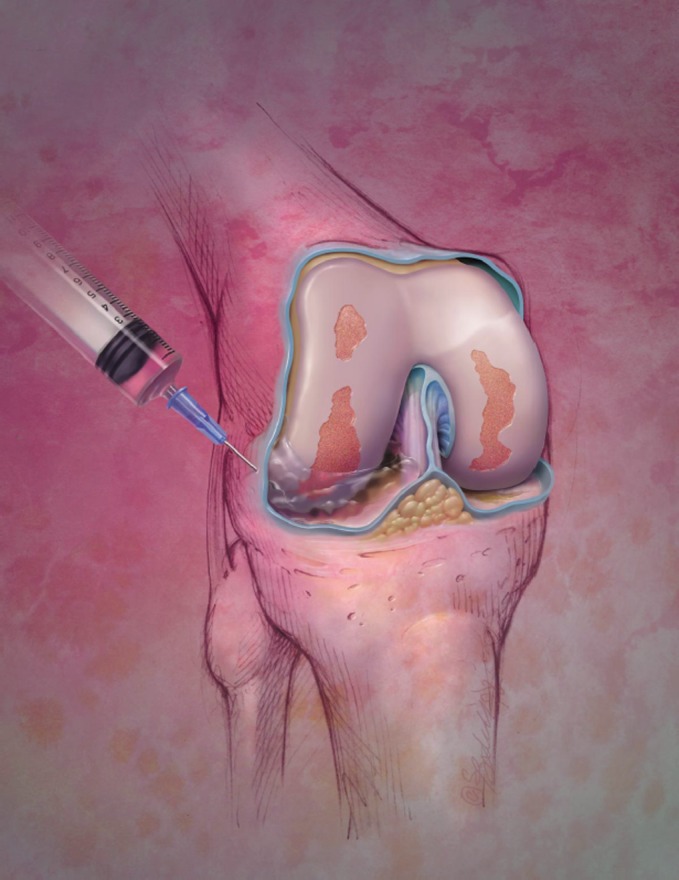
Intraarticular treatments such as corticosteroid injection have been found to be effective for pain relief in patients with OA. Photo © Bodell Communications, Inc./PhototakeUSA.com
Results
A total of 37 articles met the eligibility criteria and were reviewed in detail. The following sections summarize the efficacy and safety data on oral, topical, and intraarticular agents and synthesize recommendations on their use from various guidelines.
Oral Agents. We identified 19 studies examining the use of oral analgesics in the treatment of OA-related pain in older adults.
Acetaminophen (Tylenol and others) is one of the analgesics most commonly taken by people with OA.13 How it works remains unclear, but it’s thought to be a weak cyclooxygenase (COX) inhibitor. In a recent meta-analysis of seven RCTs comparing acetaminophen with placebo, acetaminophen (up to 4 g daily) was found to be modestly effective in reducing pain (standardized mean difference, −0.13; 95% CI, −0.22 to −0.04) and less effective at reducing pain or improving function than nonsteroidal antiinflammatory drugs (NSAIDs).17 Similar results were reported in another meta-analysis that analyzed the results of 10 RCTs.18 With respect to safety, acetaminophen toxicity was the leading cause of acute liver failure in the United States from 1998 to 2003.19 Unintentional overdose is the leading cause of acetaminophen-induced hepatotoxicity; the vast majority of these cases had taken acetaminophen to treat pain.19 Also, a large population-based retrospective cohort study found that older adults combining NSAIDs and acetaminophen had an increased risk of hospitalization for gastrointestinal events compared with those using either acetaminophen or an NSAID alone.20 That study was limited by an inability to account for over-the-counter analgesic use. Such data led the FDA to recommend in January 2011 that manufacturers cap the amount of acetaminophen in prescribed combination products at 325 mg and that public awareness campaigns focus on the risk of acetaminophen-related liver injury.21 Despite its modest impact on pain, given its low cost and relative safety profile, acetaminophen is recommended as first-line therapy for the treatment of mild-to-moderate pain.13
NSAIDs, both prescribed and over-the-counter agents, continue to be among the most commonly consumed analgesics.22, 23 Drugs in this class include ibuprofen (Advil, Motrin), naproxen (Aleve, Naprosyn), etodolac (Lodine), and indomethacin (Indocin). A meta-analysis of 23 RCTs examining the efficacy of oral NSAIDs reported an effect size of 0.32 (0.24 to 0.39) for pain reduction.24 While oral NSAIDs are considered to be more effective than acetaminophen, they carry the risk of cardiovascular, renal, and gastrointestinal toxicity. The use of NSAIDs, whether or not they’re selective for the cyclooxygenase-2 (COX-2) isoenzyme, is associated with increased risk of hospitalization, renal toxicity, myocardial infarction, stroke, and death.25–27 In addition, roughly 20% of all congestive heart failure admissions between 1993 and 1995 were attributed to NSAID use.28 The finding that NSAIDs conferred increased cardiovascular risk was initially demonstrated with COX-2-inhibitor drugs, which include rofecoxib (Vioxx), valdecoxib (Bextra), and celecoxib (Celebrex). Both rofecoxib and valdecoxib were removed from the market because of this risk. In light of these data, the American Geriatrics Society’s clinical practice guidelines on the pharmacologic management of pain recommend the use of oral NSAIDs sparingly and “with extreme caution.”13
Opioids are strong analgesics usually given when other drug and nondrug interventions have failed.29 This class includes morphine (MS Contin), hydrocodone (Vicodin), oxycodone (OxyContin) hydromorphone (Dilaudid), and fentanyl (Duragesic patch). In a meta-analysis of 40 studies examining opioids in the treatment of chronic noncancer pain in older adults, Papaleontiou and colleagues found that most investigators had looked at OA of the hip or knee.30 Positive effects were recognized for reduction in pain (effect size = −0.56, P < 0.001) and physical disability (−0.43, P < 0.001), but not for improvement in quality of life (0.19, P = 0.171). Adverse effects occurred commonly and included constipation, nausea, and dizziness, prompting opioid discontinuation in about 25% of cases. Furthermore, Solomon and colleagues used Medicare claims data (1999 through 2005) to examine the safety of COX-2-selective and nonselective NSAIDs versus opioids for noncancer pain.31 Subjects receiving COX-2-selective NSAIDs or opioids were at increased risk for adverse cardiovascular outcomes compared with those receiving nonselective NSAIDs. Both NSAID groups had similar fracture risks, while opioid users had significantly increased risks of fracture, adverse events requiring hospitalization, and death from any cause.31
Concerns about potential opioid misuse or abuse and harm persist, and its use for OA-related pain remains controversial.32 As increasing evidence shows the risk of NSAID-related toxicity, one recent guideline for managing persistent pain recommends that clinicians consider opioids for older adults who fail acetaminophen therapy and whose moderate-to-severe pain or functional impairment is unrelieved.13
Glucosamine and Chondroitin.
Glucosamine is a major building block of proteoglycans, a key component of cartilage, and chondroitin is purported to protect cartilage by providing joint lubrication and compressive resistance.33–36 Both products are sold over the counter in the United States but require prescriptions in Europe. A recent Cochrane review reported that the most rigorously conducted RCTs of glucosamine used to treat OA found no benefits (pain reduction or improved function), but that benefits were found in less rigorously conducted studies and in trials using one brand of glucosamine (Rotta).33 Studies lasting longer than 12 months show some evidence that long-term glucosamine use leads to less joint space loss, but the clinical significance of this finding is unclear.34 In a meta-analysis examining the effects of chondroitin, benefits were found in small trials with poor methodologic quality but not in three large-scale, well-conducted RCTs.35 Both glucosamine and chondroitin appear to be safe for use.33–36 Because the FDA considers them supplements rather than drugs, production is not regulated, and quality and cost can vary.
Topical Treatments. We identified 11 studies examining topical therapies for OA pain in older adults. Most, but not all, studies enrolled older adults.
Topical NSAIDs.
Given the established risks associated with oral NSAID use,25–28, 37 attention has focused on whether the use of topical NSAIDs can mitigate these risks. Over-the-counter topical NSAIDs have been on the market for some time. These products (such as Aspercreme, Bengay Arthritis Formula, and Sportscreme) contain salicylic acid, a type of NSAID. Two prescription-strength topical NSAIDs (diclofenac [Voltaren Gel, Flector Patch] and ketoprofen [Actron]) have been approved by the FDA in both gel and patch formulations, and a number of trials are testing other topical NSAID formulations.38 In one meta-analysis of 13 RCTs, prescription-strength topical NSAIDs were found to be superior to placebo in relieving OA-related pain for up to two weeks but not longer and less effective than oral NSAIDs; the researchers concluded that no evidence supports their long-term use.39 Baraf and colleagues conducted a post-hoc analysis using data from three RCTs and found that topical diclofenac was superior to placebo (as an inert gel) in patients of all ages with knee OA.40 Relative to placebo, it reduced pain scores, on average, by 1 point (range: 0 to 20), whereas function scores improved, on average, by 3 points (range: 0 to 68). Topical NSAIDs appear to be safer than oral NSAIDs.38–41 But one systematic review found that about 20% of subjects receiving a prescription-strength topical NSAID reported a systemic adverse event such as gastrointestinal problems and headache.42 (In 2009 the FDA added warnings about the potential for hepatotoxicity to the label of Voltaren; see http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm193047.htm.)
Lidocaine is FDA approved for the treatment of neuropathic pain and is thought to provide analgesia by blocking sodium channels in sensory nerves. Several small studies have examined its use in treating OA-related pain.43–45 One post-hoc analysis of data from an RCT comparing lidocaine patch 5% and celecoxib 200 mg/day for the treatment of OA-related knee pain found that they were equally effective in reducing pain (an approximate 35% reduction from baseline) and improving physical function (an approximate 38% improvement from baseline) at six weeks. The patch appeared to be well tolerated.45
Capsaicin is derived from chili peppers. Mason and colleagues identified three RCTs that examined its effects on musculoskeletal pain and found that 38% of those receiving capsaicin 0.025% gel reported pain relief of 50% or greater, compared with 25% of those receiving placebo.46 In a recent RCT, Kosuwon and colleagues found that 0.0125% capsaicin gel was more effective than placebo in treating mild-to-moderate pain associated with knee OA.47 Roughly half of all patients reported local adverse effects—burning, stinging, and erythema—but discontinuation rates were low.47
Intraarticular Treatments. We identified seven studies examining these therapies for OA in older adults.
Corticosteroid injection into the joint has commonly been performed for five decades.48–51 A Cochrane meta-analysis found it more effective than placebo in reducing pain at one to two weeks (measured on a 0-to-100-mm–point scale, with a weighted mean difference of −21.91 points [95% CI, −13.89 to −29.93])—an effect not seen at the four-week follow-up.48 No self-reported improvements in physical function were observed. When compared with hyaluronan and hylan products, steroids were found to produce similar short-term results (to four weeks), but hyaluronan and hylan products were superior in terms of pain relief and improvement in function beyond four weeks. Most adverse events from corticosteroid injection were rated as mild or moderate. Overall, steroid injections were deemed to be safe but provided only short-term benefit. The question of how frequently patients can receive intraarticular steroid injections remains unknown.
Hyaluronan and hylan derivatives (also called viscosupplements) are thought to work by improving the elastoviscous properties of synovial fluid, which progressively diminish in joints affected by OA.52–54 A Cochrane review that synthesized results from 76 trials examining outcomes in those with knee OA found that when compared with placebo, viscosupplementation provides moderate-to-large treatment effects for pain and function, with maximal benefit detected between five and 13 weeks after injection.54 No major safety issues were identified.
Findings Summary. With few exceptions, the identified studies examined analgesic safety and efficacy for 12 weeks or less. Most investigations were sponsored by pharmaceutical companies. This is important because research has shown that studies sponsored by industry are more likely to report positive findings than studies funded by other sources.55 In addition, older patients were frequently excluded from studies, most often because of coexisting chronic conditions. OA of the knee or hip were by far the most frequently studied conditions, and patients with comorbidities were often excluded. Few studies enrolled patients from diverse racial or ethnic backgrounds or reported results stratified by other potentially important predictors such as age and gender.
Discussion
Among adults with OA, evidence indicates that most of the available pharmacologic agents provided mild-to-moderate pain relief. Modest analgesic effects were observed for acetaminophen, while the largest effects were reported for opioids and viscosupplements. Topical agents and acetaminophen have safety profiles superior to those of other analgesics, including oral NSAIDs and opioids.
This review highlights several gaps in our knowledge. First, the long-term safety and effectiveness of commonly prescribed analgesics remain undetermined for adults with OA-related pain. Second, safety and efficacy data for minority populations and older adults, particularly those ages 75 and older are lacking. There is an urgent need to address these gaps, given established disparities in the management of OA-related pain among patients of different races and ethnicities 56 and in those of older age.30 A recent Institute of Medicine report, Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, identified the groups that are most at risk for undertreatment of pain: older adults, racial and ethnic minorities, women, those with cognitive impairment, and those at the end of life.57 Prior research has shown that vulnerable groups are less likely to have their pain assessed and less likely to be offered treatment for pain.56 Finally, the uncertainty about the long-term benefits and safety of medications for OA pain indicates a great need to also develop and test disease-modifying OA drugs, including growth factors, cytokine manipulation, and gene therapy.4, 5
Our study has several implications for nursing. Because analgesics can lead to adverse events when taken in quantities both large (acetaminophen) and small (opioids), routine practice must involve
taking careful medication histories, including asking about over-the-counter analgesic use and the ways in which all types of analgesics are consumed.
assessing for adverse effects, particularly in patients newly started on an analgesic (for instance, constipation in patients started on an opioid).
asking patients how their attitudes and beliefs and barriers to use (such as cost, stigma, fear of addiction) affect their analgesic regimen; these can contribute to the undertreatment of pain.32
teaching patients with OA (and their caregivers) about the risks and benefits of analgesics and how to improve medication safety.
Medication safety training is available,58–60 can be easily presented by nurses, and could help to improve the quality of life and outcomes of care for the millions of people with OA.
Acknowledgments
M. Carrington Reid is an associate attending physician at New York–Presbyterian Hospital and an associate professor of medicine at Weill Cornell Medical College (WCMC) in New York City. Rouzi Shengelia is a research assistant at WCMC. Samantha J. Parker is a former research assistant at WCMC and a current medical student at Tulane University Schoolof Medicine in New Orleans, LA. This research project was supported by an unrestricted educational grant from the National Institute on Aging: an Edward R. Roybal Center Grant (P30AG022845). Contact author: M. Carrington Reid, mcr2004@med.cornell.edu. The authors have disclosed no potential conflicts of interest, financial or otherwise.
Footnotes
This article was produced by the American Journal of Nursing (AJN), which is publishing it in its March 2012 issue. For access to all the articles in this special AJN supplement on osteoarthritis, go to www.AJNonline.com.
Reprinted with permission from American Journal of Nursing, 112(3), S38-S43, 2012
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of disabilities and associated health conditions among adults—United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50(7):120–5. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2003–2005. MMWR Morb Mortal Wkly Rep. 2006;55(40):1089–92. [PubMed] [Google Scholar]
- 3.Dillon CF, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33(11):2271–9. [PubMed] [Google Scholar]
- 4.Qvist P, et al. The disease modifying osteoarthritis drug (DMOAD): Is it in the horizon? Pharmacol Res. 2008;58(1):1–7. doi: 10.1016/j.phrs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Sarzi-Puttini P, et al. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35(1 Suppl 1):1–10. doi: 10.1016/j.semarthrit.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43(9): 1905–15. [DOI] [PubMed]
- 7.Austrian JS, et al. Perceived barriers to trying self-management approaches for chronic pain in older persons. J Am Geriatr Soc. 2005;53(5):856–61. doi: 10.1111/j.1532-5415.2005.53268.x. [DOI] [PubMed] [Google Scholar]
- 8.Sarzi-Puttini P, et al. Do physicians treat symptomatic osteoarthritis patients properly? Results of the AMICA experience. Semin Arthritis Rheum. 2005;35(1 Suppl 1):38–42. doi: 10.1016/j.semarthrit.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Barry LC, et al. Identification of pain-reduction strategies used by community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60(12):1569–75. doi: 10.1093/gerona/60.12.1569. [DOI] [PubMed] [Google Scholar]
- 10.Jordan KM, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62(12):1145–55. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim SA. Racial variations in the utilization of knee and hip joint replacement: an introduction and review of the most recent literature. Curr Orthop Pract. 2010;21(2):126–31. doi: 10.1097/BCO.0b013e3181d08223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AGS Panel on Persistent Pain in Older Persons The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50(6 Suppl):S205–S224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 13.AGS Panel on Pharmacological Management of Persistent Pain in Older Persons Pharmacological Management of Persistent Pain in Older Persons. J Am Geriatr Soc. 2009;57(8):1331–46. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 14.Leveille SG, et al. Disabling symptoms: what do older women report? J Gen Intern Med. 2002;17(10):766–73. doi: 10.1046/j.1525-1497.2002.20229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid MC, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011;12(9):1336–57. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- 17.Towheed TE, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2006(1):CD004257. [DOI] [PMC free article] [PubMed]
- 18.Zhang W, et al. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta-analysis of randomised controlled trials. Ann Rheum Dis. 2004;63(8):901–7. doi: 10.1136/ard.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson AM, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 20.Rahme E, et al. Hospitalizations for upper and lower GI events associated with traditional NSAIDs and acetaminophen among the elderly in Quebec, Canada. Am J Gastroenterol. 2008;103(4):872–82. doi: 10.1111/j.1572-0241.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. Drugs: acetaminophen information 2011. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Accessed 1 July 2011.
- 22.Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120(3):594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- 23.Talley NJ, et al. Nonsteroidal antiinflammatory drugs and dyspepsia in the elderly. Dig Dis Sci. 1995;40(6):1345–50. doi: 10.1007/BF02065549. [DOI] [PubMed] [Google Scholar]
- 24.Bjordal JM, et al. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;329(7478):1317. doi: 10.1136/bmj.38273.626655.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trelle S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez Gutthann S, et al. Nonsteroidal anti-inflammatory drugs and the risk of hospitalization for acute renal failure. Arch Intern Med. 1996;156(21):2433–9. doi: 10.1001/archinte.156.21.2433. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe MM, et al. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340(24):1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 28.Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med. 2000;160(6):777–84. doi: 10.1001/archinte.160.6.777. [DOI] [PubMed] [Google Scholar]
- 29.Nüesch E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2009(4):CD003115. [DOI] [PubMed]
- 30.Papaleontiou M, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353–69. doi: 10.1111/j.1532-5415.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon DH, et al. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170(22):1968–76. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 32.Spitz A, et al. Primary care providers’ perspective on prescribing opioids to older adults with chronic non-cancer pain: a qualitative study. BMC Geriatr. 2011;11:35. doi: 10.1186/1471-2318-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towheed TE, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001(1):CD002946. [DOI] [PubMed]
- 34.Black C, et al. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation. Health Technol Assess. 2009;13(52):1–148. doi: 10.3310/hta13520. [DOI] [PubMed] [Google Scholar]
- 35.Reichenbach S, et al. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med. 2007;146(8):580–90. doi: 10.7326/0003-4819-146-8-200704170-00009. [DOI] [PubMed] [Google Scholar]
- 36.Poolsup N, et al. Glucosamine long-term treatment and the progression of knee osteoarthritis: systematic review of randomized controlled trials. Ann Pharmacother. 2005;39(6):1080–7. doi: 10.1345/aph.1E576. [DOI] [PubMed] [Google Scholar]
- 37.Taylor RS, et al. Safety profile of topical diclofenac: a meta-analysis of blinded, randomized, controlled trials in musculoskeletal conditions. Curr Med Res Opin. 2011;27(3):605–22. doi: 10.1185/03007995.2010.550606. [DOI] [PubMed] [Google Scholar]
- 38.Haroutiunian S, et al. Topical NSAID therapy for musculoskeletal pain. Pain Med. 2010;11(4):535–49. doi: 10.1111/j.1526-4637.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin J, et al. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trials. BMJ. 2004;329(7461):324. doi: 10.1136/bmj.38159.639028.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baraf HS, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28(1):27–40. doi: 10.2165/11584880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Altman RD. New guidelines for topical NSAIDs in the osteoarthritis treatment paradigm. Curr Med Res Opin. 2010;26(12):2871–6. doi: 10.1185/03007995.2010.533650. [DOI] [PubMed] [Google Scholar]
- 42.Makris UE, et al. Adverse effects of topical nonsteroidal antiinflammatory drugs in older adults with osteoarthritis: a systematic literature review. J Rheumatol. 2010;37(6):1236–43. doi: 10.3899/jrheum.090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kivitz A, et al. Comparison of the effectiveness and tolerability of lidocaine patch 5 % versus celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12 week, prospective, randomized, active-controlled, open-label, parallel-group trial in adults. Clin Ther. 2008;30(12):2366–77. doi: 10.1016/j.clinthera.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Galer BS, et al. Topical lidocaine patch 5 % may target a novel underlying pain mechanism in osteoarthritis. Curr Med Res Opin. 2004;20(9):1455–8. doi: 10.1185/030079904X2754. [DOI] [PubMed] [Google Scholar]
- 45.Gammaitoni AR, et al. Lidocaine patch 5 % and its positive impact on pain qualities in osteoarthritis: results of a pilot 2-week, open-label study using the Neuropathic Pain Scale. Curr Med Res Opin. 2004;20(Suppl 2):S13–S19. doi: 10.1185/030079904X12951. [DOI] [PubMed] [Google Scholar]
- 46.Mason L, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328(7446):991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosuwon W, et al. Efficacy of symptomatic control of knee osteoarthritis with 0.0125 % of capsaicin versus placebo. J Med Assoc Thai. 2010;93(10):1188–95. [PubMed] [Google Scholar]
- 48.Bellamy N, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006(2):CD005328. [DOI] [PubMed]
- 49.Heyworth BE, et al. Hylan versus corticosteroid versus placebo for treatment of basal joint arthritis: a prospective, randomized, double-blinded clinical trial. J Hand Surg Am. 2008;33(1):40–8. doi: 10.1016/j.jhsa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Atchia I, et al. Efficacy of a single ultrasound-guided injection for the treatment of hip osteoarthritis. Ann Rheum Dis. 2011;70(1):110–6. doi: 10.1136/ard.2009.127183. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu M, et al. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15(1):51–6. doi: 10.1007/s00776-009-1421-0. [DOI] [PubMed] [Google Scholar]
- 52.Chevalier X, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1):113–9. doi: 10.1136/ard.2008.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarke S, et al. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK) Knee. 2005;12(1):57–62. doi: 10.1016/j.knee.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Bellamy N, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2005(2):CD005321. [DOI] [PubMed]
- 55.Bourgeois FT, et al. Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Intern Med. 2010;153(3):158–66. doi: 10.1059/0003-4819-153-3-201008030-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green CR, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 57.Committee on Advancing Pain Research, Care, and Education, Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. http://www.nap.edu/catalog.php?record_id=13172. Accessed 1 July 2011. [PubMed]
- 58.MUST for Seniors. Medication use safety training for seniors [landing page]. National Council on Patient Information and Education. 2011. http://www.mustforseniors.org. Accessed 1 July 2011.
- 59.Hall J, et al. Effectiveness of interventions designed to promote patient involvement to enhance safety: a systematic review. Qual Saf Health Care. 2010;19(5):e10. doi: 10.1136/qshc.2009.032748. [DOI] [PubMed] [Google Scholar]
- 60.Paparella SF, et al. Patient safety simulation: learning about safety never seemed more fun. J Nurses Staff Dev. 2004;20(6):247–52. doi: 10.1097/00124645-200411000-00001. [DOI] [PubMed] [Google Scholar]


