Abstract
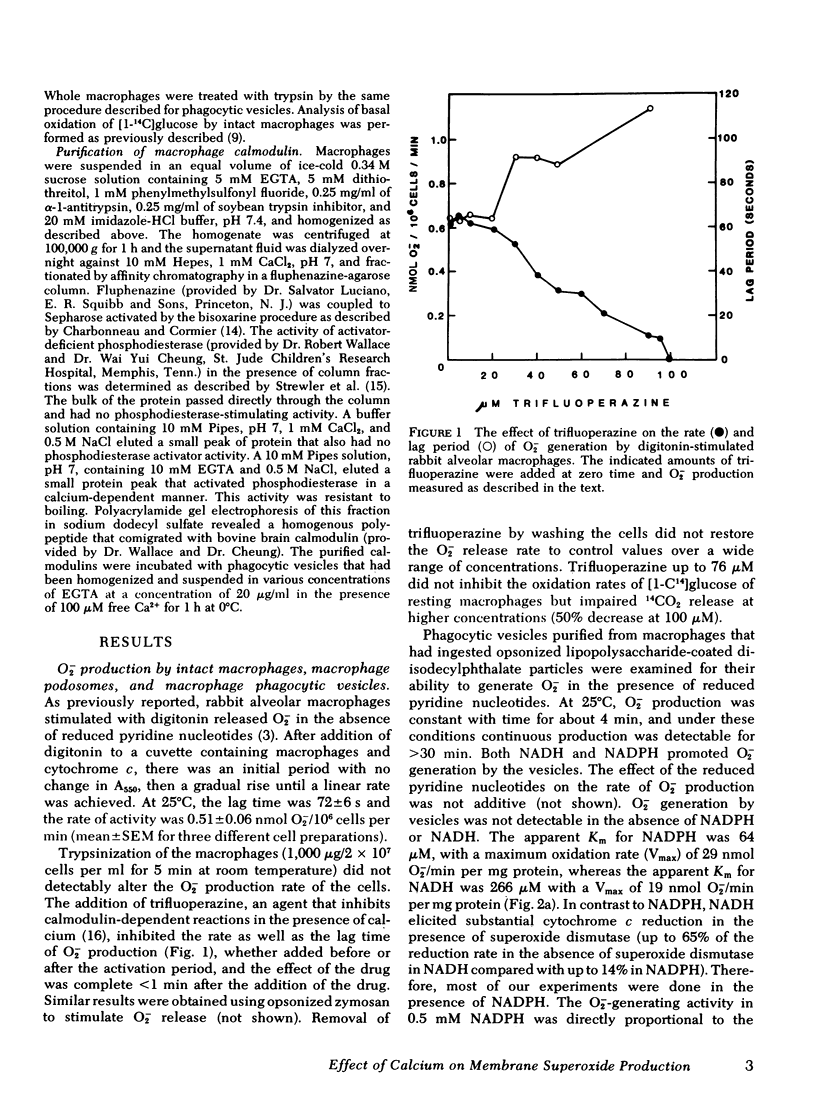
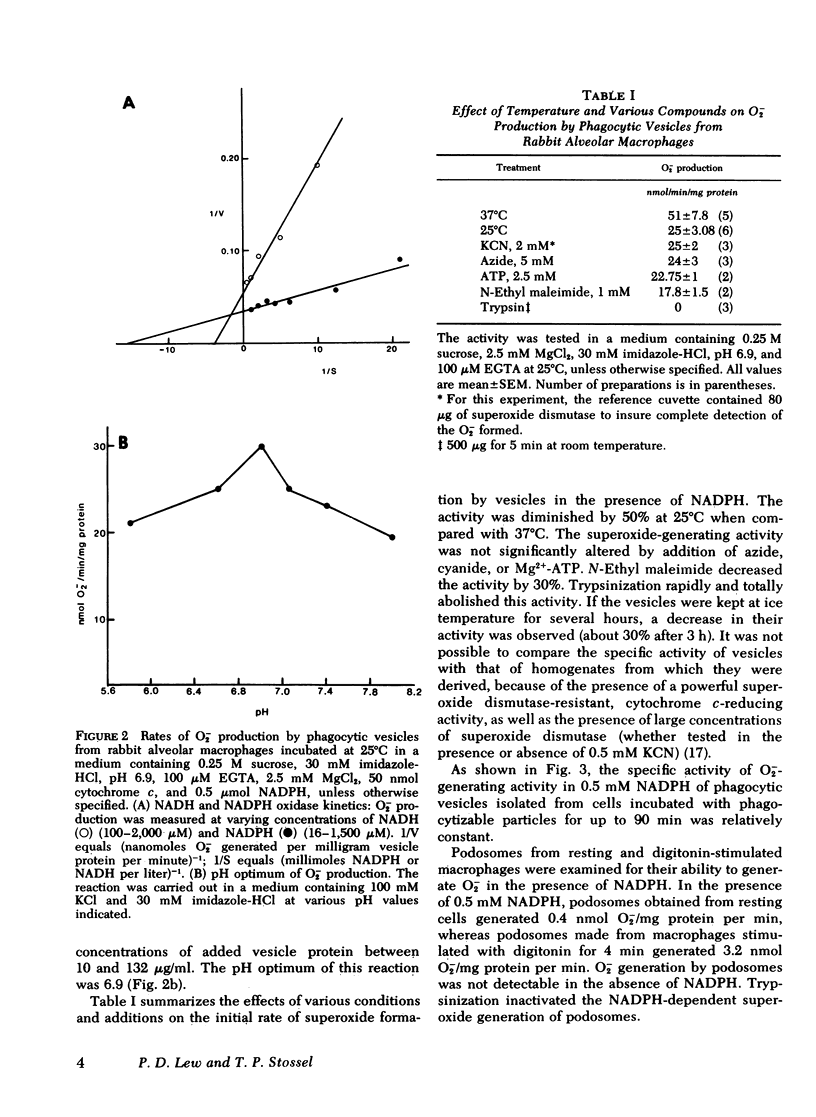
Phagocytic vesicles from rabbit lung macrophages produced superoxide in the presence of NADH or NADPH. At 37°C, these vesicles generated 51±7.8 nmol O2−/min per mg protein in the presence of 0.5 mM NADPH. The apparent Km for NADPH and NADH (66 and 266 μM, respectively), the pH optimum for the reaction (6.9), and the cyanide insensitivity were similar to properties of plasma membrane-rich fractions of stimulated polymorphonuclear leukocytes studied by others. The activity of the phagocytic vesicles was trypsin sensitive. The specific superoxide-generating activity of macrophage phagocytic vesicles isolated from cells incubated up to 90 min with phagocytic particles remained constant.
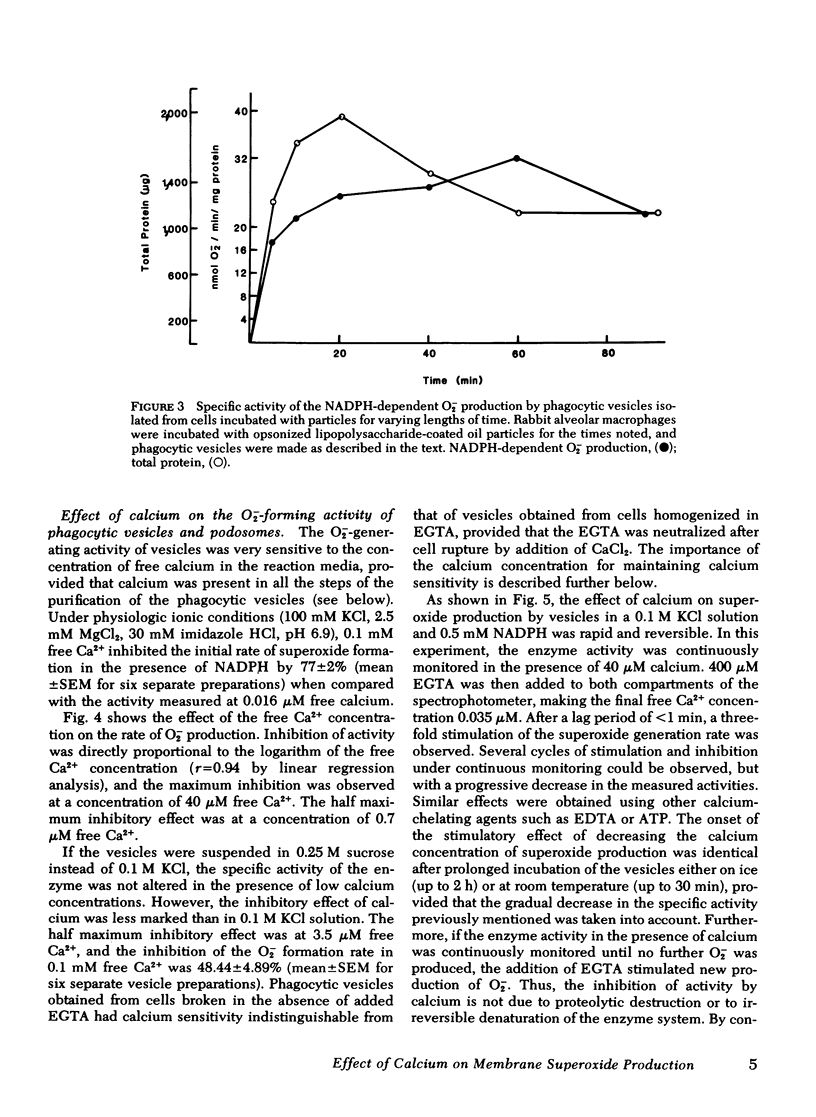
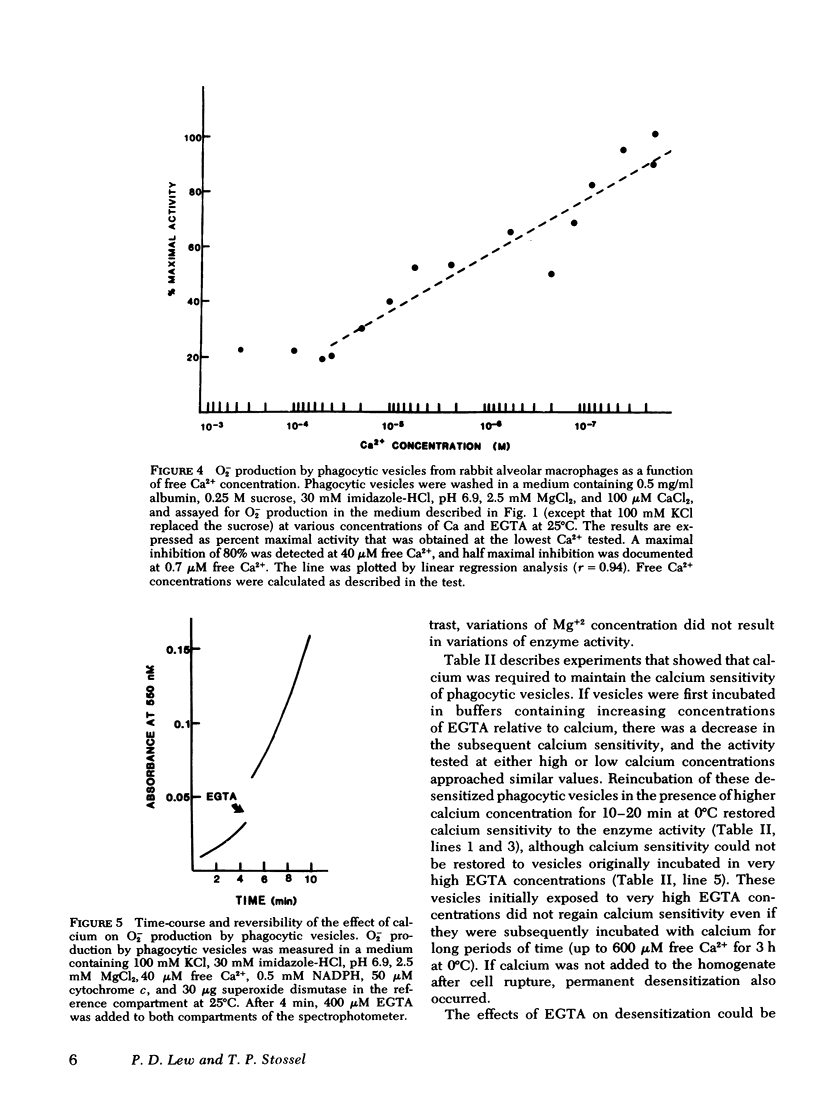
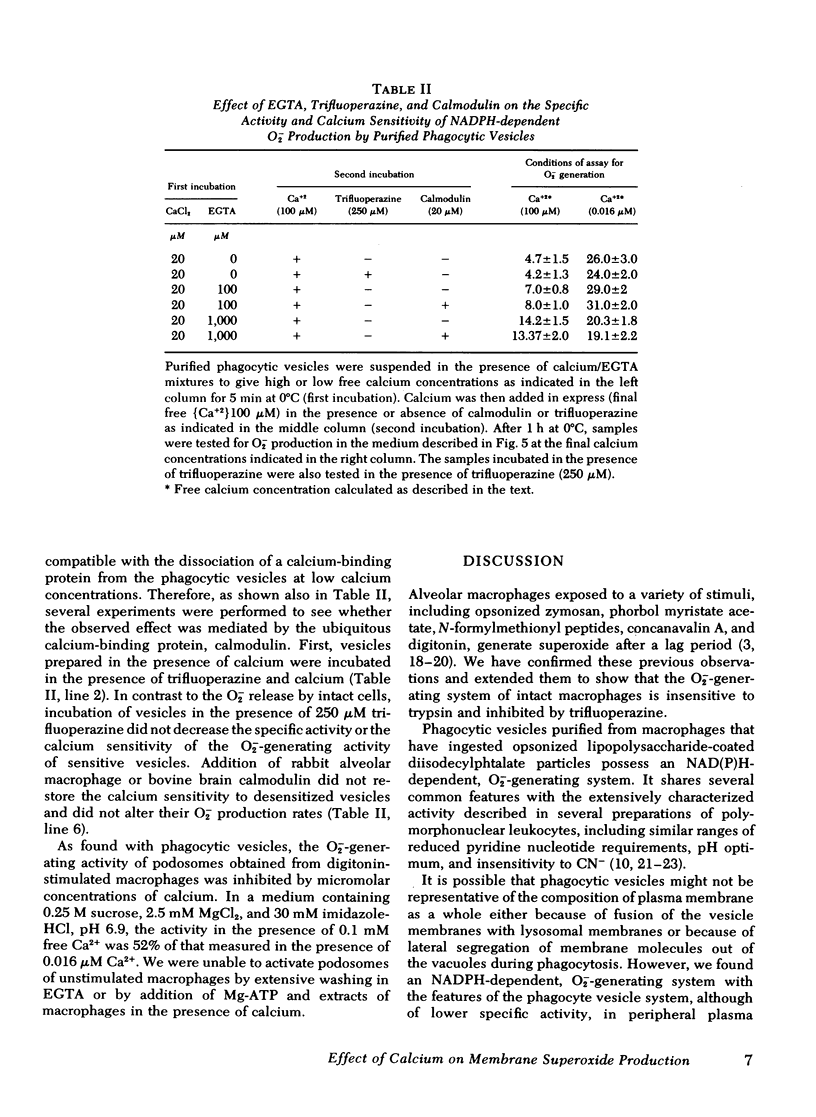
Calcium in micromolar concentrations inhibited the NADPH-dependent O2−-generating activity of phagocytic vesicles. In a physiological ionic medium (100 mM KCl, 2.5 mM MgCl2, 30 mM imidazole-HCl, pH 6.9), a maximal inhibition of O2− generation by phagocytic vesicles of 80% was observed at 40 μM free Ca2+. The half maximum inhibitory effect was at 0.7 μM Ca2+. Variations of the calcium concentration resulted in rapid and reversible alterations in O2−-forming activity. Preincubation of phagocytic vesicles in the presence of EGTA rendered their O2− generation rate in the presence of NADPH insensitive to alterations in the free calcium concentration. This desensitization by low EGTA concentrations (≤100 μM) was reversible by the addition of excess calcium, but desensitization by high EGTA concentrations (>1 mM) was not reversible by the addition of calcium either in the presence or absence of purified rabbit lung macrophage or bovine brain calmodulins. Furthermore, trifluoperazine, a drug that inhibits calmodulin-stimulated reactions, did not alter the activity or the calcium sensitivity of the superoxide-generating system of sensitive phagocytic vesicles.
Peripheral plasma membrane vesicles (podosomes) prepared by gentle sonication of macrophages possessed on O2−-generating system with similar properties to those of phagocytic vesicles.
We conclude that the activated O2−-generating system of rabbit lung macrophages has its initial localization in the plasmalemma and undergoes subsequent internalization into phagocytic vesicles, where it can function for prolonged periods of time. Calcium at concentrations likely to exist in macrophage cytoplasm exerts a regulatory effect on the activated system.
Full text
PDF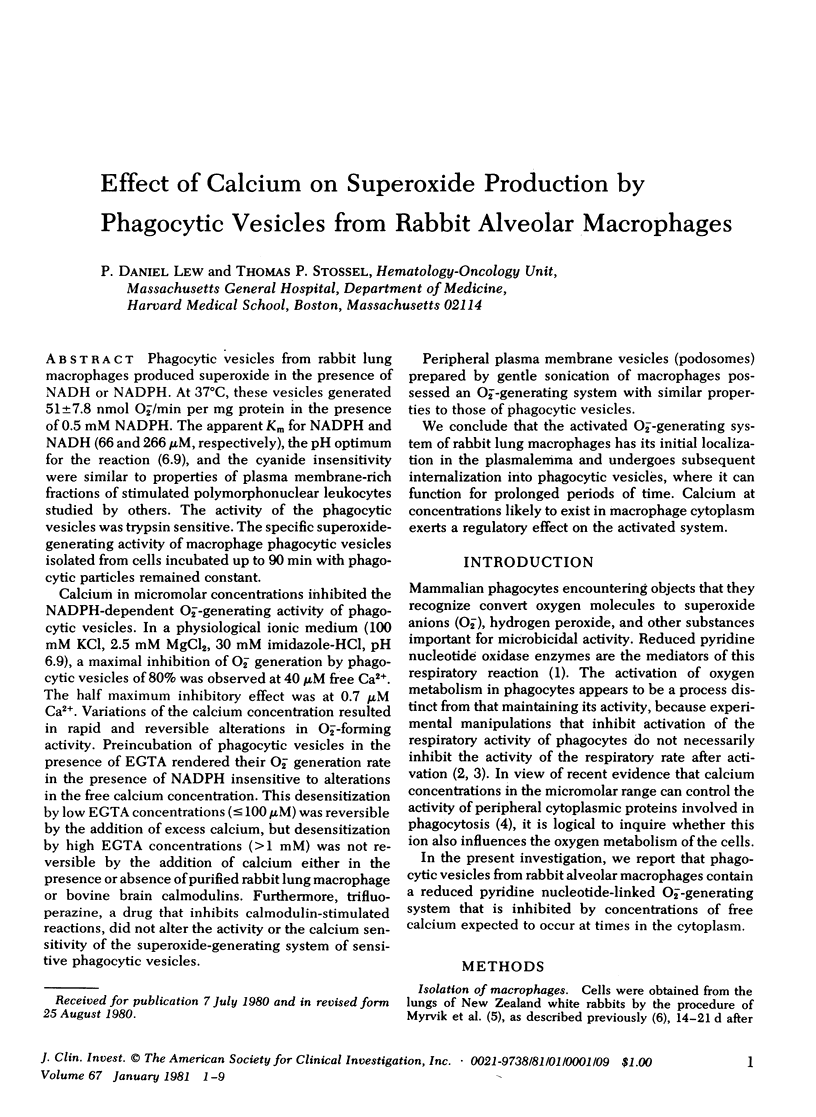



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. Calcium ion regulation in barnacle muscle fibers and its relation to force development. Ann N Y Acad Sci. 1978 Apr 28;307:308–329. doi: 10.1111/j.1749-6632.1978.tb41959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Production of superoxide and hydrogen peroxide by an NADH-oxidase in guinea pig polymorphonuclear leukocytes. Modulation by nucleotides and divalent cations. J Biol Chem. 1979 Nov 25;254(22):11530–11537. [PubMed] [Google Scholar]
- Boxer L. A., Ismail G., Allen J. M., Baehner R. L. Oxidative metabolic responses of rabbit pulmonary alveolar macrophages. Blood. 1979 Mar;53(3):486–491. [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E., Davies W. A. Activation of the guinea pig granulocyte NAD(P)H-dependent superoxide generating enzyme: localization in a plasma membrane enriched particle and kinetics of activation. Blood. 1980 Mar;55(3):355–363. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E., Ellis S. E. Chlorpromazine inhibition of granulocyte superoxide production. Blood. 1980 Jul;56(1):23–29. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations; and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J Clin Invest. 1978 Apr;61(4):1088–1096. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Newburger P. E., Chovaniec M. E. NAD(P)H-dependent superoxide production by phagocytic vesicles from guinea pig and human granulocytes. J Biol Chem. 1980 Jul 25;255(14):6584–6588. [PubMed] [Google Scholar]
- Davies W. A., Stossel T. P. Peripheral hyaline blebs (podosomes) of macrophages. J Cell Biol. 1977 Dec;75(3):941–955. doi: 10.1083/jcb.75.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holian A., Daniele R. P. Stimulation of oxygen consumption and superoxide anion production in pulmonary macrophages by N-formyl methionyl peptides. FEBS Lett. 1979 Dec 1;108(1):47–50. doi: 10.1016/0014-5793(79)81176-x. [DOI] [PubMed] [Google Scholar]
- Jandl R. C., André-Schwartz J., Borges-DuBois L., Kipnes R. S., McMurrich B. J., Babior B. M. Termination of the respiratory burst in human neutrophils. J Clin Invest. 1978 May;61(5):1176–1185. doi: 10.1172/JCI109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lew P. D., Stossel T. P. Calcium transport by macrophage plasma membranes. J Biol Chem. 1980 Jun 25;255(12):5841–5846. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Rasmussen H., Gustin M. C. Some aspects of the hormonal control of cellular calcium metabolism. Ann N Y Acad Sci. 1978 Apr 28;307:391–401. doi: 10.1111/j.1749-6632.1978.tb41964.x. [DOI] [PubMed] [Google Scholar]
- Rister M., Baehner R. L. A comparative study of superoxide dismutase activity in polymorphonuclear leukocytes, monocytes, and alveolar macrophages of the guinea pig. J Cell Physiol. 1976 Mar;87(3):345–355. doi: 10.1002/jcp.1040870310. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Field R. J., Gitlin J. D., Alper C. A., Rosen F. S. The opsonic fragment of the third component of human complement (C3). J Exp Med. 1975 Jun 1;141(6):1329–1347. doi: 10.1084/jem.141.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Pollard T. D., Vaughan M. Isolation and properties of phagocytic vesicles. II. Alveolar macrophages. J Clin Invest. 1972 Mar;51(3):604–614. doi: 10.1172/JCI106850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strewler G. J., Manganiello V. C., Vaughan M. Phosphodiesterase activator from rat kidney cortex. J Biol Chem. 1978 Jan 25;253(2):390–394. [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]



