Abstract
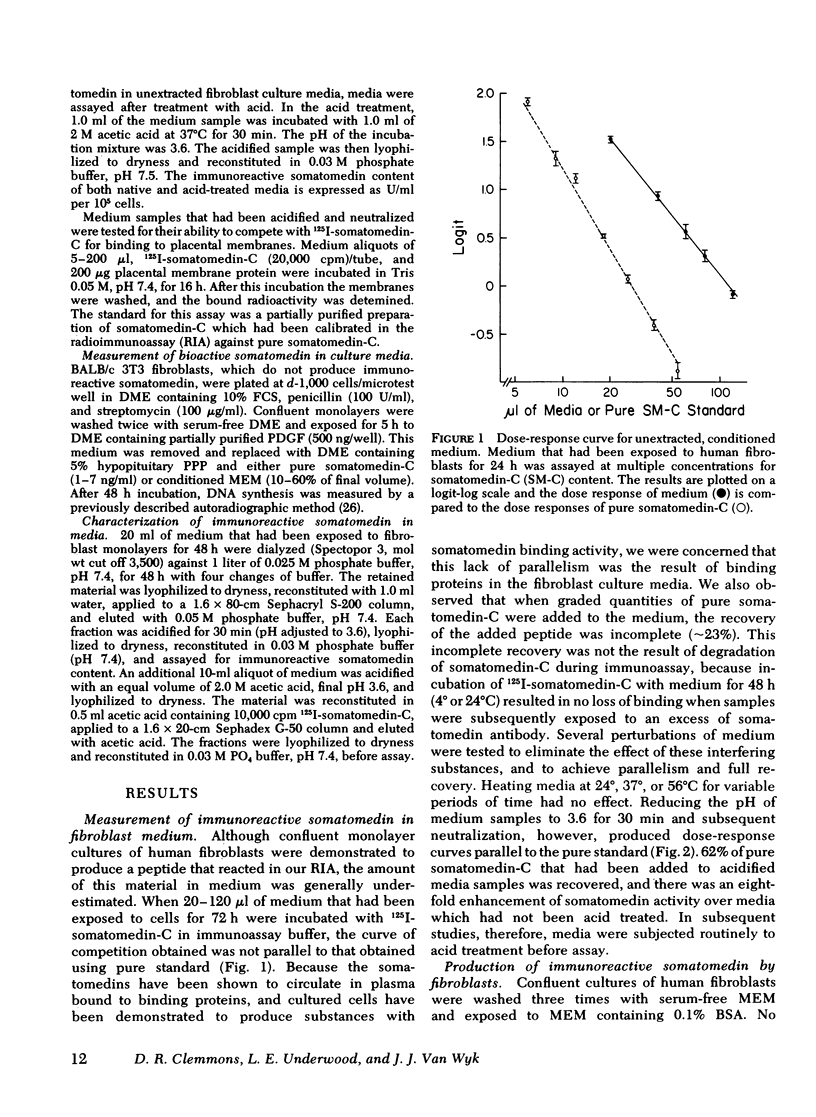
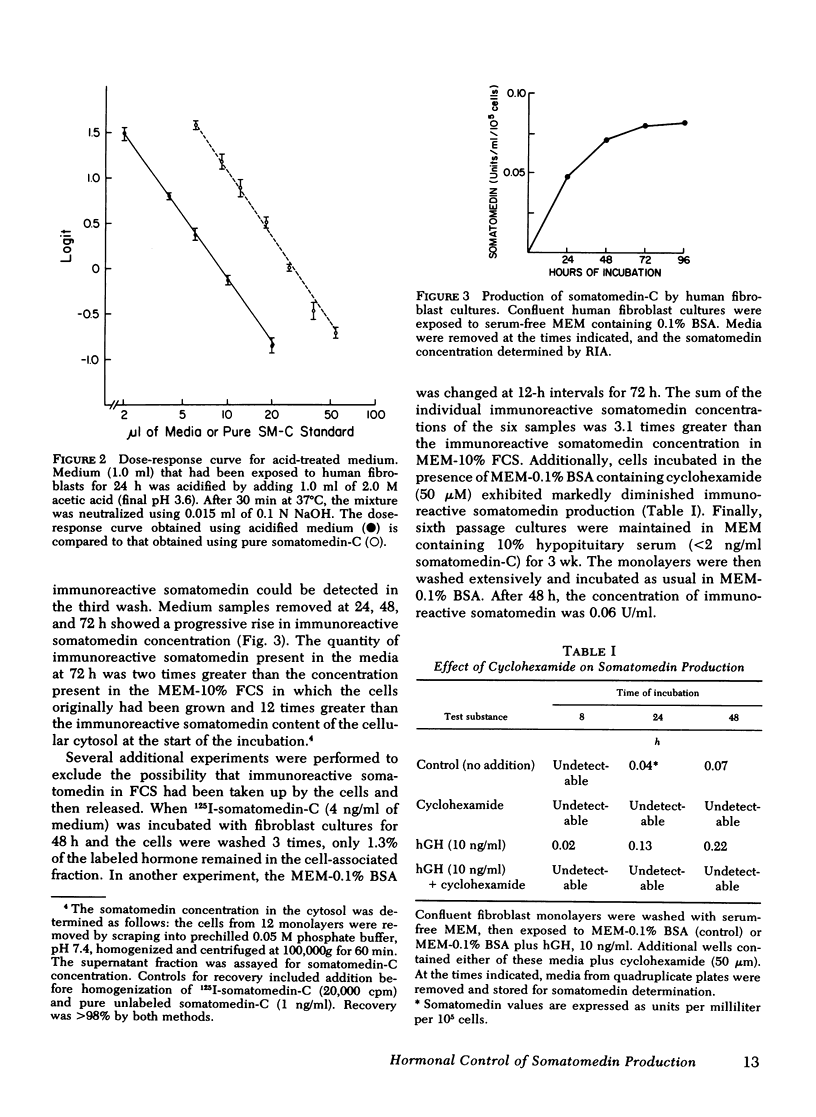
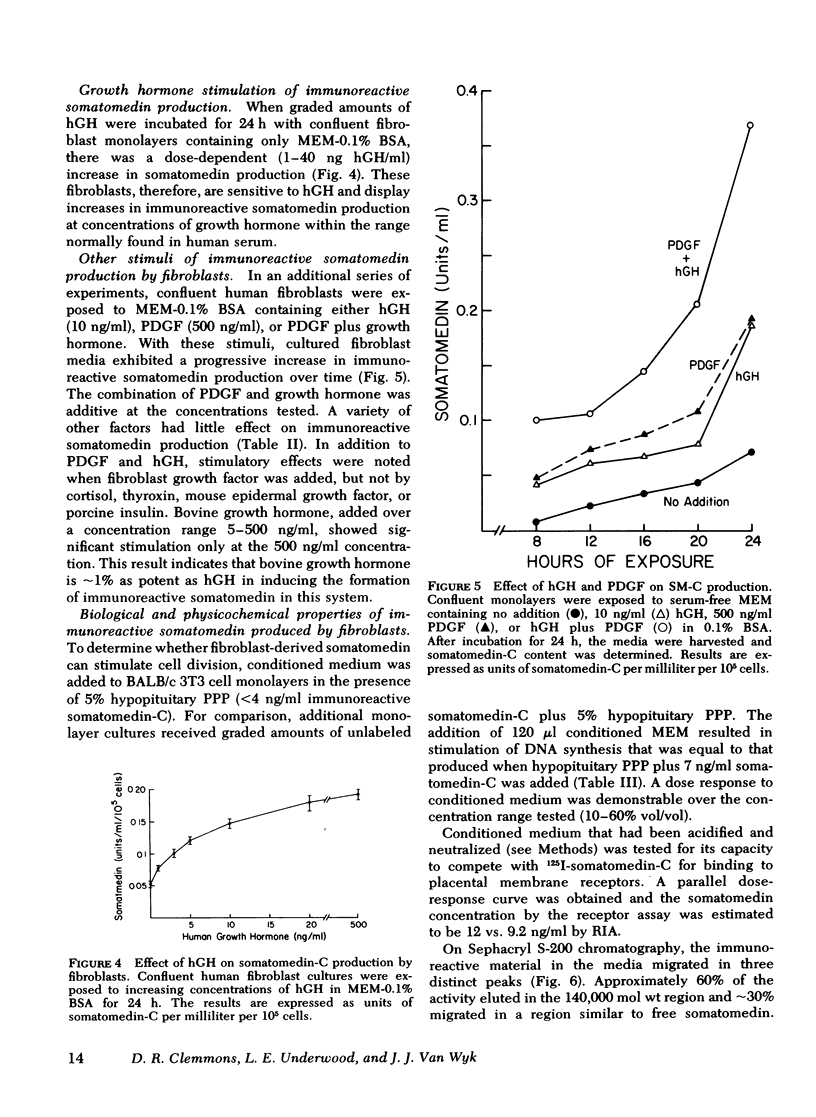
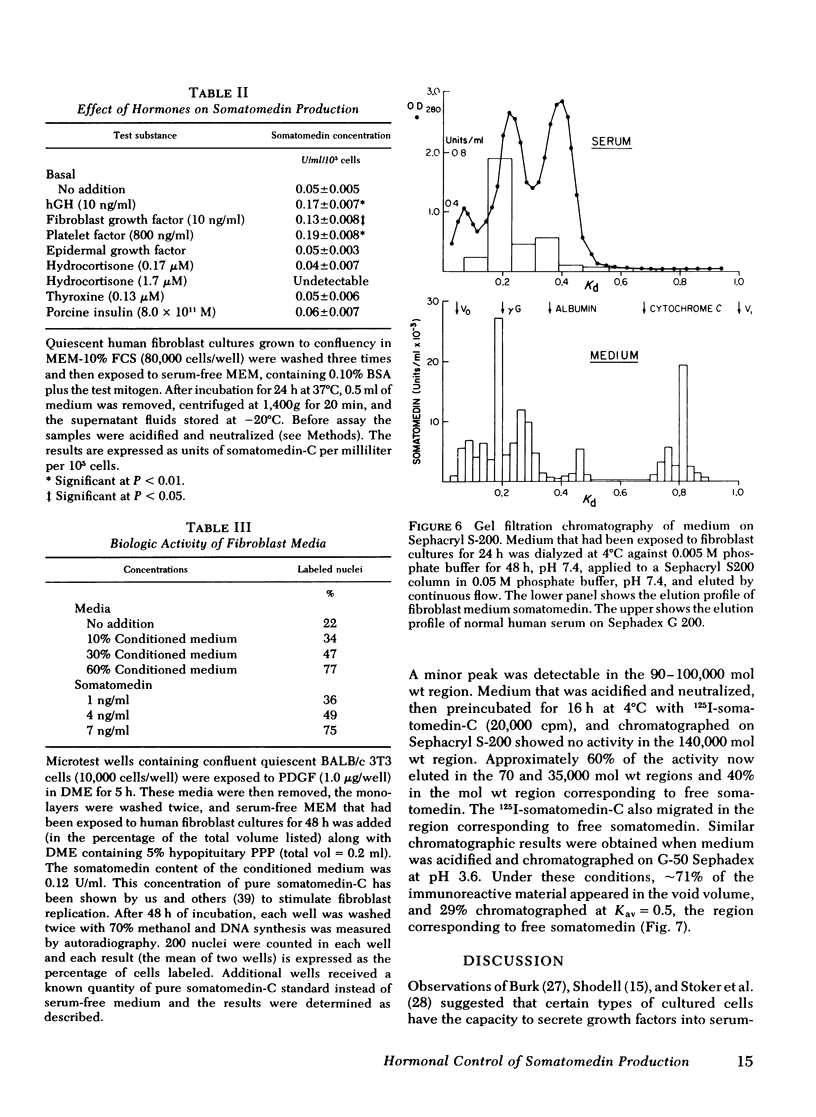
Human growth hormone (hGH) is known to be a potent stimulator of somatomedin secretion in vivo. The induction of somatomedin by growth hormone has been difficult to study in vitro, however, because no organ containing a high concentration of somatomedin has been identified. Because fetal mouse explants have been shown to produce somatomedin in vitro, we have undertaken studies to determine whether postnatal human fibroblast monolayers also produce somatomedin, and if so, whether its production is regulated by other hormones. Quiescent human fibroblasts were exposed to serum-free minimum essential medium, and the medium was assayed for somatomedin concentration using a specific radioimmunoassay for somatomedin-C. A progressive rise in immunoreactive somatomedin to 0.08 U/ml per 105 cells per 24 h was observed over 72 h of incubation. This was an underestimation of the actual concentration of immunoreactive somatomedin since the amount measured following acid treatment was at least fourfold higher than in the untreated medium. Growth hormone stimulated immunoreactive somatomedin production in a dose-dependent manner: 5 ng hGH/ml = 0.1 U/ml per 105 cells; 50 ng hGH/ml = 0.25 U/ml per 105 cells. Platelet-derived growth factor and fibroblast growth factor were also stimulatory, but epidermal growth factor, thyroxine, or cortisol had no effect. Media that had been exposed to human fibroblasts stimulated DNA synthesis in BALB/c 3T3 fibroblasts (a cell type that does not produce somatomedin). Medium-derived immuno-reactive somatomedin eluted from Sephacryl S-200 in two major peaks (150,000 and 8,000 mol wt). The higher molecular weight peak is similar to the one observed when whole serum was used. These studies provide a model system for studying the humoral and nonhumoral factors that control the biosynthesis of somatomedin by human tissues. Since immunoreactive somatomedin production may be a rate-limiting factor for fibroblast growth, the delineation of the hormonal control of somatomedin production should lead to a better understanding of the mechanisms controlling human fibroblast growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Stathakos D., Scher C. D. Isolation of a cationic polypeptide from human serum that stimulates proliferation of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2635–2639. doi: 10.1073/pnas.72.7.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkison P. R., Weidman E. R., Bhaumick B., Bala R. M. Release of somatomedin-like activity by cultured WI-38 human fibroblasts. Endocrinology. 1980 Jun;106(6):2006–2012. doi: 10.1210/endo-106-6-2006. [DOI] [PubMed] [Google Scholar]
- August G. P., Cheng R. F., Hung W., Houck J. C. Fibroblast proliferative activity in the sera of growth hormone deficient patients. Horm Metab Res. 1973 Sep;5(5):340–341. doi: 10.1055/s-0028-1093939. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürk R. R. A factor from a transformed cell line that affects cell migration. Proc Natl Acad Sci U S A. 1973 Feb;70(2):369–372. doi: 10.1073/pnas.70.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Applewhite G. T., Underwood L. E. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980 Mar 15;75(2):315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Tadaro G. J. A human fibrosarcoma cell line producing multiplication stimulating activity (MSA)-related peptides. Nature. 1978 Mar 23;272(5651):356–358. doi: 10.1038/272356a0. [DOI] [PubMed] [Google Scholar]
- Dulak N. C., Temin H. M. A partially purified polypeptide fraction from rat liver cell conditioned medium with multiplication-stimulating activity for embryo fibroblasts. J Cell Physiol. 1973 Apr;81(2):153–160. doi: 10.1002/jcp.1040810204. [DOI] [PubMed] [Google Scholar]
- Dulak N. C., Temin H. M. Multiplication-stimulating activity for chicken embryo fibroblasts from rat liver cell conditioned medium: a family of small polypeptides. J Cell Physiol. 1973 Apr;81(2):161–170. doi: 10.1002/jcp.1040810205. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Nicholson M. L., Dulak N. C. Effects of peptide anabolic hormones on growth of myoblasts in culture. Endocrinology. 1977 Jul;101(1):32–41. doi: 10.1210/endo-101-1-32. [DOI] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim A. A. Isolation of a growth-stimulating agent from human skin fibroblast cultures. Experientia. 1978 Nov 15;34(11):1515–1517. doi: 10.1007/BF01932385. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Ross R., Slichter S. J., Scott C. R. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976 Sep;58(3):731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Fryklund L. Insulin and somatomedins A and B: comparisons of biological activities in cultured human skin-derived fibroblasts. Life Sci. 1977 Oct 1;21(7):943–950. doi: 10.1016/0024-3205(77)90260-0. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J. Production of macrophage-dependent fibroblast-stimulating activity (M-FSA) by murine macrophages. Effects on BALBc 3T3 fibroblasts. Exp Cell Res. 1978 Apr;113(1):47–56. doi: 10.1016/0014-4827(78)90086-1. [DOI] [PubMed] [Google Scholar]
- Lesniak M. A., Gorden P., Roth J. Reactivity of non-primate growth hormones and prolactins with human growth hormone receptors on cultured human lymphocytes. J Clin Endocrinol Metab. 1977 May;44(5):838–849. doi: 10.1210/jcem-44-5-838. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Genereux D. P., Ham R. G. Assay and partial purification of factors from serum that control multiplication of human diploid fibroblasts. Biochem Biophys Res Commun. 1978 Feb 28;80(4):1013–1021. doi: 10.1016/0006-291x(78)91346-3. [DOI] [PubMed] [Google Scholar]
- Millis A. J., Hoyle M., Field B. Human fibroblast conditioned media contains growth-promoting activities for low density cells. J Cell Physiol. 1977 Oct;93(1):17–24. doi: 10.1002/jcp.1040930104. [DOI] [PubMed] [Google Scholar]
- Morell B., Froesch E. R. Fibroblasts as an experimental tool in metabolic and hormone studies. II. Effects of insulin and nonsuppressible insulin-like activity (NSILA-S) on fibroblasts in culture. Eur J Clin Invest. 1973 Mar;3(2):119–123. doi: 10.1111/j.1365-2362.1973.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Cohen K. L., Johnsonbaugh R., Nissley S. P. Contribution of human somatomedin activity to the serum growth requirement of human skin fibroblasts and chick embryo fibroblasts in culture. J Clin Endocrinol Metab. 1978 Jun;46(6):937–946. doi: 10.1210/jcem-46-6-937. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Eisen H. J., Higa O. Z., Nissley P., Moses A. C., Schilling E. E., Fennoy I., Bruni C. B., Phillips L. S., Baird K. L. Characterization of a somatomedin (insulin-like growth factor) synthesized by fetal rat liver organ cultures. J Biol Chem. 1979 Aug 25;254(16):7942–7950. [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- Shodell M. Environmental stimuli in the progression of BHK-21 cells through the cell cycle. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1455–1459. doi: 10.1073/pnas.69.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Temin H. M. Purified multiplication-stimulating activity from rat liver cell conditioned medium: comparison of biological activities with calf serum, insulin, and somatomedin. J Cell Physiol. 1974 Oct;84(2):181–192. doi: 10.1002/jcp.1040840204. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M. G., Clarke G. D., Thornton M. Density dependent stimulation of thymidine incorporation in BHK21 cells by active material released from the same cells. J Cell Physiol. 1971 Dec;78(3):345–354. doi: 10.1002/jcp.1040780304. [DOI] [PubMed] [Google Scholar]
- Svoboda M. E., Van Wyk J. J., Klapper D. G., Fellows R. E., Grissom F. E., Schlueter R. J. Purification of somatomedin-C from human plasma: chemical and biological properties, partial sequence analysis, and relationship to other somatomedins. Biochemistry. 1980 Feb 19;19(4):790–797. doi: 10.1021/bi00545a027. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E. Growth factors produced by sarcoma virus-transformed cells. Cancer Res. 1978 Nov;38(11 Pt 2):4147–4154. [PubMed] [Google Scholar]
- Van Wyk J. J., Svoboda M. E., Underwood L. E. Evidence from radioligand assays that somatomedin-C and insulin-like growth factor-I are similar to each other and different from other somatomedins. J Clin Endocrinol Metab. 1980 Jan;50(1):206–208. doi: 10.1210/jcem-50-1-206. [DOI] [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Quadracci L. J., Striker G. E. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol. 1978 Aug;96(2):203–213. doi: 10.1002/jcp.1040960209. [DOI] [PubMed] [Google Scholar]
- Young M., Oger J., Blanchard M. H., Asdourian H., Amos H., Arnason B. G. Secretion of a nerve growth factor by primary chick fibroblast cultures. Science. 1975 Jan 31;187(4174):361–362. doi: 10.1126/science.1167427. [DOI] [PubMed] [Google Scholar]
- Zapf J., Waldvogel M., Froesch E. R. Binding of nonsuppressible insulinlike activity to human serum. Evidence for a carrier protein. Arch Biochem Biophys. 1975 Jun;168(2):638–645. doi: 10.1016/0003-9861(75)90296-9. [DOI] [PubMed] [Google Scholar]
- van Wyk J. J., Underwood L. E., Baseman J. B., Hintz R. L., Clemmons D. R., Marshall R. N. Explorations of the insulinlike and growth-promoting properties of somatomedin by membrane receptor assays. Adv Metab Disord. 1975;8:127–150. doi: 10.1016/b978-0-12-027308-9.50015-9. [DOI] [PubMed] [Google Scholar]


