Abstract
The current paper examines the effect of administering Dehydroepiandrosterone (DHEA) on visual-spatial performance in post-menopausal women (N=24, ages 55-80). The concurrent reduction of serum DHEA levels and visual-spatial performance in this population, coupled with the documented effects of DHEA’s androgenic metabolites on visual-spatial performance, suggest that DHEA administration may enhance visual-spatial performance. The current experiment used a double-blind placebo-controlled crossover design in which 50 mg of oral DHEA was administered daily in the drug condition to explore this hypothesis. Performance on the Mental Rotation, Subject-Ordered Pointing, Fragmented Picture Identification, Perceptual Identification, Same-Different Judgment, and Visual Search tasks and serum levels of DHEA, DHEAS, testosterone, estrone and cortisol were measured in the DHEA and placebo conditions. In contrast to prior experiments using the current methodology that did not demonstrate effects of DHEA administration on episodic and short-term memory tasks, the current experiment demonstrated large beneficial effects of DHEA administration on Mental Rotation, Subject-Ordered Pointing, Fragmented Picture Identification, Perceptual Identification and Same-Different Judgment. Moreover, DHEA administration enhanced serum levels of DHEA, DHEAS, testosterone and estrone, and regression analyses demonstrated that levels of DHEA and its metabolites were positively related to cognitive performance on the visual-spatial tasks in the DHEA condition
Keywords: Dehydroepiadrosterone (DHEA), post-menopausal women, cognition, visual-spatial tasks, androgens
The current paper examines the effect of administering dehydroepiandrosterone (DHEA) on visual-spatial performance in post-menopausal women . Both DHEA levels and visual-spatial performance decline with age, raising the possibility that declines in DHEA levels may contribute to age-related impairments in visual spatial performance. Specifically, peak levels of DHEA occur in the mid 20s for females and by age 70, plasma levels of DHEA are approximately 30% of their original peak levels (Sulcova, Hill, Hampl, & Starka, 1997; Labrie Belanger, Cusan, Gomez, Candas, 1997; Orentreich, Brind, Vogelman, Andres & Baldwin, 1992). Similarly, performance in the mental rotation, visual search, subject-ordered pointing and visual-spatial memory tasks decline with age (Beigneux, Plau & Isingrini, 2007; Chaytor & Schmitter-Edgecombe, 2004; Jansen & Heil, 2010; Lorenzo-Lopez, Amenedo and Cadaveira, 2008; West, Welch & Knabb, 2002).
DHEA’s metabolism provides possible mechanisms for a beneficial effect of DHEA replacement on visual-spatial performance. DHEA is an adrenal steroid that can be metabolized into its sulfated version, DHEA-S and androgens like testosterone. These androgens, in turn, can be aromatized into estrogens such as estrone and estradiol (Barru, Charou & Liddy, 1997). Androgens may affect performance on visual-spatial tasks through their effects on receptors in the neo cortex (Puy, MacLusky, Becker, Karsan, Trachtenberg, & Brown, 1995; Shaywitz., Shaywitz, Pugh, Fulbright, Skudlarski & Mencl, 1999), while estrogens may affect neurotransmitter levels that have demonstrated effects on cognition (e.g., dopamine (Becker, 1990), acetylcholine (Gibbs, 2000)). It has also been observed that sex steroids support growth and survival of neurons in various brain regions involved in cognitive processes (e.g. estrogens in the hippocampus (Woolley, Weiland, McEwen, & Schwarthroin, 1997), androgens in the pre-frontal cortex (Kolb & Stewart, 1995)). Relevant evidence on the experimental and observational effects of DHEA on cognitive tasks and the effects of androgens and estrogens on visual-spatial tasks in post-menopausal women are presented next.
Experimental and Observational Studies of DHEA
Experimental and observational studies exploring the effects of DHEA on cognitive performance in post-menopausal women have reported mixed results, raising important questions regarding DHEA’s potential therapeutic benefits. Hirshman, Wells, Wierman, Anderson, Butler, Senholzi and Fisher (2003) reported that DHEA administration improved verbal recognition memory performance in post-menopausal women, but this improvement only occurred when items were presented for 800 ms. or less at study. This pattern suggested that DHEA’s beneficial effect on recognition memory might have been mediated by its effect on item perception at study. Consistent with this view, Stangl, Hirshman and Verbalis (2010a) found no effects of DHEA administration on recognition memory in post-menopausal women when items were presented for 1 second during study. Similarly, Stangl, Hirshman and Verbalis (2010b) examined the effect of DHEA administration on short term memory tasks in this population and found no significant benefits. Wolf, Naumann, Hellhammer, Geiben, Strasburger, Dressendorfer, Pirke & Kirschbaum (1997) observed that DHEA administration enhanced picture recall in post-menopausal women, but a later study revealed that this effect disappeared after a stressful event (Wolf, Kudielka, Hellhammer, D.H., Hellhammer, J., & Kirschbaum, 1998b) Last, cognitive performance was impaired on the Boston Naming Task, and the Judgment of Line Orientation task following administration of DHEA in post-menopausal women (Parsons, Kratz, Thompson, Stanczyk & Buckwalter, 2006).
Observational studies show a similarly mixed pattern. Yaffe, Ettinger, Pressman, Seeley, Whooley, Schaefer & Cummings (1998) reported that DHEA-S level in post-menopausal women was not a significant predictor of performance on the modified Mini-Mental State Exam, Trails B, or Digit Symbol subtest. Similarly Parsons et al. (2006) demonstrated that level of DHEA was negatively associated with backward digit span scores in post-menopausal women. In contrast, Kalmijn, Launer, Stolk, de Jong, Pols, Hofman, Breteler & Lamberts (1998) demonstrated that DHEA-S level inversely predicted cognitive decline on the Mini-Mental State Examination. Thus, to date, and despite its documented physiological effects, there is not compelling evidence that DHEA administration enhances cognitive performance in post-menopausal women (see also Huppert, Van Niekerk, and Herbert (2001)).
Androgen Studies
Multiple studies (Cherrier, Craft, Asthana, Plymate, Baker, Matsumoto, Peskind, Raskind, Brodkin, Brember, Petrova & LaTendresse, 2001; Cherrier, Matsumoto, Amory, Johnson, Craft, Peskind and Raskind 2007;Janowsky, Chavez & Orwoll, 2000; Janowsky, Oviatt & Orwoll, 1994) have demonstrated that testosterone administration enhances visual-spatial performance in older men. Observational studies have raised the prospect that this effect may also occur in women. Gouchie and Kimura (1991) and Moffat and Hampson reported that women with higher testosterone levels had better performance on measures of spatial ability and, spatial cognition (see also Aleman, Bronk, Kessels, Koppeschaar and van Honk (2004) for additional results with young women).
Estrogen Studies
The effects of estrogens on visual-spatial tasks in post-menopausal women are mixed. Janowsky, Chavez and Orowell (2000) found no benefit of estrogen on the SOPT. In contrast, Aveleyra, Carranza-Lira, Ulloa-Aguirre & Ostrosky-Solis (2005) demonstrated higher scores on the backwards spatial span task in post-menopausal women receiving estrogen treatment and Duff and Hampson (2000) demonstrated better performance in estrogen users on measures of short-term spatial memory. The protective effects of estrogen were further described in the Baltimore Longitudinal Study of Aging which demonstrated better performance on a measure of visual memory (Benton Visual Retention Test) with estrogen replacement therapy (Resnick, Metter & Zonderman, 1997) (see also Janowsky, Chavez, Zamboni & Orowell (1998) for mixed results from an observational study).
The Current Experiment
While prior studies of the effects of DHEA administration on cognitive tasks do not provide compelling evidence that DHEA administration enhances cognition, the reported effects of DHEA’s androgenic metabolites on visual-spatial tasks in older men and younger women motivated the current examination of the effect of DHEA administration on visual-spatial tasks in post-menopausal women.
Our experiment explores the effects of DHEA on tasks that reflect a range of visual-spatial processes. We examined two tasks, the Mental Rotation Task (MRT, Shepard & Metzler, 1971; Vandenberg & Kuse, 1978) and the SOPT (Janowsky et al, 2000), which have traditionally shown effects of androgens. We also examined the fragmented picture identification task (Snodgrass & Hirshman, 1994), the perceptual identification task (Hirshman & Mulligan, 1991) and the same-different judgments task (Krueger & Allen, 1987). These tasks involve elementary object recognition and comparison processes that may also be involved in the more complex MRT and SOPT tasks. Thus, results on these tasks will help constrain interpretations of the results on the MRT and SOPT tasks. In addition, we used the visual search task (Treisman & Gelade, 1980) as a final task. While similar to the other tasks in relying on elementary visual object recognition and comparison processes, the visual search task also relies on complex attention and visual search processes that differentiate it from simple object recognition and comparison tasks.
We chose to examine the effects of DHEA administration in post-menopausal women. We focused on this population because declines in both estrogen and testosterone are associated with menopause (Morales, Nolan, Nelson, & Yen, 1994; Rubino, Stomati, Bersi, Casarosa, Luisi, Petraglia & Genazzani, 1998). In addition, these sex steroid deficiencies, in combination with declining DHEA levels, may sensitize this population to the effects of DHEA. The hormone levels in this population contrast with those of younger women whose levels are substantially higher.
In addition to examining the experimental effect of DHEA administration, we also examined the relations between serum levels of various hormones and performance on these six tasks in both the DHEA and the placebo condition to help identify the relationship between hormone levels and cognitive performance. Specifically, levels of DHEA, DHEAS, testosterone, estrone and cortisol were measured. We did not expect cortisol to be affected by DHEA administration and we used it as a control measure.
Similarly, because we are hypothesizing that the metabolites of DHEA may have potential effects, the time of cognitive testing is significant. Our prior work (Hirshman, Merritt, Wang, Wierman, Budescu, Kohrt, Templin, & Bhasin, 2004) demonstrated that serum levels of DHEA and its metabolites peaked at approximately 10am and maintained this level for approximately 2 hours when 50 mg of DHEA was administered orally at approximately 8am. Therefore, all cognitive testing was conducted between 10am and noon in the current study to make certain that we were testing at the maximal levels of DHEA and its metabolites.
Method
Participants
We examined women ages 55-80 years old (N = 24). All participants were volunteers recruited by newspaper advertisement and were paid $300.00 for their participation. Participants met the World Health Organization’s criterion for post-menopausal status of one year’s absence of menses or bilateral ovariectomy that preceded the study by at least one year. The participants were not using any form of hormone replacement therapy. Approval to use human participants was granted by the Institutional Review Board of the Georgetown University Medical Center.
Exclusions
Participants were excluded if a pre-enrollment medical evaluation revealed contraindications to DHEA, estrogen or androgen treatment (e.g., personal history of, or active, breast cancer or other estrogen-dependent neoplasms,history of clotting disorders,). Women whose pre-enrollment assays of DHEAS, estradiol or testosterone were above the normal post-menopausal women’s range or whose body mass index (BMI) exceeded 35 were excluded. Use of substances that influence cognition (e.g., amphetamines, benzodiazepines, nicotine) was also grounds for exclusion, as was a serious physical illness within the last year. All women enrolled had a normal mammogram within the prior year and a normal Pap smear within the prior three years.
Materials
Tests were presented on a Dell Latitude D820 laptop computer. All tasks were created using E Prime (Schneider, Eschman & Zuccolotto, 2002.). All data were analyzed using SPSS 16.0 version.
Experimental Design
Type of Drug (DHEA vs. Placebo) was manipulated within subject in a crossover design. Participants received DHEA for one 4-week period and placebo for the other 4-week period with a 1-week “washout” period between treatments. Order of treatments was counterbalanced across participants.
DHEA Doses and Placebos
We administered 50 mg of oral DHEA daily. This dose was selected because it is safe in short-term clinical trials, can alter levels of estrogens and androgens, and can affect cognitive performance (Hirshman et al., 2003; Morales Haubricht, Hwangt, Asakura, & Yen, 1998). DHEA was compounded by Belmar Pharmaceutical (Lakewood, CO), the source of DHEA for numerous clinical trials (e.g., Casson, Lindsay, Pisarksa, Carson & Buster, 2000). Placebos consisted of lactose and were identical in appearance to DHEA capsules. The number of pills dispensed was greater than the number needed for purposes of retrospective verification of compliance.
Measures of Sex Steroids
Serum levels of DHEA, DHEAS, estrone, testosterone and cortisol were measured at each cognitive testing time. Each appointment required 0.25 mL of serum. Samples were collected in red top tubes without a serum separator. Blood was allowed to clot for 20 minutes. Afterward the blood samples were placed in a centrifuge at 4000 rpm for 5 minutes. The serum was then separated and frozen at −80C. Assays were all conducted on site at the General Clinical Research Center of Georgetown University Medical Center by Dr. Soldin’s laboratory. Researchers at Georgetown University (Guo, Chan & Soldin, 2004) had recently developed a variant of liquid chromatography tandem mass spectrometry relying on stable isotope dilution to measure steroid hormone levels. These methods have the significant advantage of providing rapid simultaneous quantitation of multiple steroids in a single blood sample. The lower limit of detection for the steroids ranged from 1.5 to 10 pg/ml and the coefficients of variation (between-day results) ranged from 7.6% to 12.2% at low concentration levels and 5.8% to 9.5% at high concentration levels (Guo, Taylor, Singh & Soldin, 2006).
Procedure
Participants who responded to newspaper advertisements underwent a 5-minute telephone interview by the Study Coordinator. Individuals who met the entry criteria were set up for a pre-enrollment evaluation at the General Clinical Research Center at Georgetown University Hospital. The evaluation began with an informed consent form. If the participant agreed to participate in the study, the evaluation was conducted. It began with a history, including review of illnesses, medications, and contraindications to steroid hormone administration. A physical exam was performed and blood was drawn for a complete blood count (CBC), CHEM 7 (including electrolytes, blood urea, nitrogen and creatinine), as well as baseline sex steroid assays (DHEA, DHEAS, testosterone, estrone and cortisol). The Symptom Checklist 90-R (SCL 90-R) (Derogatis & Savitz, 2000) and the Mini-Mental State Exam (Folstein , Folstein, & McHugh, 1975) were administered to supplement information obtained by history.
Participants were assigned to receive DHEA or placebo in a block-randomization scheme. All investigators and participants were blind to treatment status. Upon receipt of the appropriate pillbox, participants were instructed to take one pill each morning with breakfast and to write any significant adverse events on a provided diary sheet.
Cognitive testing occurred in 28 days and the participant was given a reminder call on the day prior to testing. Participants were instructed to fast after midnight and to not consume any caffeine. On the test day, pills were counted as a measure of compliance. At 7:30am the participant was expected to arrive and was given a breakfast. At 8:00am the participant ingested either 50 mg of DHEA or the placebo. A blood draw was performed at 9:50 am. Cognitive testing occurred between 10am and noon on each testing day. Each cognitive task was presented once during each testing session. At 12:30pm, the Beck Depression Inventory and the SCL-90 tests were administered.
At the end of the first test day, participants were given appropriate pills, an appointment for the second testing session, and new diary sheets. Participants were instructed to begin taking the pills in one week and a reminder call was made prior to the second testing day. Testing procedures were identical on the second test day, except participants were debriefed at the end of this day.
Cognitive Measures
We tested participants on cognitive tasks designed to measure visual-spatial processes. Order of task presentation was randomized across participants.
Cognitive Tasks
Mental Rotation Task
Each trial showed a target figure consisting of adjoining cubes (see Vandenberg & Kuse, 1978). Four similar figures were presented next to the target drawing and the participant’s task was to identify two figures that represented a rotation of the target figure. Participants were presented with 1 set of 10 trials on each test day and were given 3 minutes to complete the 10 trials. The dependent measure was the number of figures correctly identified.
Subject-Ordered Pointing Task
Each trial in the SOPT (Petrides & Milner, 1982) presents a set of 12 images on a display with the arrangement of images varying from trial to trial. On each trial, the participant’s task is to, within 10 seconds, point to an item which they had not pointed to on a previous trial..The dependent measures include the following: errors (pointing to an item that has been previously pointed to), percent correct, response latency, the number of time outs (did not click on an image within the 10 second time frame), and rule breaks (clicking on a space 2 consecutive times).
Fragment Completion Task
On each trial, the participant was presented with a succession of eight progressively more-complete line drawings of an object (Snodgrass & Hirshman, 1994). For each line drawing, the participant’s task is to identify the object by typing in the name of the object. The dependent measure was the degree of fragmentation at which pictures were correctly identified (i.e., 1,2,3 …8 with higher numbers representing identification of more complete drawings). There was a training phase and a testing phase. Items presented in the testing phase included items presented during the training phase, referred to as “old” items and new items not presented during the training phase. Scores were recorded for training items, old items and new items.
Perceptual Identification Task
On each trial, a word was presented for 33 ms. on a computer monitor and then covered by a backward pattern mask (i.e., a row of Xs). The presented word, as well as a distracter, were then shown and the subject was asked to identify the presented word. The dependent measures were the proportion of trials (out of 70) in which the presented item was identified and the latency of correct responses.
Same-Different Judgment Task
On each trial, two letters were presented and the participants judged whether the letters were the same or different.The presented letters were the same on half of the trials All the letters of the alphabet except for I, V, and W were used. The dependent measures were the proportion of trials (out of 92) on which the judgment was correct and the latency of correct judgments..
Visual Search Task
Each trial of the visual search task (Treisman & Gelade, 1980) began with the presentation of a fixation cross for 1000 ms. A 4 × 4 matrix consisting of 16 colored letters was then presented. The participant’s task was to determine if a pre-specified target letter (e.g., a red “O”) was present in the matrix. There were three conditions (color search, letter search, conjunctive search). In the color search condition, the target letter was presented in a different color from the distracter letters. For example, if the target was a red “O”, it would be placed in a matrix of orange “O”s. In the letter search condition, the target was a different letter from the distracters. For example, if the target was a red “O”, it was placed in a matrix of red “Q”s. In the conjunctive search condition, the target letter could differ from the distracters on the dimensions of color, letter or both color and letter. For example, if the target was a red “O”, it was placed in a matrix consisting of red “Q”s, orange “O”s, and orange “Q”s.
Trials in the three Type of Search conditions were presented in 12 blocks of 32 trials with the target being presented on half of the trials in each block (i.e., 4 in each Type of Search condition) for a total of 384 trials. Prior to each block, instructions appeared alerting the participant to the Type of Search required in the block. Two letters (e.g., “O”, “Q”) and two corresponding colors (orange, red) were used in each block and letter-color combinations were counter-balanced across blocks so that each letter-color combination was assigned to each Type of Search condition equally often. The dependent measures were the proportion of correctly identified targets and the latency of correct identifications.
Affective Measures
Mini-Mental State Exam
During the pre-enrollment period, participants were given the Mini-Mental State Exam.
Beck Depression Inventory
The Beck Depression Inventory (Steer, Cavalieri, Leonard & Beck, 1999) was used to measure whether DHEA affected depression. This self-report inventory consists of 21 questions examining a participant’s hedonic state and physiological functioning. Scores less than 10 do not generally motivate clinical inquiries.
Symptom Checklist 90-R
The Symptom Checklist 90-R (SCL 90-R) (Derogatis & Savitz, 2000) was administered during the pre-enrollment period, Test Day 1 and Test Day 2. It consisted of 90 questions about physical and mental ailments that may have bothered them in the past week including that day. The test was then scored in 9 different scales for somatization, obsessive compulsive, interpersonal sensitivity, anxiety, depression, hostility, phobic anxiety, paranoid ideation, and psychoticism.
Hormone Variables
Serum levels of DHEA, DHEAS, testosterone, estrone and cortisol were also measured.
Statistical Analysis
For the hormone measures, a paired sample t test compared levels in the DHEA and placebo conditions and correlations among the measures were computed in the DHEA and placebo conditions.
For the cognitive tasks, all reaction times that were more than 2 standard deviations above the mean were removed to provide a more accurate measure of reaction time. A paired samples t test was used to compare accuracy on the Mental Rotation task between the drug condition and placebo condition.
The measures of the SOPT were accuracy, latency to make a decision, the number of errors, the number of rule breaks (clicking on a space two consecutive times in a row), and the number of time outs (did not make a decision within the 10 second frame). A paired samples t test compared each of these measures from the drug condition and the placebo condition.
For the Fragmented Pictures task, training, old and new items were compared in a paired samples t test across the drug and placebo conditions. A priming measure (new - old) was also computed and compared across the drug and placebo conditions.
A paired samples t test was used to compare measures from the drug condition and placebo condition for the Perceptual Identification task (accuracy, reaction time, median reaction time).
For the Same-Different task, a within participants two-way analysis of variance (ANOVA) was conducted using the factor of Type of Drug (DHEA vs. Placebo) and Type of Comparison (Same vs. Different), using the measures of accuracy, reaction time, and median reaction time. For the Visual Search task, a 2 × 2 × 3 ANOVA was conducted using the factor of Type of Drug (DHEA vs. Placebo), Target Presence (present vs. absent) and Type of Stimulus (color vs. letter vs. 2 features). This was conducted on the measures of accuracy, reaction time, and median reaction time.
We also conducted regression analyses in the DHEA and placebo conditions for each cognitive task that demonstrated significant effects of DHEA administration. These analyses used the cognitive outcome measures of each task as the dependent variable and the following hormone measures as our predictor variables: DHEA, DHEA-S, testosterone, estrone, and cortisol. These analyses determined the relation between hormone levels and cognitive performance for those cases in which DHEA administration affected performance.
Results
Demographic Data
Per our selection criteria, participants were aged (M= 65.25, SD = 7.89). The participants performed well on the Mini-Mental State Exam (M = 28.79, SD =1.47) and did not have extreme Body Mass Indices (M = 27.70, SD = 3.85). As indicated in Table 1, baseline hormone levels at the pre-enrollment evaluation were in the normal range for post-menopausal women.
Table 1.
Circulating levels of DHEA, DHEAS, Testosterone, Estrone and Cortisol (means with standard deviations in parentheses)
| Type of Drug |
DHEA (ng/dL) |
DHEAS (ug/dL) |
Testosterone (ng/dL) |
Estrone (pg/dL) |
Cortisol (ug/dL) |
|---|---|---|---|---|---|
| Baseline | 120.62 (68.55) | 51.58 (29.04) | 17.51 (11.08) | 17.93 (7.63) | 9.85 (3.35) |
| DHEA | 295.79 (191.99) * | 237.71 (89.43) * | 29.86 (19.49) * | 23.19 (7.75) * | 10.45 (4.11) |
| Placebo | 114.87 (78.54) | 49.83 (29.49) | 15.45 (10.55) | 15.62 (5.74) | 10.64 (5.28) |
indicates p < .001 for the comparison of hormone levels in the DHEA and Placebo onditions. Hormone levels did not differ between the Baseline and Placebo conditions.
Circulating Levels of Steroid Hormones
Table 1 represents circulating levels of DHEA, DHEAS, testosterone, estrone and cortisol as a function of Type of Drug (DHEA vs Placebo). DHEA administration affected serum levels as indicated by the substantially higher levels of circulating DHEA (t(23) = 5.48, p < .001). While there is substantial variability among participants, DHEA levels in the DHEA condition are in the range of physiological values among younger adults (Sulcova et al., 1997). The results with DHEAS, testosterone and estrone provide support that DHEA was metabolized into these related hormones (t(23) = 10.45, p < .001, t(23) = 4.85, p < .001, and t(23) = 5.54, p < .001, respectively). Levels of cortisol did not differ significantly across the conditions (p > .5). The increase in serum levels of DHEA provides strong evidence of the efficacy of our manipulation. In contrast, cortisol, another adrenal product, was not affected by DHEA administration.
Table 2 represents the correlations (Pearson r’s) between the hormones in the DHEA and placebo conditions. The changes in these patterns of correlations across the DHEA and placebo conditions provide a secondary measure of the effects of DHEA administration.
Table 2.
Correlations (Pearson r’s) Between Serum Levels of Hormones as a Function of Type of Drug (DHEA vs. Placebo)
| Type of Drug |
Hormone | DHEA (ng/dL) | DHEAS (ug/dL) |
Testosterone (ng/dL) |
Estrone (pg/dL) | Cortisol (ug/dL |
|---|---|---|---|---|---|---|
| DHEA | DHEA (ng/dL) | 1 | .60** | .38 | .19 | .36 |
| DHEAS (ug/dL) | 1 | .46* | .32 | .48* | ||
| Testosterone (ng/dL) | 1 | .38 | 66** | |||
| Estrone (pg/dL) | 1 | .04 | ||||
| Cortisol (ug/dL) | 1 | |||||
| DHEA (ng/dL) |
DHEAS
(ug/dL) |
Testosterone
(ng/dL) |
Estrone (pg/dL) |
Cortisol
(ug/dL) |
||
| Placebo |
DHEA
(ng/dL) |
1 | .73** | −.03 | .47* | .17 |
| DHEAS (ug/dL) | 1 | −.20 | .53* | −.20 | ||
| Testosterone (ng/dL) | 1 | .10 | .13 | |||
| Estrone (pg/dL) | 1 | −.06 | ||||
| Cortisol (ug/dL) | 1 |
= p < .05
= p < .01
In the placebo condition, there is a very strong correlation between DHEA and DHEAS. There are also significant correlations between estrone and DHEA/DHEAS. In contrast, in the DHEA condition, the correlations between testosterone and DHEAS/DHEA become substantially larger, while the correlations betweens estrone and DHEA/DHEAS diminish. Presumably, these changes reflect DHEA’s direct androgenic metabolism, a process that is enhanced with increasing levels of DHEA.
An additional and unexpected result is that while cortisol levels were not correlated with levels of the other hormones in the placebo condition, substantial correlations between cortisol and DHEAS/testosterone emerge in the DHEA condition. Since cortisol levels did not differ between the drug conditions, this difference may reflect participant differences in which participants with higher levels of cortisol were able to metabolize greater amounts of DHEAS and testosterone from the ingested drug.
Affective Measures
A paired samples t test revealed that the Beck Depression Inventory (BDI) scores were not significantly different (p > .5) between the DHEA condition (M= 2.08, SD = 2.04) and the placebo condition (M = 2.63, SD = 2.93). The Symptoms Checklist 90 (SCL-90) and its nine sub-scales (Somatization, Obsessive Compulsive, Interpersonal Sensitivity, Anxiety, Depression, Hostility, Phobic Anxiety, Paranoid Ideation, and Psychoticism) did not differ between the DHEA and the placebo conditions. These null effects may reflect our selection criteria that precluded enrollment of participants with extreme scores on these measures.
Mental Rotation Task
A paired samples t test was used to compare the number of items correct in the DHEA and placebo conditions on the Mental Rotation task. Participants scored significantly higher in the DHEA condition than the placebo condition (Mean DHEA = 8.50, SD = 2.63, Mean Placebo = 5.83, SD = 3.07, t(23) = 5.88, p < .001) .
SOPT
Table 3 presents the mean accuracy, latency, number of errors, number of rule breaks (clicking on a space two consecutive times in a row), and number of time outs (did not make a decision within the 10 second frame) in the DHEA and placebo conditions on the SOPT. Each measure demonstrated better performance in the DHEA than the placebo condition. A paired samples t test compared the DHEA and placebo conditions on each of these measures. Accuracy significantly improved in the DHEA condition, while the number of errors significantly decreased in the DHEA condition (t(23) = 3.11, p = .005, and t(23) = −2.56, p = .02, respectively).
Table 3.
SOPT Accuracy, Latency, Number of Errors, Number of Rule Breaks, and Number of Time Outs as a Function of Type of Drug (DHEA vs. Placebo) (means with standard deviations in parentheses)
| Type of Drug | DHEA | Placebo |
|---|---|---|
| Measure | ||
| Accuracy | .87 (.10) ** | .83 (.09) |
| Latency (s) | 4.79 (1.17) | 4.97 (1.20) |
| Number of Errors | 1.38 (1.10) * | 1.71 (.91) |
| Number of Rule Breaks | .25 (.44) | .38 (.49) |
| Number of Time Outs | .17 (.38) | .29 (.46) |
p < .05
p < .01
Fragmented Pictures Task
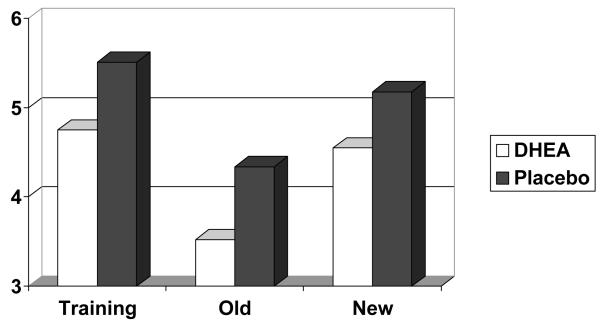
Figure 1 presents mean performance in the DHEA and placebo conditions for training, old and new items on the fragmented pictures task. Lower values indicate superior performance. Measures from the DHEA and placebo condition were compared in paired samples t tests. Performance in the DHEA condition was superior to performance in the placebo condition for training, old and new items (t(23) = 6.09 , p < .001, t(23) = 6.76 , p < .001, t(23) = 7.27, p < .001, respectively). A 2 × 2 ANOVA using the factor of Type of Drug (DHEA vs. Placebo) and Type of Task (old vs. new) demonstrated that there was a significant priming or learning effect. Participants identified old pictures better than new pictures (F(1,23) = 256.47, p < .01). Priming was not significantly different between the DHEA and placebo conditions (p > .1) for the interaction of Type of Task and Type of Drug).
Figure 1.
Mean level of Identification for Fragmented Pictures as a Function of Type of Drug (DHEA vs. Placebo) and Prior Exposure (Training vs. Old vs. New)
A t-test comparing the training and the new condition was also conducted to ascertain whether there were general practice effects on the fragmented pictures task. This comparison indicated that performance on new items was superior to performance on the training items (t(23) = 35.91, p < .001), demonstrating general effects of practice on the fragmented pictures task.
Perceptual Identification Task
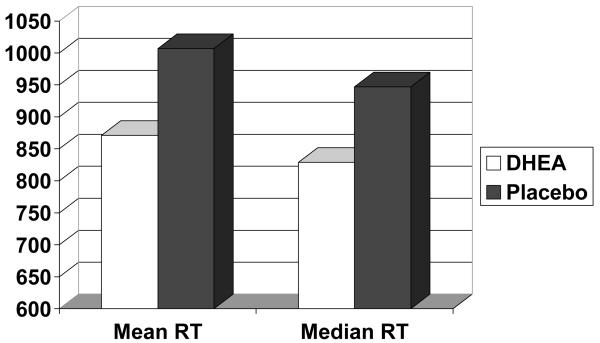
Mean accuracy of perceptual identification was near the performance ceiling (DHEA = .95, Placebo = .95). Figure 2 presents the mean and median reaction times in the DHEA and placebo conditions on the Perceptual Identification task. A paired samples t test was used to compare each measure in the DHEA and placebo conditions. Both reaction time measures were significantly faster in the DHEA than the placebo condition (t(23) = 3.01 , p < .01, t(23) = 2.95, p < .01, respectively).
Figure 2.
Perceptual Identification Performance (mean reaction time, median reaction time) as a Function of Type of Drug (DHEA vs. Placebo)
Same-Different Judgment Task
Table 4 presents accuracy and reaction times for the DHEA and placebo conditions for same and different letter pairs. Accuracy was near the performance ceiling and did not differ between the DHEA and placebo conditions. A within participants 2 × 2 ANOVA using the factor of Type of Drug (DHEA vs. Placebo) and Type of Comparison (same vs. different) was carried out on the reaction time measures. Reaction times were faster in the DHEA than the placebo condition (F(1,23) = 13.04, p < .01; F(1,23) = 9.07, p < .01, respectively). Moreover, while there was no significant difference in reaction time between the same and different conditions, Type of Drug and Type of Comparison interacted so that the reaction time advantage of DHEA was larger in the same than the different condition ((F(1,23) = 5.43, p < .05).
Table 4.
Performance on the Same-Different Letter Comparison Task (Accuracy, Mean Reaction Time, and Median Reaction Time) as a Function of Type of Drug (DHEA vs. Placebo) (means with standard deviations in parentheses)
| Type of Drug | DHEA | Placebo |
|---|---|---|
| Measure | ||
| SAME | ||
| Accuracy | .95 (.05) | .95 (.07) |
| Reaction Time (ms) | 682.88 (134.73) ** | 816.81 (194.59) |
| Median Reaction Time (ms) | 660.35 (134.60) ** | 789.06 (205.07) |
| DIFFERENT | ||
| Accuracy | .98 (.03) | .97 (.04) |
| Reaction Time (ms) | 687.87 (151.84) ** | 790.76 (183.71) |
| Median Reaction Time (ms) | 664.52 (158.34) ** | 742.42 (174.75) |
p < .01
As discussed earlier, we used the visual search task (Treisman & Gelade, 1980) as a final control task. While similar to the other tasks in relying on elementary visual object recognition and comparison processes, the visual search task also relies on complex attention and visual search processes that differentiate it from simple object recognition and comparison tasks.
Visual Search Task
Table 5 presents accuracy and reaction time (mean) measures for the visual search task in our experimental conditions. (Mean and median reaction time measures produce identical conclusions so we only present the former analysis here for ease of exposition.) We conducted a 2 × 2 × 3 ANOVA using the factor of Type of Drug (DHEA vs. Placebo), Target Presence (present vs. absent) and Type of Stimulus (color vs. letter vs. conjunction) on the accuracy and reaction time measures. Critically, there was no effect of Type of Drug or any interaction involving this variable on any of the performance measures. Thus, even though we have demonstrated repeated beneficial effects of DHEA administration on visual-spatial tasks, these effects do not occur on the visual search task.
Table 5.
Visual Search Task Accuracy, Reaction Time, and Median Reaction Time as a Function of Type of Drug (DHEA vs. Placebo) Target Presence (present vs. absent) and Type of Stimulus (color vs. letter vs. 2 features) (means with standard deviations in parentheses)
| Type of Drug | DHEA | PLACEBO | ||||
|---|---|---|---|---|---|---|
| Color | Letter | 2 Feature | Color | Letter | 2 Feature | |
| Target Present | ||||||
|
|
||||||
| Accuracy | .99 (.02) | .97 (.03) | .90 (.09) | .98 (.04) | .96 (.04) | .88 (.13) |
| Reaction Time (ms) | 910.97 (327.57) |
1164.45 (297.73) |
1662.73 (461.43) |
891.19 (277.32) |
1100.95 (284.28) |
1671.17 (373.28) |
| Median Reaction Time (ms) |
805.85 (214.10) |
1003.65 (235.24) |
1499.65 (410.19) |
796.33 (194.97) |
966.23 (200.52) |
1527.10 (323.55) |
|
|
||||||
| Target Absent | ||||||
|
|
||||||
| Accuracy | .99 (.02) | .99 (.01) | .94 (.09) | .97 (.03) | .99 (.03) | .94 (.11) |
| Reaction Time (ms) | 1126.84 (478.71) |
1703.89 (464.95) |
2752.26 (695.31) |
1099.28 (304.28) |
1620.51 (400.39) |
2753.99 (876.49) |
| Median Reaction Time (ms) |
972.79 (294.56) |
1608.81 (452.74) |
2634.94 (642.17) |
947.92 (222.01) |
1528.02 (404.80) |
2645.89 (855.63) |
Importantly, we replicated traditional findings in the visual search task. There was an effect of target presence on accuracy in that participants were less accurate on trials when the target was present than on trials when the target was absent (F(1,23) = 10.04, p < .01). There was also an effect of stimulus type in that participants had the highest accuracy in the color condition, followed by the letter condition, and the conjunction condition, (F(2,46) = 25.16, p < .001).
Reaction time measures also showed traditional effects. Participants were faster when the target was present (F(1,23) = 207.84, p < .001). There was also an effect of stimulus type (F(2,46) = 204.28?, p < .001) showing faster reaction times in the color condition than in the letter condition which, in turn, was faster than the conjunction condition. An interaction between target presence and stimulus type (F(,2,46) = 56.00, p < .001) showed the traditional finding that the negative effects of searching in the letter and conjunction conditions were more pronounced when the target was absent.
Regression Results
Table 6 presents the results of the regression analyses that use hormone measures to predict cognitive performance in the DHEA and placebo conditions. Consistent with our results demonstrating that DHEA administration enhances cognitive performance, the table demonstrates a substantial number of relatively large relationships in which higher DHEA/DHEAS levels are associated with better cognitive performance in the DHEA condition. These relationships occur on both accuracy and reaction time measures and across multiple tasks and conditions. Moreover, these relationships do not generally occur in the placebo condition where the level of DHEA and its metabolites are substantially lower. The one exception to this latter generality is the positive relationship between estrone levels and fragment completion performance, a relationship that holds in both the placebo and the DHEA conditions.
Table 6.
Intercepts and Regression Coefficients for Regression Model Predicting Cognitive Performance as a Function of Hormone Levels
| Model | Intercept | DHEA | DHEA-S | Testosterone | Estrone | Cortisol | R2 |
|---|---|---|---|---|---|---|---|
| DHEA | |||||||
| MRT Total | 6.21 (.85) |
.56 (.002)** |
- | - | - | - | .32 |
| SOPT Errors | 4.23 (.57) |
- | −.51 (.002)** |
- | −.42 (.02)* |
- | .41; .57 |
| Fragpix Train | 6.15 (.38) |
−.51 (.001)** |
- | - | −.38 (.02)* |
- | .34; .47 |
| Fragpix Old | 5.22 (.49) |
−.39 (.001)** |
- | - | −.44 (.02)* |
- | .27; .41 |
| Fragpix New | 5.9 (.50) | - | - | - | −.51 (.02)* |
- | .26 |
| PID Mean Reaction Time |
1079.22 (111.51) |
−.43 (.32)* |
- | - | - | - | .18 |
| PID Median Reaction Time |
1021.71 (108.05) |
−.41 (.31)* |
- | - | - | - | .17 |
| S-D Mean Reaction Time_Same |
783.89 (45.80) |
−.49 (.13)* |
- | - | - | - | .24 |
| S-D Mean Reaction Time Different |
812.33 (50.03) |
−.53 (.14)** |
- | - | - | - | .28 |
| S-D Median Reaction Time Different |
791.89 (52.55) |
−.52 (.15)** |
- | - | - | - | .27 |
| Placebo | - | - | - | ||||
| Fragpix Train | 6.54 (.43) |
- | - | - | −.48 (.03)* |
- | .23 |
| Fragpix Old | 5.97 (.49) |
- | - | - | −.60* (.03) |
- | .36 |
| Fragpix New | 6.35 (.34) |
- | - | - | −.62 (.02)* |
- | .38 |
= p <.05
= p < .01
R2 presented as measure of model fit (standard error in parentheses)
Measures of Effect Size (Cohen’s d)
Table 7 presents Cohen’s d for each of the significant results reported here. Using conventional interpretations, the results demonstrate large effect sizes in Mental Rotation, Fragment Completion and Same-Different Judgment and medium effect sizes in SOPT and Perceptual Identification.
Table 7.
Measures of Effect Size (Cohen’s d) For Significant Effects Reported
| Cognitive Task | Effect Size |
|---|---|
| Mental Rotation | .94 |
| SOPT-Accuracy | .42 |
| SOPT-Errors | .33 |
| Frag. Comp.Train | .99 |
| Frag. Comp. Old | .88 |
| Frag. Comp. New | .80 |
|
Percept.Id. RT-
mean |
.40 |
|
Percept.IdRT-
median |
.37 |
| Same-Different- | .80 |
|
SameCondition-
RT mean |
|
| Same-Different- | .61 |
|
Diff. Condition-RT
mean |
|
| Same-Different- | .75 |
|
SameCondition-
RT median |
|
| Same-Different- | .46 |
|
Diff. Condition-RT
RT median |
Discussion
The current results on the mental rotation, SOPT, fragment completion, perceptual identification and Same-Different tasks present the first systematic and compelling evidence that administration of DHEA substantially enhances visual-spatial cognition in post-menopausal women. These results occur on both accuracy and latency measures, across multiple experimental conditions, and on tasks of varying cognitive complexity (e.g., Mental rotation vs Same-Different letter judgment). The results of regression analyses, demonstrating multiple relationships between serum levels of hormones and cognitive performance in the DHEA condition, provide converging evidence of DHEA’s influence on cognition. Moreover, conventional interpretations of measures of effect size indicate the effects reported here are medium-to-large
Multiple lines of evidence suggest that DHEA’s beneficial effect in the current studies are specific to a subset of visual-spatial processes. First, prior studies in our laboratory (Hirshman et al., 2003; Hirshman et al., 2004; Stangl et al., 2010a; Stangl et al., 2010b), using the same methodology as the current study, failed to provide systematic evidence that DHEA administration enhances episodic or short-term memory performance on measures of accuracy or reaction time. Moreover, these studies tested double the number of participants as the current study, suggesting that differences in the experimental power to detect effects are not responsible for the differing results of the two sets of studies. The current finding that DHEA administration did not affect priming in fragment completion is also consistent with the view that DHEA administration does not affect learning. Second, there is no evidence that DHEA substantially influenced affective processes as measured by the BDI or SCL-90. Thus, the observed effects on visual-spatial tasks are not due to changes in the affective processes measured by these tests.
Third, DHEA administration did not enhance visual search in the current experiment. Thus, while it is clear from the overall pattern of findings that DHEA administration enhances some aspects of visual-spatial performance, the null effect on visual search suggests that all visual-spatial processes are not enhanced. The larger effect of DHEA in the same than the different condition on the same-different task is also consistent with the view that DHEA’s effect is specific to selected visual-spatial processes. Without speculating excessively, we note that this effect suggests that DHEA may enhance processes that involve the comparison of multiple representations of (purportedly) the same object. This is a provocative hypothesis for further exploration given the central role mental comparisons of object representations play in the tasks that demonstrate beneficial effects here. In mental rotation, the target object is compared to objects that may be rotated versions of the target. In self-ordered pointing, objects in the presented array on a trial are being compared to objects in short-term memory that were pointed to on a prior trial. In picture fragment completion and perceptual identification, representations of presented visual objects (pictures, words) are being compared to representation in semantic memory to identify the object’s name.
The finding that DHEA administration does not enhance visual search provides a potential boundary condition for the hypothesis that DHEA may enhance processes for the comparison of multiple representations. Visual search, by the definition of the task, must involve such comparison processes, but complex attention and visual search processes may be the predominant influences on this task and DHEA administration may not affect these attention and visual search processes. Thus, the current results suggest the hypothesis that administration of DHEA will enhance performance only on visual-spatial tasks in which the comparison of multiple representations is a dominant influence on task performance.
The current study raises a number of questions for further study. Given that the study participants have a broad range of ages, one can ask whether beneficial effects of DHEA are more pronounced in women who are younger (i.e., is the benefit larger if DHEA replacement is provided closer to menopause?). Unfortunately, the number of participants tested in the current study (24) is not sufficiently large to provide a definitive answer to this question.
A second important question concerns whether DHEA and/or its androgenic/estrogenic metabolites are responsible for the enhancements observed here. The regression analyses (see Table 7) suggest that DHEA is the predominant influence. Similarly, while prior observational studies have demonstrated stronger association with the ratio of DHEA to cortisol than DHEA alone (e.g., Kalmijn et al., 1998), cortisol did not change between the DHEA and placebo conditions in the current study, making it impossible for variation in cortisol levels to produce the current effects. Nonetheless, the relatively high correlation between DHEA and testosterone (see Table 2) means we can not rule out the possibility that testosterone is an important influence. Moreover, given the possibility of local steroid conversion, correlations involving serum levels of hormones are suggestive, but not conclusive.
In conclusion, the current experiments demonstrate important effects of DHEA administration on visual-spatial performance and raise important questions about the cognitive and hormonal mechanism mediating these effects.
Acknowledgments
The work presented here was supported by the National Institute on Aging of the National Institutes of Health grant (27675-1-CCLS-2035F, 5RO1AG020543).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Aleman A, Bronk E, Kessels R, Koppeschaar H, van Honk J. A single administration of testosterone improves visuospatial ability in young women. Psychoneuroendocrinology. 2004;29(5):612–617. doi: 10.1016/S0306-4530(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Aveleyra E, Carranza-Lira S, Ulloa-Aguirre A, Ostrosky-Solis F. Cognitive effects of hormone therapy in early postmenopausal women. International Journal of Psychology. 2005;40(5):314–323. [Google Scholar]
- Becker J. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during micro-dialysis. Neuroscience Letters. 1990;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein S. A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: The rancho bernardo study. Journal of the American Geriatrics Society. 1994;42(4):420–423. doi: 10.1111/j.1532-5415.1994.tb07491.x. [DOI] [PubMed] [Google Scholar]
- Beigneux K, Plaie T, Isingrini M. Aging effect on visual and spatial components of working memory. International Journal of Aging and Human Development. 2007;65(4):301–314. doi: 10.2190/AG.65.4.b. [DOI] [PubMed] [Google Scholar]
- Barrou Z, Charru P, Liddy C. Dehydroepiandrosterone (DHEA) and aging. Archives of Gerontology and Geriatrics. 1997;24:233–241. doi: 10.1016/s0167-4943(96)00761-3. [DOI] [PubMed] [Google Scholar]
- Carlson L, Sherwin B, Chertkow H. Relationships between dehydroepiandrosterone sulfate (DHEAS) and cortisol (crt) plasma levels and everyday memory in alzheimer’s disease patients compared to healthy controls. Hormones and Behavior. 1999;35(3):254–263. doi: 10.1006/hbeh.1999.1518. [DOI] [PubMed] [Google Scholar]
- Casson P, Lindsay M, Pisarksa M, Carson S, Buster J. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Human Reproduction. 2000;15:2129–2132. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M. Working memory and aging: A cross sectional and longitudinal analysis using a self-ordered pointing task. Journal of the International Nueropsychological Society. 2004;10(4):489–503. doi: 10.1017/S1355617704104013. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Brember W, Petrova A, LaTendresse S, Craft S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57(1):80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32(1):72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Savitz KL. The SCL-90-R and Brief Symptom Inventory (BSI) in primary care. In: Maruish ME, editor. Handbook of psychological assessment in primary care settings. Lawrence Erlbaum Associates; Mahwah, NJ, US: 2000. pp. 297–334. [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Hormones and Behavior. 2000;38(4):262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fonda SJ, Bertrand R, O’Donnell A, Longcope C, McKinlay JB. The Journals of Gerontology. (Series A).Biological Sciences and Medical Sciences. 2005;60(3):385–390. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;6:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Guo T, Chan M, Soldin S. Steroid profiles using liquid chromatography tandem mass spectrometry with atmospheric pressure photoionization source. Archives of Pathology and Laboratory Medicine. 2004;128(4):469–475. doi: 10.5858/2004-128-469-SPULCM. [DOI] [PubMed] [Google Scholar]
- Guo T, Taylor R, Singh R, Soldin S. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clinica Chimica Acta. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Merritt P, Wang CCL, Wierman M, Budescu DV, Kohrt W, Templin JL, Bhasin S. Evidence that androgenic and estrogenic metabolites contribute to the effects of dehydroepiandrosterone on cognition in postmenopausal women. Hormones & Behavior. 2004;45(2):144–155. doi: 10.1016/j.yhbeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Mulligan N. Perceptual interference improves explicit memory, but does not enhance data-driven processing. Journal of Experimental Psychology: Learning Memory and Cognition. 1991;17:507–513. doi: 10.1037//0278-7393.17.3.507. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Wells E, Wierman M, Anderson B, Butler A, Senholzi M, Fisher J. The effect of dehyroepiandrosterone (DHEA) on recognition memory decision processes and discrimination in postmenopausal women. Psychonomic Bulletin & Review. 2003;10(1):125–134. doi: 10.3758/bf03196476. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral Neuroscience. 1994;108(2):325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Zamboni BD, Orwoll E. The cognitive neuropsychology of sex hormones in men and women. Developmental Neuropsychology. 1998;14:421–440. [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. Journal of Cognitive Neuroscience. 2000;12(3):407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Jansen P, Heil M. Gender differences in mental rotation across adulthood. Experimental Aging Research. 2010;36(1):94–104. doi: 10.1080/03610730903422762. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Launer L, Stolk R, de Jong F, Pols H, Hofman A, Breteler M, Lamberts S. A Prospective Study on Cortisol, Dehydroepiandrosterone Sulfate, and Cognitive Function in the Elderly. The Journal of Clinical Endocrinology & Metabolism. 1998;83:3487–3492. doi: 10.1210/jcem.83.10.5164. [DOI] [PubMed] [Google Scholar]
- Kolb B, Stewart J. Changes in the neonatal gonadal hormonal environment prevent behavioral sparing and alter cortical morphogenesis after early frontal cortex lesions in male and female rats. Behavioral Neuroscience. 1995;109:285–294. doi: 10.1037//0735-7044.109.2.285. [DOI] [PubMed] [Google Scholar]
- Krueger L, Allen P. Same-different judgments of foveal and parafoveal letter pairs by older adults. Perception & Psychophysics. 1987;41(4):329–334. doi: 10.3758/bf03208234. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Cusan L, Gomez J-L, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. Journal of Clinical Endocrinology and Metabolism. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- Lorenzo-López L, Amenedo E, Cadaveira F. Feature processing during visual search in normal aging: Electrophysiological evidence. Neurobiology of Aging. 2008;29(7):1101–1110. doi: 10.1016/j.neurobiolaging.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Moffat S, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: Possible influence of hand preference. Psychoneuroendocrinology. 1996;21(3):323–337. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Morales A, Nolan J, Nelson J, Yen S. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. Journal of Clinical Endocrinology and Metabolism. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- Morales AJ, Haubricht RH, Hwangt JY, Asakura H, Yen SSC. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clinical Endocrinology. 1998;49:421–432. doi: 10.1046/j.1365-2265.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. Journal of Endocrinology and Metabolism. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- Parsons T, Kratz K, Thompson E, Stanczyk F, Buckwalter J. DHEA supplementation and cognition in postmenopausal women. International Journal of Neuroscience. 2006;116(2):141–155. doi: 10.1080/00207450500341506. [DOI] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Puy L, MacLusky N, Beker L, Karsan N, Trachtenberg J, Brown T. Immunocytochemical detection of androgen receptors in human temporal cortex. Journal of Steroid Biochemistry and Molecular Biology. 1995;55:197–209. doi: 10.1016/0960-0760(95)00165-v. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49(6):1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Rubino S, Stomati M, Bersi C, Casarosa E, Luisi M, Petraglia F, Genazzani A. Neuroendocrine effect of a short-term treatment with DHEA in post-menopausal women. Maturitas. 1998;28:251–257. doi: 10.1016/s0378-5122(97)00086-8. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Psychology Software Tools Inc.; Pittsburgh: 2002. [Google Scholar]
- Shaywitz S, Shaywitz B, Pugh K, Fulbright K, Skudlarski P, Mencl W, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171(3972):701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Snodgrass J, Hirshman E. Dissociations among implicit and explicit memory tasks: The role of stimulus similarity. Journal of Experimental Psychology: Learning Memory and Cognition. 1994;20:150–160. doi: 10.1037//0278-7393.20.1.150. [DOI] [PubMed] [Google Scholar]
- Stangl B, Hirshman E, Verbalis J. Administration of Dehydroepiandrosterone (DHEA) Increases Serum Levels of Androgens and Estrogens but Does Not Enhance Recognition Memory in Post-Menopausal Women. In: Benjamin Aaron S., editor. Successful Remembering and Successful Forgetting: A Festschrift in Honor of Robert A. Bjork. Psychology Press; 2010a. [Google Scholar]
- Stangl B, Hirshman E, Verbalis J. Administration of dehydroepiandrosterone (DHEA) increases serum levels of androgesn and estrogens but does not enhance short-term memory in post menopausal women. 2010b. [DOI] [PMC free article] [PubMed]
- Steer RA, Cavalieri TA, Leonard DM, Beck AT. Use of the Beck depression inventory for primary care to screen for major depression disorders. General Hospital Psychiatry. 1999;21(2):106–111. doi: 10.1016/s0163-8343(98)00070-x. [DOI] [PubMed] [Google Scholar]
- Sulcova J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulfate in normal subjects. Journal of Endocrinology. 1997;154:57–62. doi: 10.1677/joe.0.1540057. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Turvey C, Schultz S, Arndt S, Wallace R, Herzog R. Memory complaints in a community sample aged 70 and older. Journal of the American Geriatrics Society. 2000;48:1435–1441. doi: 10.1111/j.1532-5415.2000.tb02634.x. [DOI] [PubMed] [Google Scholar]
- Van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: Association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26(6):591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualization. Perceptual & Motor Skills. 1978;47(2):599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- West RL, Welch DC, Knabb PD. Gender and aging: Spatial self-efficacy and location recall. Basic and Applied Social Psychology. 2002;24:71–80. [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998b;23:617–629. doi: 10.1016/s0306-4530(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Naumann O, Hellhammer D, Geiben AC, Strasburger CJ, Dressendorfer RA, Pirke KM, Kirschbaum C. Effects of a two week physiological dehydroepiandrosterone substitution on cognitive performance and well being in healthy elderly women and men. Journal of Clinical Endocrinology and Metabolism. 1997;82(7):2363–2367. doi: 10.1210/jcem.82.7.4056. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Naumann O, Hellhammer D, Kirschbaum C. Effects of dehydroepiandrosterone replacement in elderly men on event-related potential, memory and well being. Journal of Gerontology: Medical Sciences. 1998a;53A:M385–M390. doi: 10.1093/gerona/53a.5.m385. [DOI] [PubMed] [Google Scholar]
- Woolley C, Weiland N, McEwen B, Shwarthroin P. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: Correlation with dendritic spine density. Journal of Neuroscience. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Ettinger B, Pressman A, Seeley D, Whooley M, Schaefer C, Cummings S. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: A prospective study. Biological Psychiatry. 1998;43(9):694–700. doi: 10.1016/s0006-3223(97)00303-x. [DOI] [PubMed] [Google Scholar]