Abstract
G protein-coupled receptors (GPCRs) comprise the largest family of membrane proteins in the human genome and mediate cellular responses to an extensive array of hormones, neurotransmitters, and sensory stimuli. While some crystal structures have been determined for GPCRs, most are for modified forms, showing little basal activity, and are bound to inverse agonists or antagonists1. Consequently, these structures correspond to receptors in their inactive states. The visual pigment rhodopsin is the only GPCR for which structures exist that are thought to be in the active state2,3. However, these structures are for the apoprotein or opsin form that does not contain the agonist all-trans retinal.
We present here a crystal structure for the constitutively active rhodopsin mutant E113Q4-6 in complex with a peptide derived from the C-terminus of the G protein transducin (the GαCT peptide). Importantly, the protein appears to be in an active conformation, and retinal is retained in the binding pocket after photoactivation. Comparison with the structure of ground state rhodopsin7 suggests how translocation of the retinal β-ionone ring leads to a rotational tilt of transmembrane helix 6 (TM6), the critical conformational change upon activation8. A key feature of this conformational change is a reorganization of water mediated hydrogen-bonding networks between the retinal-binding pocket and three of the most conserved GPCR sequence motifs. For the first time we thus show how an agonist ligand can activate its GPCR.
In the dark, rhodopsin contains a covalently bound 11-cis-retinal chromophore that preferentially binds to the inactive state and therefore functions as an inverse agonist in the visual system. Upon exposure to light, the retinal isomerizes to the all-trans-form, initiating a series of conformational changes leading to the transient intermediate metarhodopsin-II (meta-II), responsible for activating the G protein. All-trans-retinal thus functions in this scenario as an agonist for activation of the receptor.
The first structures of bovine rhodopsin were determined for the inactive-state of the native protein bound to the 11-cis-retinal chromophore9. Other dark-state structures followed7,10, including those for rhodopsin containing non-native 9-cis-retinal11 and squid rhodopsin12. Importantly, several structures have been determined for crystals of the protein following photoisomerisation of the chromophore to the all-trans-form13. However, these are thought to be for early photointermediates in the activation pathway and have not yet undergone the critical conformational alteration required for activation of the G protein.
Recently, two structures thought to represent the active state have been determined for the apoprotein form opsin2,3. The two structures are very similar and are thought to represent activated forms of the receptor because one was determined for opsin bound to a peptide derived from the C-terminal tail of the α-subunit of the G protein transducin (GαCT) known to bind preferentially and stabilize the active intermediate meta-II14. While the opsin structures are of immense value to our understanding of the active state, a complete understanding of the active state will require structures in which the all-trans-retinal agonist is included in the ligand-binding pocket of the receptor.
In an attempt to obtain active-state crystals of rhodopsin, which still contain the all-trans-retinal ligand, we used the crystallization conditions described for opsin but substituted a previously described mutant, E113Q3.28 (superscripts denote the Ballesteros-Weinstein general GPCR numbering), for the native protein. E113Q3.28 incorporates a change of the Schiff base counterion4,5, and there were three reasons for choosing it for these studies. First, hydrolysis and dissociation of all-trans-retinal from the photoactivated protein is dramatically slowed in the mutant5. Second, fourier transform infrared spectroscopy (FTIR) shows that the mutant lacks a classical meta-I/meta-II equilibrium, instead favouring the active meta-II state, and that the normally inactive post-meta-II intermediate meta-III is in fact in an active conformation15. Third, the opsin form of the E113Q3.28 mutant is constitutively active6, and addition of all-trans-retinal to the apoprotein can activate the mutant to levels comparable to those of light-activated wild-type rhodopsin5,16, essentially turning rhodopsin into a GPCR that binds and is activated by diffusible agonists. Unfortunately, the E113Q3.28 mutant is also significantly less stable than wild-type rhodopsin (Supplementary Figure 1), and for this reason we used the E113Q mutation in the context of another rhodopsin mutant, N2C/D282C, containing an engineered disulfide bond known to enhance thermal stability of the protein without affecting activity15,17. We previously solved the structure of this stabilized mutant and showed it to be virtually identical with that of the native protein with the important exceptions that the oligosaccharide chain normally linked to Asn2 is missing, and the two engineered Cys residues are linked by a disulfide bond18.
The E113Q/N2C/D282C triple mutant (henceforth referred to as simply the E113Q3.28 mutant) was expressed and purified using a previously described immunoaffinity procedure in which rhodopsin is reconstituted with 11-cis-retinal while still immobilized on the affinity matrix18. The purified protein was then mixed with lipids and the GαCT peptide before light was used to isomerise retinal and activate the protein just before crystallization. In independent experiments, crystals were also grown in the dark from E113Q3.28 opsin in the presence of all-trans-retinal. Crystals from both samples displayed a faint yellow colour (Supplementary Figure 2) indicative of bound retinal. While complete data sets were generated from both samples, we present data obtained from light-activated rhodopsin as selective illumination of protonated retinal minimises formation of unwanted isomers and resembles activation in the retina more closely.
The structure, solved by molecular replacement, contains residues 1-326 of the mutant opsin and all eleven residues of the GαCT peptide, including the Lys341→Leu mutation introduced to increase affinity for the receptor19. In addition, clear electron density is observed for two partially ordered lipid molecules, one molecule of octylglucoside, several previously unresolved water molecules, and most strikingly, retinal within the ligand-binding pocket of the receptor (see below). With the exception of the missing oligosaccharyl chain at position 2, the recombinantly produced protein contains all post-translational modifications observed with the native protein purified from bovine retina including acetylation of the N-terminus, palmitoylation of Cys residues at positions 322 and 323, and glycosylation of Asn at position 15. Finally, the engineered disulfide between Cys residues at positions 2 and 282 is clearly visible in the mutant.
The structure of the E113Q/GαCT complex deviates significantly from the ground-state structure of rhodopsin (1GZM)7, but displays high similarity to the active-state structure of the opsin/GαCT complex (3DQB)3, with respective Cα RMS deviations of 2.42 Å and 0.58Å (supplementary Figure 3). The E113Q/GαCT structure is also in good agreement with the results of high-resolution distance mapping using double-electron-electron resonance (DEER) spectroscopy to investigate movement undergone by rhodopsin in the photoactivation event where pairs of nitroxide spin labels (in particular at positions 241 and 252) were used to quantify a 5-Å outward movement of TM6 critical to the activation process20. For comparison, the Cα atoms of Arg2416.42 and Ala2526.35 of TM6 in the E113Q/GαCT structure are shifted by 5.1Å and 4.1Å, respectively, with regard to the same reference point within ground-state rhodopsin used in the DEER experiments (supplementary Table1), providing excellent agreement with the biophysical studies. Other residues not showing significant change in the DEER experiments also do not show significant difference from ground-state rhodopsin in the E113Q/GαCT structure.
On the basis of constitutive activity of the E113Q3.28 mutant, presence of the GαCT peptide in the structure, and agreement with the results from DEER spectroscopy, we conclude that the structure of the E113Q/GαCT complex reported here is in fact that of the active state.
As is shown in Figure 1, electron density for retinal is clearly observed in the general area where 11-cis-retinal is found in ground-state rhodopsin, confirming the presence of the chromophore in the ligand-binding pocket of the E113Q/GαCT complex. However, density for the nearby side-chain of Lys2967.43 is weak indicating that retinal is not bound to the protein covalently by a Schiff base, as in ground-state rhodopsin or the meta-II state. In addition, density for the β-ionone ring and most of the polyene chain is well defined but broadens after position C9 suggesting that the retinal is present as a mixture of isomers. We have modelled retinal in the all-trans conformation, but based on occupancy refinement we estimate a mixture composed of 60% all-trans-retinal and 40% of various isoforms. However, it is important to note that we cannot distinguish between a model based upon a mixture of cis and trans isomers about double bonds, catalyzed for example by phosphatidylethanolamine lipids21 or Lys2967.43, and one based upon a mixture of conformers arising from rotations about single bonds in the polyene chain. In fact, the latter might be more likely as similar electron density was observed for the retinal in crystals grown from E113Q3.28 opsin and all-trans-retinal in the dark, in this case the chromophore never having been exposed to light.
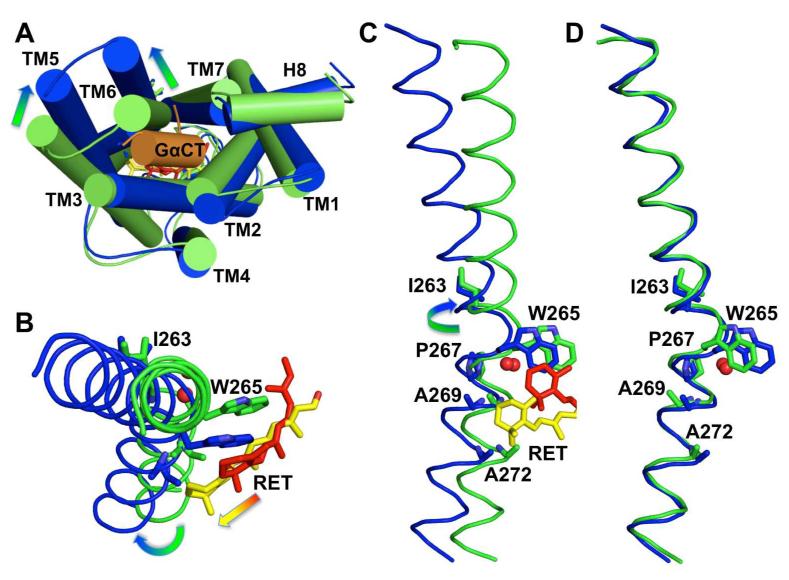
Figure 1. Conformational changes in the retinal binding pocket.
A: 2Fo-Fc map (contoured at 1.5 sigma) of the retinal-binding pocket. The retinal β-ionone ring is well resolved while density of the polyene chain broadens towards the end facing K2967.43. Occupancy refinements indicated a mixture of 60% all-trans-retinal and 40% of isomers rotated around single bonds or double bonds after position C9. An acetate molecule is packed between F2085.43 and F2766.59 and blocks a potential retinal entry/exit channel2. B: Superposition of E113Q/GαCT (blue, 2×72) with ground state rhodopsin (green, 1GZM)7. Compared to the β-ionone ring of 11-cis-retinal (red) in ground state rhodopsin, the β-ionone ring of all-trans-retinal (yellow) in the active E113Q/GαCT structure is shifted 4.3 Å towards the cleft between TM5 and TM6, where it makes contact with both helices. Simultaneously W2656.48 of the CWxP motif is released from its locked position in the ground state, which disrupts a water-mediated interaction to S2987.45 and breaks the restraining TM6-TM7 link26. The salt bridge between E1133.28 as counterion to the protonated Schiff base and K2967.43 is broken in the E113Q/GαCT structure removing a link known to restrain TM3 and TM7 in the inactive, ground-state conformation6. By alteration of these three key interactions retinal can induce the rotation of TM6 that opens the G protein-binding site and simultaneously can facilitate a reorganization of hydrogen-bonding networks in the NPxxY and E(D)RY at the cytoplasmic end of TM3 and TM7.
These conclusions, that retinal is bound non-covalently to the protein and that it is composed of a mixture of isomers, are rather surprising as E113Q3.28 is known to contain a covalently bound all-trans-retinal ligand under similar conditions in solution studies4,5. Clearly, the structure represents an active conformation but is not identical to metarhodopsin-II because the covalent bond to Lys2967.43 is not present. We suspect that it probably corresponds to a trapped intermediate in which the retinal is either entering or exiting the ligand-binding pocket. It is well established that wild-type opsin transiently activates as retinal enters the binding pocket but before a covalent bond to Lys2967.43 has been formed22, and that both all-trans and 11-cis retinol can act as potent agonists23. In this regard, the ionone ring-end of retinal and part of the polyene chain in the E113Q/GαCT structure is probably in the same position that it occupies in meta-II despite the absence of a covalent bond to Lys2967.43. The location of the β-ionone ring is shifted 4.3 Å from its position in ground-state rhodopsin (Figure 1) and inserted in the cleft between TM5 (residues Met2075.42, Phe2085.43, and Phe2125.47) and TM6 (residues Trp2656.48, Ala2696.52, and Ala2726.55). This position is in excellent agreement with (2D) dipolar-assisted rotational resonance (DARR) NMR experiments of the retinal position in the meta-II state24.
The transition from inactive to active states evident upon comparison of the ground-state and active-state structures is accompanied by global rearrangement of the seven transmembrane helix bundle (Figure 2) that produces an apparent tilt of the cytoplasmic end of TM6 (and to a lesser extent TM5) away from the bundle core (TM1-TM4 and TM7). The apparent tilt is not achieved through a hinge movement but by rotation about the main axis of TM6 that leaves the shape of the helix intact. On the cytoplasmic side, the rotational tilt is amplified through the characteristic bend caused by Pro2676.50, the most conserved residue in TM6 among GPCRs and part of the ubiquitous CWxP motif. Pro2676.50 is in close contact with water7 that is hydrogen bonded to Cys2646.47, Tyr2686.51, and Pro2917.38, an arrangement similar to that observed in ground-state rhodopsin. This water is found also in structures of the beta1 adrenergic, beta2 adrenergic, and adenosine receptors, and likely forms an important architectural element in formation of the bend in TM6.
Figure 2. Rearrangement of the heptahelix bundle and rotation of TM6.
A: Superposition of Cα traces of ground state rhodopsin (green) and E113Q (blue) with bound G protein peptide (orange), as seen from the cytoplasmic side. The main rearrangements of the seven transmembrane helices (cylinders) that open the G protein-binding site are indicated as arrows. The loop regions have been smoothed for clarity. B, C: Cytoplasmic and membrane side views of TM6 (Cα traces in ribbon representation) from ground state rhodopsin and the E113Q/GαCT structure illustrates how 11-cis (red) to all-trans (yellow) isomerisation of retinal can release W2656.48 from its locked position in the ground state and insert the β-ionone ring between F2085.43/F2125.47 in H5 and A2696.52/A2726.55 in H6. This leads to a rotation of TM6 that is amplified towards its cytoplasmic end by the characteristic bend of the helix introduced by P2676.50 and water7. D: Superposition of TM6 alone shows that the shape of the helix is preserved during the rearrangements.
Trp2656.48 of the CWxP motif is a highly conserved amino acid that is tightly packed against retinal in ground-state rhodopsin and has been identified as important for GPCR activation through early mutagenesis studies. Trp2656.48 occupies a central role in the toggle-switch model for activation of GPCRs25 and, in rhodopsin, is thought to be released by cis/trans isomerisation of the chromophore allowing H6 to swing out and expose the G protein-binding site7,24. Superposition of the ground-state (1GZM) and E113Q/GαCT structures indicates that the indole group of Trp2656.48 has moved 3.6 Å away from its ground-state position as a consequence of rhodopsin activation. However, we do not observe the rotamer change that has been originally proposed based on computer simulations. Instead, Trp2656.48 follows the β-ionone ring, maintaining contact with the C18 methyl group. Importantly, the spectral changes measured for Trp2656.48 by DARR spectroscopy24 are in good agreement to the movement of Trp2656.48 revealed by our structure, which involves a translation without rotation of the side chain. It is tempting then to suggest that the critical movement of TM6 stems from a motion of the β-ionone ring against TM6 just beneath the CWxP motif, while Trp2656.48 is simultaneously released from its locked ground-state position.
Of special interest in the E113Q/GαCT structure is a cluster of densities that indicate the presence of structural water molecules (Figure 3) between some of the most conserved residues in GPCRs. While modelling of water is difficult in the 3Å resolution range, omission of this potential water cluster during refinement resulted in clear difference peaks (panel A), presumably due to tight hydrogen bonding typical for structural waters in the interior of membrane proteins. Our interpretation of these difference peaks as waters is further strengthened by the presence of a similar hydrogen-bonding network found in ground state rhodopsin that begins at Trp2656.48, involves the conserved Asp832.50 in TM2 and Ser2987.45 and Asn3027.49 (of the NPxxY motif) in TM7, and ends just below a hydrophobic barrier formed by six residues (Leu762.43 and Leu792.46 in TM2; Leu1283.43 and Leu1313.46 in TM3; Met2536.36 and Met2576.40 in TM6), of which many are conserved in rhodopsin-class GPCRs.
Figure 3. Rearrangement of water mediated hydrogen-bonding networks.
A: The E113Q structure reveals potential water mediated hydrogen-bonding networks that connect the retinal-binding region with the GαCT binding site. Strong electron density (Blue mesh represents 2Fo-Fc map contoured at 2.0 sigma. Green mesh shows the Fo-Fc map contoured at 3.0 sigma) difference peaks are observed when waters are omitted during simulated annealing refinement, demonstrating a high degree of local order. B: In ground-state rhodopsin, the hydrogen-bonding network connects W2656.48 in the retinal-binding pocket with N3027.49 of the NPxxY motif (blue) and the carboxyl of M2576.40, via the conserved residues S2987.45 and D832.50. M2576.40 is part of a region called the hydrophobic barrier (green) that separates the ionic lock interaction between the E1343.49/R1353.50 pair in the E(D)RY motif (salmon) of TM3 and E2476.30 in TM6. C: In the active E113Q/GαCT structure, TM6 is rotated including W2656.48, which breaks the water-mediated link to S2987.45 in TM7. The TM1-TM2-TM7 network between N551.50, D832.50 and S2987.45 is reorganized but maintains and stabilizes a similar distortion of TM7 induced by P3037.50 of the NPxxY motif (blue)26. The hydrophobic barrier that separates the E(D)RY motif (salmon) in TM3 from the membrane core is opened. Y3067.53 of the NPxxY motif (blue) in TM7 and Y2235.58 in TM5 rearrange to fill the resulting gap and extend the hydrogen-bonded network towards R1353.50 of the E(D)RY motif (salmon). R1353.50 is released from its ground state bent interaction with E1343.49, which opens the ionic lock and facilitates the outward movement of TM6 that allows binding of the GαCT peptide (orange ribbon). In the indicated location the water cluster can bridge the retinal-binding core with the cytoplasmic side of the receptor and is directly mediating interactions to the G protein peptide.
The E113Q/GαCT structure indicates a rearrangement of this water cluster through rotation of TM6. The water-mediated link between Trp2656.48 in TM6 and Ser2987.45 in TM7 is broken, while Ser2987.45 together with water16, Asn551.50, Asp832.50 and Asn3027.49 continues to stabilize the unusual Pro-kink introduced in TM726 by Pro3037.50. While these reorganizations are comparatively minor, they directly link changes in the CWxP motif in the retinal-binding pocket with the two most conserved residues in TM1 and TM2, and the NPxxY motif in TM7. On the cytoplasmic site of the reorganized hydrogen-bonding network, the rotation of TM6 in the activated state breaks the hydrophobic barrier between the retinal- and G protein-binding sites. Two conserved residues, Tyr2235.58 of TM5 and Tyr3067.53 of the NPxxY motif, swing into the protein interior from their membrane-facing positions in the ground state to fill the gap created by removal of Met2576.40. The phenolic side chains allow additional interactions with water molecules (waters 2+14) and extension of the hydrogen-bonding network through the opened hydrophobic barrier up to the highly conserved E(D)RY motif at the cytoplasmic surface of TM3. Residue Glu1343.49 and Arg1353.50 of this motif are released from the bent conformation in the ground state, which opens the ionic lock interactions27 of Glu1343.49/Arg1353.50 and Glu2476.30 and allows binding of the G protein peptide in a position that is occupied by TM6 in the ground state. Thus, rotation of TM6 and displacement of Trp2656.48 results in a hydrogen-bonding network connecting residues from the protein interior in contact with retinal to those at the cytoplasmic surface critical to activation of the G protein. The hydrogen-bonding network is further extended towards the GαCT peptide by water13 bridging Tyr3067.53 and Arg1353.50 to Asn3108.47 in the TM7-H8 junction (Figure 3). Asn3108.47 in turn interacts with water9, which forms hydrogen bonds to Asn732.40 of the receptor and the backbone carbonyl of Asp346 in the GαCT peptide. Asp346 of the peptide appears to be a key residue for binding as the backbone carbonyl is linked to Thr702.37 of cytoplasmic loop1 through water8, and the side chain is linked to Asn343 of the peptide and Arg1473.62 of the receptor through water5.
This newly recognized extension of the water mediated hydrogen-bonding network provides a beautiful structural context (Figure 4) for interpretation of results from several biochemical studies. First, mutations of L792.46, Glu1343.49, Arg1353.50 and Met2576.40 are known to strongly favour the meta-II state or constitutively activate rhodopsin. Second, the active-state reorganization of the cytoplasmic ends of TM3, TM6 and TM7 connecting three of the most conserved sequence motifs in GPCRs is expected to be restrained by a salt bridge between Glu1133.28 and Lys2967.43; mutation of either residue leads to constitutive activation6. Third, the rhodopsin double mutant Y306C/F313C is unable to form meta-II under oxidizing conditions but does so under reducing conditions, albeit with severely reduced ability to activate the G protein28. Finally, mutation of Tyr2275.58 in the beta1 adrenergic receptor (Tyr2235.58 in rhodopsin) to Ala leads to strong stabilization of the antagonist binding conformation, an effect that has been attributed to an impaired ability to form the inherently less stable active conformation29.
Figure 4. Activation of rhodopsin by the agonist all-trans-retinal.
The protein backbone of E113Q/GαCT is shown in cyan with predominant conformational changes in TM5 and TM6 (RMSD of Cα atoms with respect to 1GZM > 3.5Å) in blue. The key regions involved in rhodopsin activation and discussed in the text are highlighted. The side-chains of the CWxP motif close to the retinal-binding site are coloured red. Side-chains of the NPxxY motif are coloured, blue and extend the hydrogen-bonding network through the green hydrophobic barrier. Side-chains of the E(D)RY motif, as part of the ionic lock and the G protein-binding site are coloured salmon. The GαCT peptide is shown as an orange ribbon. The engineered disulfide bond in the extracellular domain is well isolated from the structural motifs involved in rhodopsin activation, an explanation for its neutral stabilizing characteristics15.
In summary, we describe here how translocation of the retinal β-ionone ring can lead to the conformational changes that allow rhodopsin to bind its G protein. For the first time we show how an agonist is bound to the active state of a GPCR and how activation is accompanied by a reorganization of hydrogen-bonding networks between some of the most conserved residues among GPCRs. Additional structures of activated GPCRs, and their thorough analysis using structural bioinformatics tools will show to which extent this activation mechanism is conserved throughout this important class of signal transducers comprising more than 1% of the human genome.
Materials and Methods
Preparation of stable cell line
The rhodopsin gene containing the stabilizing N2C/D282C and E113Q counterion mutation was cloned into the pACMVtetO vector for tetracycline-inducible expression in mammalian cells30 using NotI and KpnI restriction sites. HEK293S-GnTI− cells with restricted and homogenous N-glycosylation were stably transfected with this vector as described31. Both vector and cells were a kind gift from Philip J. Reeves, University of Essex, UK.
Large-scale expression in wave bag bioreactors
Cells were expanded as adhesion cultures in DMEM/F12 medium supplemented with FBS (10%), PenStrep (Gibco), Geneticin-G418 (200μg/ml) and blasticidin (5μg/ml). Cells from 5 fully confluent 75cm2 flasks were harvested and further expanded into a 300ml suspension culture in Freestyle Medium (Invitrogen) supplemented with FBS (5%) and PenStrep (Gibco). A wave bioreactor (GE Healthcare) was used to further expand the initial suspension cultures to a volume of 9.5l with a cell density of 2×106 cells per millilitre. Protein expression was induced by 0.5l medium supplemented with tetracycline and sodium butyrate for final concentrations in the wave bag of 2μg/ml and 5mM. Cells were harvested 72h after induction at a density of 4-5×106 cells per millilitre. Cell pellets were washed with PBS buffer containing protease inhibitor cocktail (complete protease inhibitor cocktail tablets, Roche) and stored at −80°C. The modelled N-glycan in the final structure is based on the homogenous glycosylation pattern of the HEK293-GnTI− cell line31 used for expression and has been built as GlcNAc2-Man3 with two disordered mannose sugars. Crystal contacts between two N-glycans and palmitoyl chains that fill the cavity between the two rhodopsin molecules in the crystallographic dimer suggest the homogeneous posttranslational modifications as an important factor in crystal formation.
Purification
Cell pellets were solubilized for 1 hour at 4°C with PBS buffer containing protease inhibitor tablets (complete protease inhibitor cocktail tablets, Roche) and 1.25% DM (β-decyl-maltoside). Nuclei and other unsolubilized material were removed by centrifugation and the supernatant incubated with 1D4 antibody coupled to CnBr activated sepharose (Amersham Biosciences). After 3-4 hours the matrix was washed with PBS pH 7, 0.125% DM. Ground state rhodopsin was reconstituted by adding 11-cis retinal (50μM) to the matrix and overnight incubation at 4°C. All steps involving retinal were performed under dim red light.
The matrix was washed with PBS pH 7.0, 0.125% DM followed by 10mM Hepes pH 7.0, 1% OG (β-octyl-glycoside). The purified protein was eluted in the same buffer supplemented with an elution peptide resembling the C-terminus of rhodopsin (TETSQVAPA, 80μM). The eluate was reduced to 0.5ml by a 50kD cutoff concentrator prior to gel filtration on a Superdex200 column. The gel filtration step was used to exchange the buffer for 10mM Mes pH 5.0, 100mM NaCl, 1% OG which leads to protonation of the Schiff base in the E113Q counterion mutant and shifts the absorption maximum from 382nm to 498nm4,5,15.
Light activation and crystallization
Reconstituted E113Q was concentrated to 5-7.5 mg/ml (Vivaspin, 50kD cutoff concentrator) and mixed with dried brain lipid extract (Avanti Polar Lipids, 1 w/w, 10μM all-trans retinal). The sample was briefly sonicated and incubated for 30 minutes in presence of a 10 fold molar excess of peptide resembling the last eleven amino-acids of the Gα subunit of the G protein carrying the mutation K341L (ILENLKDCGLF, Advanced Biomedical). The receptor was activated with for 5 minutes using a >515nm long pass filter that prevented light exposure of free retinal and meta-II.
Sample was mixed 1:1 with 3.0-3.4 M ammonium sulphate, 100 mM sodium acetate pH 4.5 and crystallized by sitting drop vapour diffusion in the dark. Crystals were harvested under dim-red light and soaked in crystallization buffer containing 10% trehalose prior to freezing in liquid nitrogen.
Data Collection and Structure determination
Diffraction quality of crystals was assessed at several synchrotron X-ray sources (SLS, Villigen, ESRF, Grenoble and DIAMOND, Didcot). The best data with a maximum resolution of 2.9Å was collected at the micro-focus beamline ID23eh-2 (ESRF, Grenoble) by the helical data collection method. Data were integrated using XDS32 and brought onto a common scale using SCALA from the CCP4 program suite33. Dataset statistics are given in supplementary table 2.
Phases were obtained by molecular replacement using the program PHASER37 and the polypeptide of the opsin structures (3CAP2 and 3DQB3) or the ground state rhodopsin structure (1GZM7) as search model. The resulting solution was refined using iterative cycles of model building in COOT34 and refinement (rigid body, energy minimization, simulated annealing, individual B-factor refinement) with the PHENIX program suite35. Ordered water molecules were added to the model based on five criteria. First, clear difference peaks in a 2.5 sigma contoured Fo-Fc electron density map calculated after simulated annealing refinement in which water molecules have been omitted (Figure 3). Second, density above 1 sigma in 2Fo-Fc maps refined including waters. Third, two or more hydrogen bonds to the protein or other water molecules. Fourth, a B-factor cutoff within 20% of the average and finally, interpretable density in maps calculated with both datasets.
Geometric restraints for lipids and heteroatoms were prepared using the PRODRG2 server38 (http://davapc1.bioch.dundee.ac.uk/prodrg/) and Hic-Up database39 (http://xray.bmc.uu.se/hicup/). Coordinates for all-trans and 9-cis retinal were obtained from the Cambridge Small Molecule Database. Retinal geometry restraints used in the refinement were prepared by carefully adjusting torsion angles and planarity restraints in the retinal parameter file distributed as part of the CCP4 program suite. Coordinates and structure factors have been deposited under pdb code 2×72 in the case of light activated E113Q rhodopsin and xxxx of constitutively active opsin.
Supplementary Material
Acknowledgements
We thank Phil Evans, Xavier Deupi and Reiner Vogel for discussions and reading of the manuscript. We thank Rosalie Crouch at the University of South Carolina for the kind gift of 11-cis retinal. Phil J. Reeves at the University of Essex we thank for providing the pACMVtetO vector and the HEK293S-GnTI− cells and for his advice on creating stable cell lines and tetracycline-inducible expression. We also thank staff at the macromolecular crystallography beamlines at the European Synchrotron Radiation Facility (ESRF), the Diamond Light Source and the Swiss Light Source (SLS). The work was financially supported by NIH grant EY007965 (to D.O.), the Human Frontier Science Project (HFSP) program grant RG/0052 (to D.O. and G.S.), the European Commission FP6 specific targeted research project LSH-2003-1.1.0-1 (to G.S.), the Marie Curie Intra European Fellowship MEIF-CT-2006-039171 (to J.S.) and the EMBO long-term fellowship ALTF 198-2006 (to J.S.).
Abbreviations
- GPCR
G protein-coupled receptor
- TM
transmembrane helices
- meta
metarhodopsin
- GαCT
peptide derived from the C terminus of the G protein α subunit
References
- 1.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, et al. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454(7201):183. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 3.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455(7212):497. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 4.Zhukovsky EA, Oprian DD. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989;246(4932):928. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- 5.Sakmar TP, Franke RR, Khorana HG. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989;86(21):8309. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD. Constitutively active mutants of rhodopsin. Neuron. 1992;9(4):719. doi: 10.1016/0896-6273(92)90034-b. [DOI] [PubMed] [Google Scholar]
- 7.Li J, et al. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343(5):1409. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 8.Farrens DL, et al. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274(5288):768. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 9.Unger VM, Hargrave PA, Baldwin JM, Schertler GF. Arrangement of rhodopsin transmembrane alpha-helices. Nature. 1997;389(6647):203. doi: 10.1038/38316. [DOI] [PubMed] [Google Scholar]; Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 10.Okada T, et al. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol. 2004;342(2):571. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Nakamichi H, Buss V, Okada T. Photoisomerization mechanism of rhodopsin and 9-cis-rhodopsin revealed by x-ray crystallography. Biophys J. 2007;92(12):L106. doi: 10.1529/biophysj.107.108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Kouyama T. Crystal structure of squid rhodopsin. Nature. 2008;453(7193):363. doi: 10.1038/nature06925. [DOI] [PubMed] [Google Scholar]
- 13.Ruprecht JJ, et al. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 2004;23(18):3609. doi: 10.1038/sj.emboj.7600374. [DOI] [PMC free article] [PubMed] [Google Scholar]; Salom D, et al. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci U S A. 2006;103(44):16123. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nakamichi H, Okada T. Local peptide movement in the photoreaction intermediate of rhodopsin. Proc Natl Acad Sci U S A. 2006;103(34):12729. doi: 10.1073/pnas.0601765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamm HE, et al. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988;241(4867):832. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]; Vogel R, Siebert F. Conformations of the active and inactive states of opsin. J Biol Chem. 2001;276(42):38487. doi: 10.1074/jbc.M105423200. [DOI] [PubMed] [Google Scholar]
- 15.Standfuss J, Zaitseva E, Mahalingam M, Vogel R. Structural impact of the E113Q counterion mutation on the activation and deactivation pathways of the G protein-coupled receptor rhodopsin. J Mol Biol. 2008;380(1):145. doi: 10.1016/j.jmb.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen GB, Oprian DD, Robinson PR. Mechanism of activation and inactivation of opsin: role of Glu113 and Lys296. Biochemistry. 1992;31(50):12592. doi: 10.1021/bi00165a008. [DOI] [PubMed] [Google Scholar]
- 17.Xie G, Gross AK, Oprian DD. An opsin mutant with increased thermal stability. Biochemistry. 2003;42(7):1995. doi: 10.1021/bi020611z. [DOI] [PubMed] [Google Scholar]
- 18.Standfuss J, et al. Crystal structure of a thermally stable rhodopsin mutant. J Mol Biol. 2007;372(5):1179. doi: 10.1016/j.jmb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin EL, Rens-Domiano S, Schatz PJ, Hamm HE. Potent peptide analogues of a G protein receptor-binding region obtained with a combinatorial library. J Biol Chem. 1996;271(1):361. doi: 10.1074/jbc.271.1.361. [DOI] [PubMed] [Google Scholar]
- 20.Altenbach C, et al. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc Natl Acad Sci U S A. 2008;105(21):7439. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groenendijk GW, Jacobs CW, Bonting SL, Daemen FJ. Dark isomerization of retinals in the presence of phosphatidylethanolamine. Eur J Biochem. 1980;106(1):119. doi: 10.1111/j.1432-1033.1980.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 22.Kefalov VJ, Crouch RK, Cornwall MC. Role of noncovalent binding of 11-cis-retinal to opsin in dark adaptation of rod and cone photoreceptors. Neuron. 2001;29(3):749. doi: 10.1016/s0896-6273(01)00249-5. [DOI] [PubMed] [Google Scholar]
- 23.Kono M, Goletz PW, Crouch RK. 11-cis- and all-trans-retinols can activate rod opsin: rational design of the visual cycle. Biochemistry. 2008;47(28):7567. doi: 10.1021/bi800357b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahuja S, et al. Location of the retinal chromophore in the activated state of rhodopsin*. J Biol Chem. 2009;284(15):10190. doi: 10.1074/jbc.M805725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, et al. Beta2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J Biol Chem. 2002;277(43):40989. doi: 10.1074/jbc.M206801200. [DOI] [PubMed] [Google Scholar]; Schwartz TW, et al. Molecular mechanism of 7TM receptor activation--a global toggle switch model. Annu Rev Pharmacol Toxicol. 46(2006):481. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- 26.Pardo L, et al. The role of internal water molecules in the structure and function of the rhodopsin family of G protein-coupled receptors. Chembiochem. 2007;8(1):19. doi: 10.1002/cbic.200600429. [DOI] [PubMed] [Google Scholar]
- 27.Vogel R, et al. Functional role of the “ionic lock”--an interhelical hydrogen-bond network in family A heptahelical receptors. J Mol Biol. 2008;380(4):648. doi: 10.1016/j.jmb.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Fritze O, et al. Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc Natl Acad Sci U S A. 2003;100(5):2290. doi: 10.1073/pnas.0435715100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tate CG, Schertler GF. Engineering G protein-coupled receptors to facilitate their structure determination. Curr Opin Struct Biol. 2009;19(4):386. doi: 10.1016/j.sbi.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Reeves PJ, Kim JM, Khorana HG. Structure and function in rhodopsin: a tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc Natl Acad Sci U S A. 2002;99(21):13413. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A. 2002;99(21):13419. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 1993;26:795. [Google Scholar]
- 33.The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 5):760. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 34.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Adams PD, et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 11):1948. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 36.Alexandrov AI, et al. Microscale fluorescent thermal stability assay for membrane proteins. Structure. 2008;16(3):351. doi: 10.1016/j.str.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 37.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 4):458. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 38.Schuttelkopf AW, van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 8):1355. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 39.Kleywegt GJ. Crystallographic refinement of ligand complexes. Acta Crystallogr D Biol Crystallogr. 2007;63(Pt 1):94. doi: 10.1107/S0907444906022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.