Abstract
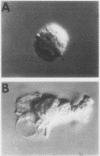
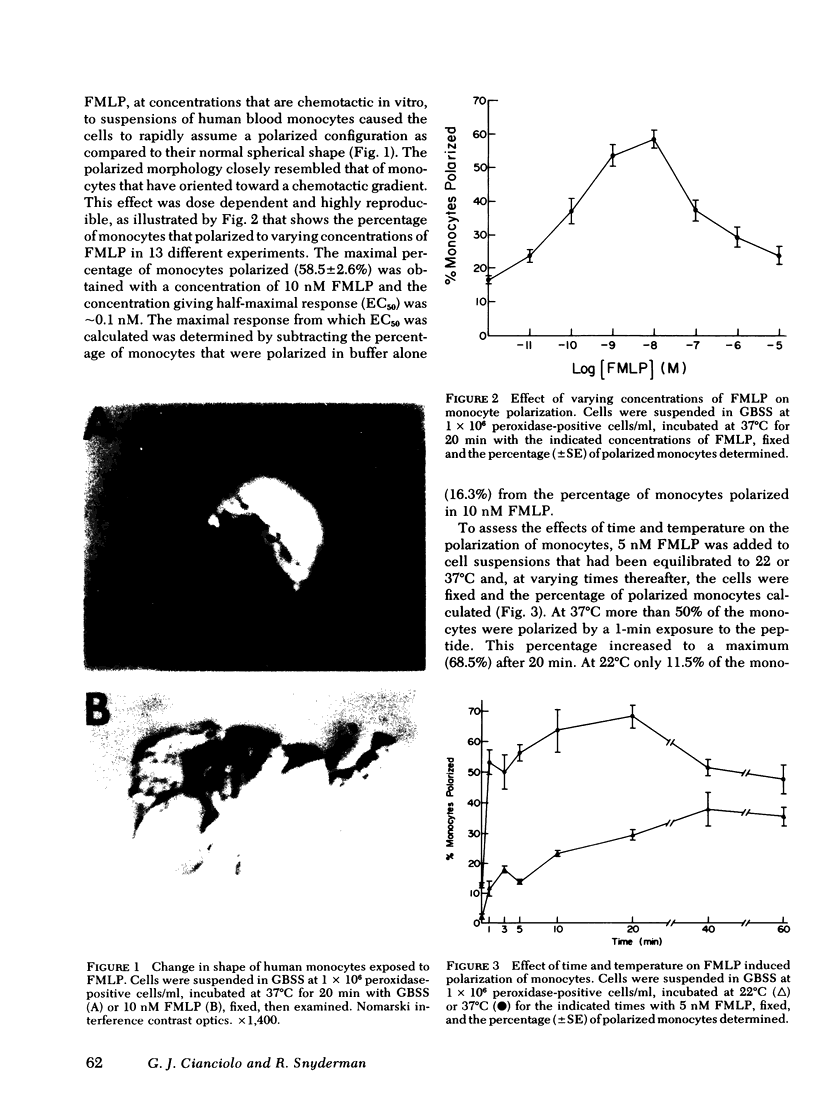
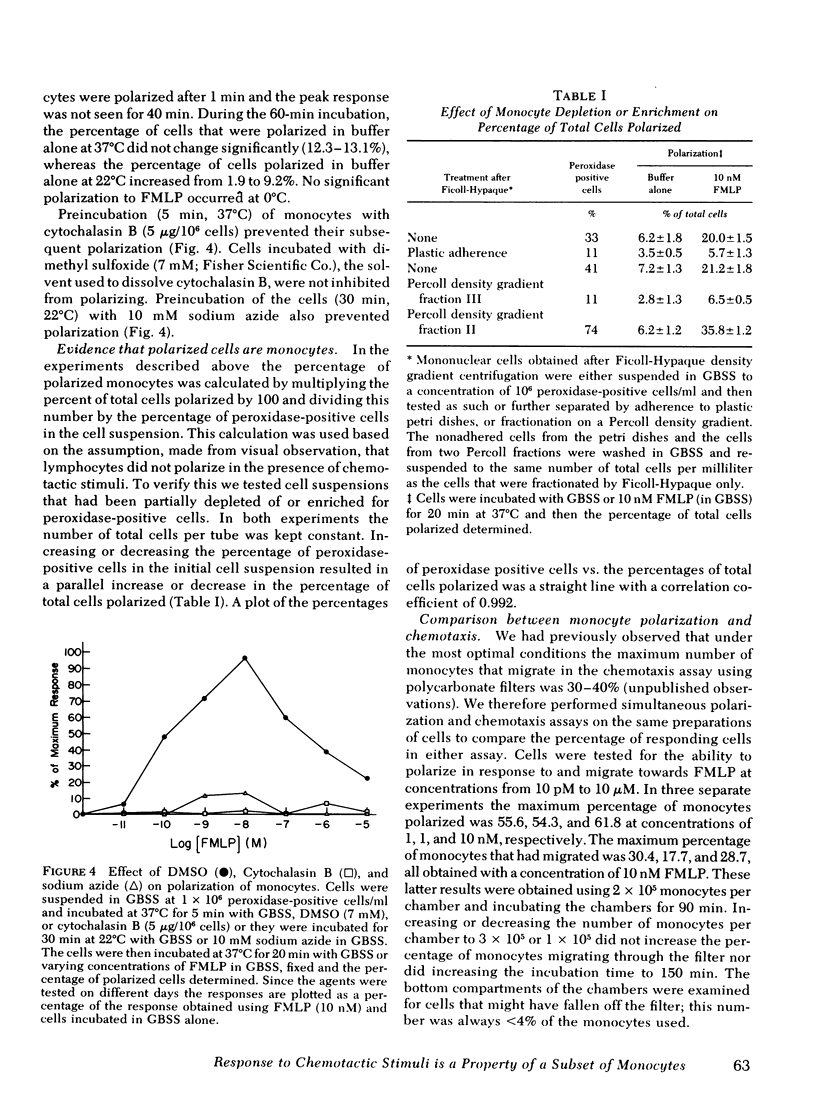
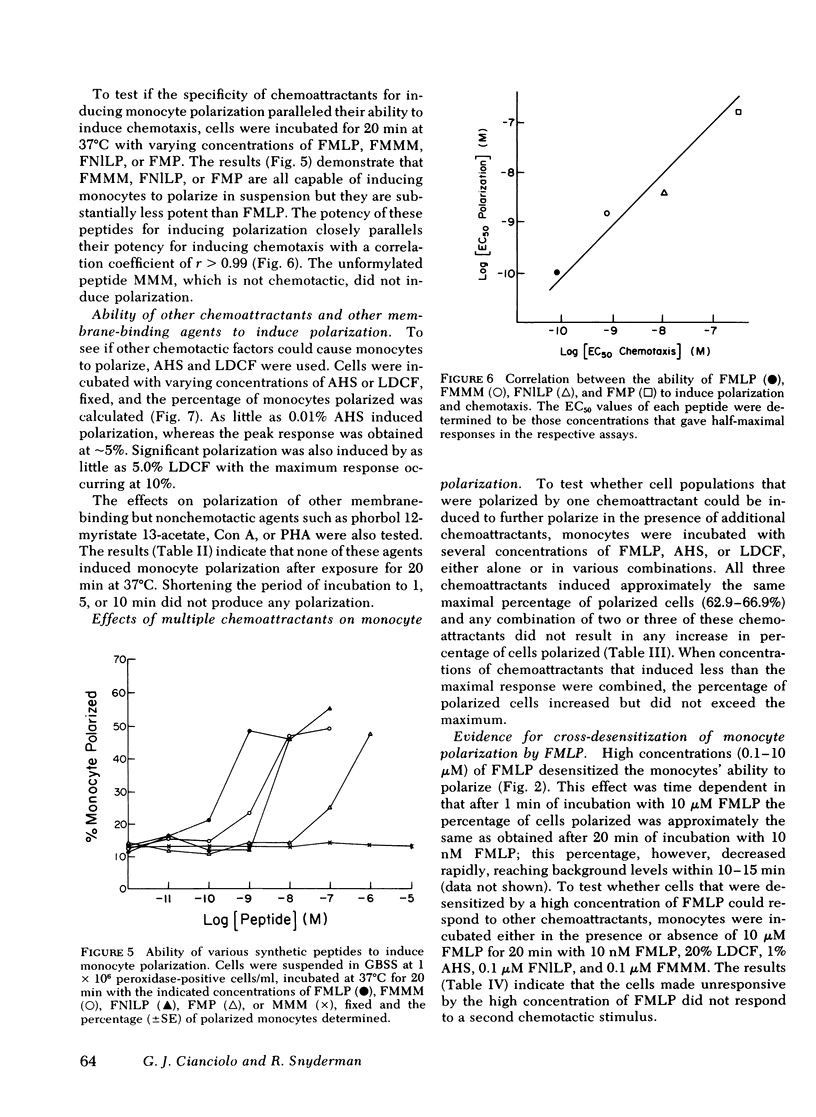
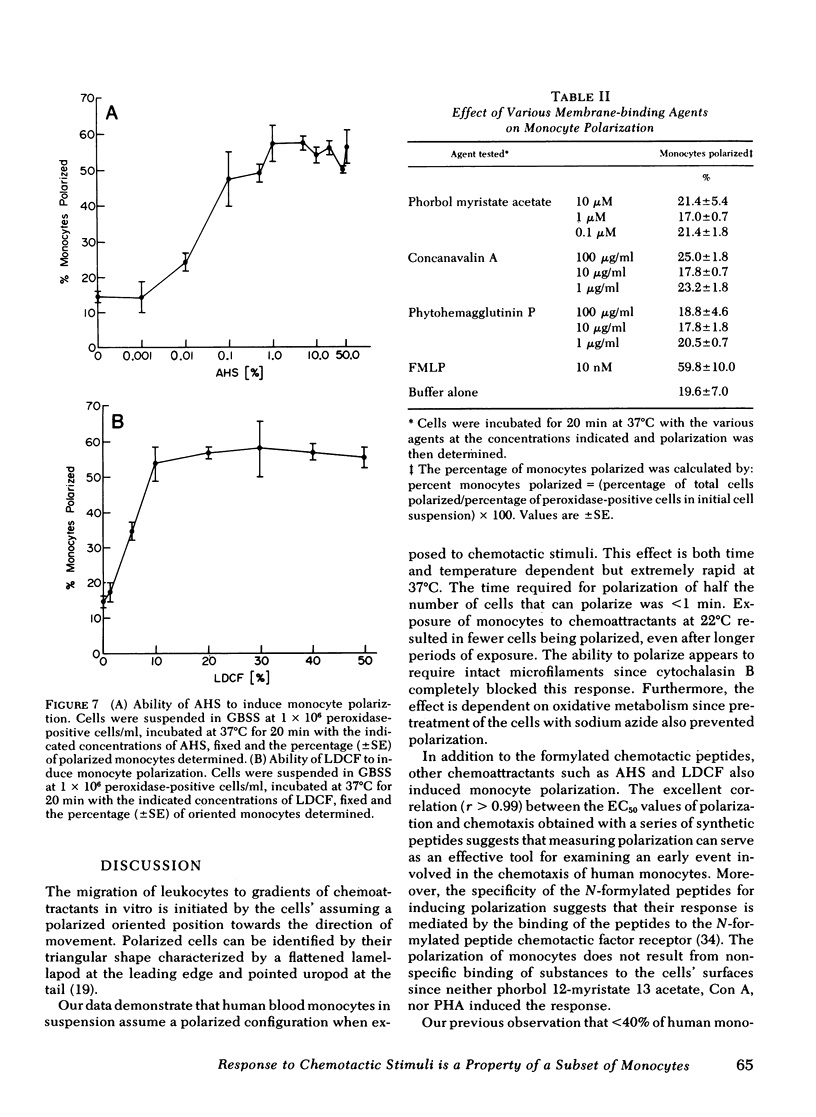
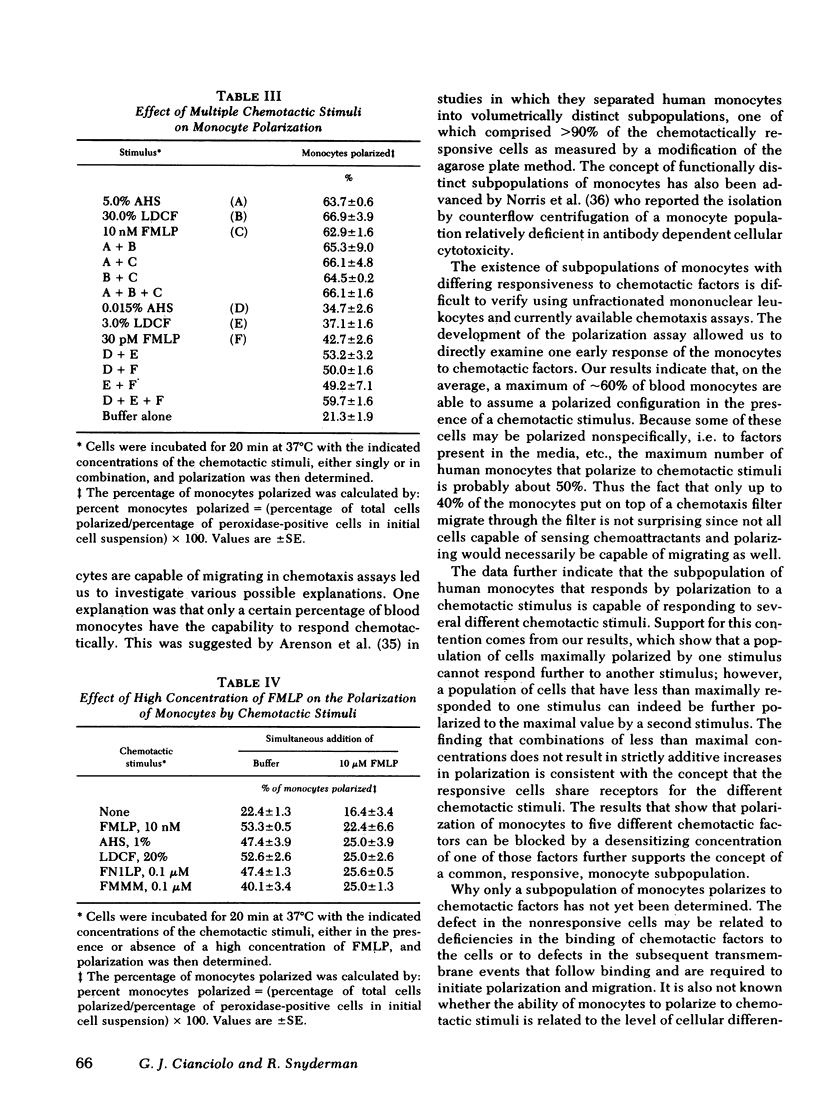
The chemotactic migration of leukocytes is preceded by an alteration in the cells' shape from round to a characteristic polar configuration. We have developed an assay that shows that human monocytes, when exposed to chemoattractant in suspension, assume this polarized shape. The three types of chemo-attractants studied, a chemotactic lymphokine, complement-activated serum, and the N-formylated oligopeptides, all induced polarization in a time, temperature, and dose-dependent fashion. Nonchemotactic agents such as mitogens or phorbol myristate acetate did not induce polarization. At 37°C, polarization was rapid (<1 min) and was inhibitable by cytochalasin B, sodium azide, or low temperature. A series of N-formylated oligopeptides were studied and their activity in inducing polarization correlated closely (r > 0.99) with their chemotactic activity. Of the entire population of circulating monocytes there is a subpopulation of cells that is capable of polarizing in response to chemotactic stimuli. The maximum percentage of monocytes which polarized to any chemotactic factor was ∼60%. Furthermore, the combination of several chemotactic factors could not increase the percentage of polarized monocytes above the maximum obtained with an optimal dose of any single chemoattractant. The data also demonstrate that high doses of a chemoattractant can induce a state of cross-desensitization in monocytes that blocks the response of the cells to other types of chemotactic factors. These results support the concept that the monocytes that do respond to chemotactic stimuli are capable of responding to any of several attractants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. C., Snyderman R., Blaese R. M. Abnormalities of chemotactic lymphokine synthesis and mononuclear leukocyte chemotaxis in Wiskott-Aldrich syndrome. J Clin Invest. 1974 Aug;54(2):486–493. doi: 10.1172/JCI107784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman L. C., Snyderman R., Oppenheim J. J., Mergenhagen S. E. A human mononuclear leukocyte chemotactic factor: characterization, specificity and kinetics of production by homologous leukocytes. J Immunol. 1973 Mar;110(3):801–810. [PubMed] [Google Scholar]
- Arenson E. B., Jr, Epstein M. B., Seeger R. C. Volumetric and functional heterogeneity of human monocytes. J Clin Invest. 1980 Mar;65(3):613–618. doi: 10.1172/JCI109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Boetcher D. A., Leonard E. J. Abnormal monocyte chemotactic response in cancer patients. J Natl Cancer Inst. 1974 Apr;52(4):1091–1099. doi: 10.1093/jnci/52.4.1091. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Demarest G. B., Hudson L. D., Altman L. C. Impaired alveolar macrophage chemotaxis in patients with acute smoke inhalation. Am Rev Respir Dis. 1979 Feb;119(2):279–286. doi: 10.1164/arrd.1979.119.2.279. [DOI] [PubMed] [Google Scholar]
- Furukawa C. T., Altman L. C. Defective monocyte and polymorphonuclear leukocyte chemotaxis in atopic disease. J Allergy Clin Immunol. 1978 May;61(5):288–293. doi: 10.1016/0091-6749(78)90049-0. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., Gallin J. I. Interaction of chemotactic factors with human macrophages. Induction of transmembrane potential changes. J Cell Biol. 1977 Oct;75(1):277–289. doi: 10.1083/jcb.75.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Klimerman J. A., Padgett G. A., Wolff S. M. Defective mononuclear leukocyte chemotaxis in the Chediak-Higashi syndrome of humans, mink, and cattle. Blood. 1975 Jun;45(6):863–870. [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A method for assessing the in vitro chemotactic response of neutrophils utilizing 51cr-labeled human leukocytes. Immunol Commun. 1972;1(5):421–430. doi: 10.3109/08820137209022954. [DOI] [PubMed] [Google Scholar]
- Hausman M. S., Brosman S., Snyderman R., Mickey M. R., Fahey J. Defective monocyte function in patients with genitourinary carcinoma. J Natl Cancer Inst. 1975 Nov;55(5):1047–1054. doi: 10.1093/jnci/55.5.1047. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- Norris D. A., Morris R. M., Sanderson R. J., Kohler P. F. Isolation of functional subsets of human peripheral blood monocytes. J Immunol. 1979 Jul;123(1):166–172. [PubMed] [Google Scholar]
- Oliver J. M. Cell biology of leukocyte abnormalities--membrane and cytoskeletal function in normal and defective cells. A review. Am J Pathol. 1978 Oct;93(1):221–270. [PMC free article] [PubMed] [Google Scholar]
- Rubin R. H., Cosimi A. B., Goetzl E. J. Defective human mononuclear leukocyte chemotaxis as an index of host resistance to malignant melanoma. Clin Immunol Immunopathol. 1976 Nov;6(3):376–388. doi: 10.1016/0090-1229(76)90091-x. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. Motility and adhesiveness in human neutrophils. Redistribution of chemotactic factor-induced adhesion sites. J Clin Invest. 1980 Apr;65(4):804–812. doi: 10.1172/JCI109731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Frankel A., Blaese R. M. Defective mononuclear leukocyte chemotaxis: a previously unrecognized immune dysfunction. Studies in a patient with chronic mucocutaneous candidiasis. Ann Intern Med. 1973 Apr;78(4):509–513. doi: 10.7326/0003-4819-78-4-509. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Snyderman R., Fudman E. J. Demonstration of a chemotactic factor receptor on macrophages. J Immunol. 1980 Jun;124(6):2754–2757. [PubMed] [Google Scholar]
- Snyderman R., Gewurz H., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide. Generation of a factor chemotactic for polymorphonuclear leukocytes. J Exp Med. 1968 Aug 1;128(2):259–275. doi: 10.1084/jem.128.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Meadows L., Holder W., Wells S., Jr Abnormal monocyte chemotaxis in patients with breast cancer: evidence for a tumor-mediated effect. J Natl Cancer Inst. 1978 Apr;60(4):737–740. doi: 10.1093/jnci/60.4.737. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Seigler H. F., Meadows L. Abnormalitieis of monocyte chemotaxis in patients with melanoma: effects of immunotherapy and tumor removal. J Natl Cancer Inst. 1977 Jan;58(1):37–41. doi: 10.1093/jnci/58.1.37. [DOI] [PubMed] [Google Scholar]
- Spilberg I., Mehta J. Demonstration of a specific neutrophil receptor for a cell-derived chemotactic factor. J Clin Invest. 1979 Jan;63(1):85–88. doi: 10.1172/JCI109282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]