Abstract
Zinc is an essential trace element, whose importance to the function of the central nervous system (CNS) is increasingly being appreciated. Alterations in zinc dyshomeostasis has been suggested as a key factor in the development of several neuropsychiatric disorders. In the CNS, zinc occurs in two forms: the first being tightly bound to proteins and, secondly, the free, cytoplasmic, or extracellular form found in presynaptic vesicles. Under normal conditions, zinc released from the synaptic vesicles modulates both ionotropic and metabotropic post-synaptic receptors. While under clinical conditions such as traumatic brain injury, stroke or epilepsy, the excess influx of zinc into neurons has been found to result in neurotoxicity and damage to postsynaptic neurons. On the other hand, a growing body of evidence suggests that a deficiency, rather than an excess, of zinc leads to an increased risk for the development of neurological disorders. Indeed, zinc deficiency has been shown to affect neurogenesis and increase neuronal apoptosis, which can lead to learning and memory deficits. Altered zinc homeostasis is also suggested as a risk factor for depression, Alzheimer's disease (AD), aging, and other neurodegenerative disorders. Under normal CNS physiology, homeostatic controls are put in place to avoid the accumulation of excess zinc or its deficiency. This cellular zinc homeostasis results from the actions of a coordinated regulation effected by different proteins involved in the uptake, excretion and intracellular storage/trafficking of zinc. These proteins include membranous transporters (ZnT and Zip) and metallothioneins (MT) which control intracellular zinc levels. Interestingly, alterations in ZnT and MT have been recently reported in both aging and AD. This paper provides an overview of both clinical and experimental evidence that implicates a dysfunction in zinc homeostasis in the pathophysiology of depression, AD, and aging.
Keywords: zinc, zinc transporters, metallothioneins, depression, aging, Alzheimer's disease, neurodegeneration
Introduction
Knowledge about zinc has rapidly evolved over the years with the last two decades having brought, interesting new insights about the role of zinc in molecular and cellular processes as well as health and disease. Zinc is one of the most prevalent trace elements in the human body. It is a key structural component of a great number of proteins, and a co-factor of more than 300 enzymes that regulate a variety of cellular processes and cellular signaling pathways essential for both brain and systemic physiology (Takeda, 2000). In the brain, zinc is also present in its free ionic form (Zn2+) within synaptic vesicles, mostly at the glutamatergic terminals (Frederickson et al., 2000; Paoletti et al., 2009; Sensi et al., 2011). Synaptically released zinc, during neuronal activity, affects the activity of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptors, GABAA and glycine inotropic receptors (Smart et al., 2004). It has also been found to activate a specific metabotropic Zn2+-sensing receptor GPR39 (Besser et al., 2009). In physiological concentrations zinc exhibits neuroprotective activity, although high concentrations of zinc are neurotoxic (Choi et al., 1988; Perry et al., 1997; Cote et al., 2005; Plum et al., 2010). Therefore, an imbalance of zinc homeostasis will have complex implications in a number of brain processes then leading to the onset of chronic pathologies such as depression, schizophrenia, Alzheimer's disease (AD), Parkinson's disease, aging, or amyotrophic lateral sclerosis (ALS). Given the complex nature of zinc homeostasis in the brain, it is not surprising that several different groups of proteins are involved in managing its cellular levels. The first group consists of are membranous transporters (ZnTs) mediating the zinc efflux from cells or influx into cellular compartments or organelles (Huang and Tepaamorndech, 2013). The second group is members of the Zip family (zinc-regulated and iron-regulated transporter proteins) that promote zinc transport from the extracellular space or from intracellular vesicles to the cytoplasm (Cousins et al., 2006). So far, 10 members of the ZnT and 14 members of the ZIP protein families have been identified (Lichten and Cousins, 2009). The third group of these zinc homeostasis-regulating proteins is metallothioneins (MTs)—a group of low-molecular-weight metal-binding proteins that have a high affinity for zinc (Krezel et al., 2007). Four MT isoforms have been described so far; MT-I and MT-II are expressed in many tissues; MT-IV is exclusively expressed in some stratified squamous epithelia (Quaife et al., 1994) and MT-III. MT-III is a brain-specific member of the MTs protein family, found exclusively in neurons, and localized predominantly in neurons that sequester zinc in synaptic vesicles (Masters et al., 1994). MT-III mRNA has been found in the cortex, hippocampus, amygdala, and cerebellum (Masters et al., 1994). The role of MTs is to buffer cytoplasmic zinc following its influx into the cytoplasm, and so far it seems that temporary cellular zinc storage is the exclusive function of MTs (Krezel et al., 2007). MTs play a crucial protective role (due to their redox properties) in the presence of radiations, heavy toxic metals, lipid peroxidation, or reactive oxygen species (ROS) (Sato and Kondoh, 2002).
The understanding of the physiological functions of zinc transporters and MTs has grown dramatically during recent decade and their involvement in the pathogenesis of neurodegenerative diseases more apparent than previously. This review focuses on depression, AD, and age related pathologies, in which a specific role for zinc dyshomeostasis has been reported. Also, disease associated alterations in proteins responsible for zinc transport and zinc storage will be discussed.
The role of zinc in modulating synaptic function
There are several important aspects associated with zinc depletion; supplementation and delivery of zinc to the brain. Experimentally, zinc deficiency is reached by partaking in a diet that contains 0.5 mgZn/kg–6 mgZn/kg for at least 2–4 weeks (Tamano et al., 2009; Mlyniec et al., 2012). Zinc overdoses can be obtained at 100 mgZn/kg–180 mgZn/kg (Yang et al., 2013). The most common way for assessing the zinc level is by measuring the serum or plasma zinc. Unfortunately, elevated or lowered serum zinc does not correspond with the elevated or lowered brain zinc total. This suggests that the brain zinc total is strictly controlled and may not be easily influenced by peripheral zinc level. The other problem is the lack of sensitive methods to measure alterations in the extra or intracellular zinc levels. Available data indicates that the hippocampus seems to be the most responsive both to the deficiency as well as an overdose of zinc (Takeda et al., 2005; Suh et al., 2009; Yang et al., 2013). Because the hippocampus is the region of the brain which plays a critical role in memory, learning and neurogenesis, the impact of zinc deficiency or zinc supplementation on these processes will be critical. Indeed, it was found that a zinc deficient diet, decreases the number of progenitor cells and immature neurons in the dentate gyrus (DG) in rodents and that reversal to a normal diet containing zinc restored a number of these cells (Gao et al., 2009; Suh et al., 2009). Reduced progenitor cells were also found after zinc chelator treatment and in ZnT3 KO mice (a lack of zinc in the synaptic vesicles) (Suh et al., 2009). A growing body of evidence indicates that dietary zinc deficiency influences hippocampal learning and memory in an age-dependent manner. It was found that a decrease in dietary zinc during early development produces an irreversible deficit of learning and memory, while zinc deficient induced impairments in young adult rats can be reversed by feeding them with an adequate diet (Takeda, 2000; Keller et al., 2001). Recent data published by Gao et al. (2009, 2011) showed that the zinc-deficiency induced hippocampal learning and memory impairments is in part due to the disruption of the calmodulin (CaM), CaM-dependent protein kinase II (CaMKII), and cAMP-responsive element binding protein (CREB) signaling pathway. As was mentioned above zinc was found to modulate neural transmission through the GPR39 Zn2+-sensing receptor. Recent studies showed a significant reduction in the GPR39 protein level in the frontal cortex in mice receiving the zinc deficient diet (Mlyniec et al., 2013). This study provides evidence that the GPR39 Zn2+-sensing receptor may be involved in the pathomechanism of depression. This hypothesis was further supported by data indicating the up-regulation of the GPR39 receptor after chronic antidepressant treatment (Mlyniec and Nowak, 2013).
The other mechanism by which zinc can modulate synaptic functions is the transactivation of the tropomyosin-related kinase B (TrkB) receptor and activation of brain-derived neurotrophic factor BDNF signaling in a neurotrophic—independent manner (Huang et al., 2008). Zinc can affect BDNF signaling also by promoting the maturation of pro-BDNF to BDNF throughout the activation of metalloproteinases (MMPs) (Hwang et al., 2005).
Zinc also appears to have an effect of oxidative stress. It was found that both high and extremely low concentrations of zinc are associated with increased oxidative and nitrosative stress [by increasing the expression of neuronal nitric oxide synthase (nNOS) and NADPH oxidase] (Noh and Koh, 2000; Aimo et al., 2010), however, intermediate concentrations was found to be neuroprotective (Aimo et al., 2010). This demonstrates once again the importance of zinc homeostasis in normal brain function. Although, the effect of zinc deficiency on the brain zinc homeostasis and learning and memory has been well studied, the effect of a zinc overdose on these processes is poorly described and the data are rather conflicting. First, the effect of zinc supplementation on learning and memory impairments is dose dependent. Generally, zinc supplementation in a low dosage seems to improve the performance of animals in spatial memory tasks (Piechal et al., 2012) or the contextual discrimination task (Yang et al., 2013). However, memory deficits in rats after low dose of zinc supplementation were also observed (Flinn et al., 2005; Railey et al., 2010). Interestingly Yang et al. (2013) reported that zinc supplementation in high doses induce a dramatic decrease in hippocampal zinc levels, especially in the CA3 and DG, and impaired learning and memory due to a decreased availability of synaptic zinc and BDNF deficits.
Zinc and depression
Depression is a common mental disorder associated with functional impairment, significant disability, morbidity and mortality. Despite the extensive research that has so far been carried out on depression, its pathophysiology is still poorly understood. One of the many hypotheses proposed for depressive disorder indicates that depression is characterized by an enhanced neurodegeneration and decreased neurogenesis (Maes et al., 2009). On the other hand, there is increasing evidence linking depression or depression-related changes in brain function or cognitive performance to zinc ion availability.
Zinc levels in depression (Table 1)
Clinical studies demonstrate significantly lower serum zinc levels in patients suffering from major depression or unipolar depression than that in non-depressed patients (McLoughlin and Hodge, 1990; Maes et al., 1994, 1997; Nowak et al., 1999). In some patients, a negative correlation between the serum zinc level and severity of depression was found (Maes et al., 1994; Nowak et al., 1999). A lower serum zinc level was also found to accompany antepartum and postpartum depression. In this study the level of zinc was also negatively correlated with the severity of depressive symptoms (Wojcik et al., 2006). Low serum zinc levels have also been noted in depressed patients with end-stage renal disease undergoing hemodialysis (Roozbeh et al., 2011). Moreover, treatment-resistant depressed patients have been shown to exhibit much lower serum zinc concentrations than their non-treatment resistant depressed counterparts (Siwek et al., 2010). Thus far, only two studies have reported no differences in the zinc level between depressed and non-depressed patients (Narang et al., 1991; Irmisch et al., 2010). The paper published by Irmisch et al. (2010), however indicated that zinc concentrations might differ dependent on comorbid disorders and severity of depression. Similarly, Narang et al. (1991) reported no significant difference between control and depressed patients, however, they found that the values were significantly higher in recovered patients compared to patients with depression. Although these results do not confirm the general hypothesis of a lack of zinc in depressive disorders, however favor the existence of correlation between severity of depression or status of patients and zinc concentration.
Zinc deficiency and depression
There is a paucity of clinical studies that have examined the relationship between dietary zinc intake and depressive symptoms (Table 1). One study, carried out by Amani et al. (2010) showed that both daily zinc intake and the serum zinc levels in young depressed women were about two thirds of that observed in healthy volunteers. Moreover, an inverse correlation was found between serum zinc concentrations and depression scale scores. In another study, conducted among a group of pregnant women, the relationship between dietary zinc intake, psychosocial stress and sociodemographic factors and depression was examined. Analysis of the results showed that lower zinc intake, higher stress and social disadvantage were associated with the occurrence of depressive symptoms, which were in turn attenuated by higher zinc intake (Roy et al., 2010). Data coming from animal studies further support the hypothesis that a deficiency in zinc can lead to the induction of depressive behavioral symptoms (Table 1). Studies have shown that zinc-deficient mice exhibit an increased immobility time in the forced swim test (FST) and tail suspension test (TST) (Whittle et al., 2009; Mlyniec and Nowak, 2012; Mlyniec et al., 2012). Pro-depressive-like behavior (increased immobility in the FST or anhedonia) was also found in rats subjected to zinc-deprivation (Tassabehji et al., 2008; Tamano et al., 2009; Watanabe et al., 2010)
Table 1.
Summary of the main clinical and preclinical findings supporting the involvement of zinc in depression.
| Serum/plasma zinc status—human data | References |
|---|---|
| ↓ Major depressed patients; negative correlation between the serum zinc and severity of depression | Maes et al., 1994; Nowak et al., 1999 |
| ↓ Depressed patients vs. control | Siwek et al., 2010 |
| ↓ Patients with affective disorders | McLoughlin and Hodge, 1990 |
| ↓ Women with antepartum and postpartum depressive symptoms | Wojcik et al., 2006 |
| ↓ Depressed patients with end-stage renal disease undergoing hemodialysis | Roozbeh et al., 2011 |
| ↔ Depressed patients; zinc concentrations differ dependent on comorbid disorders and severity of depression | Irmisch et al., 2010 |
| ↔ Depressed patients; significantly higher zinc level in recovered patients compared to patients with depression | Narang et al., 1991 |
| Effect of zinc deficiency—human study | |
| Correlation between dietary zinc intake and the serum zinc concentrations; the inverse correlation between serum zinc levels and depression scales | Amani et al., 2010 |
| Zinc intake moderates the association between stress and depressive symptoms | Roy et al., 2010 |
| Effect of zinc deficiency—animal study | |
| ↑ Immobility time in FST in rats | Tassabehji et al., 2008; Tamano et al., 2009; Watanabe et al., 2010 |
| ↑ Immobility time in FST in mice | Whittle et al., 2009; Mlyniec et al., 2012 |
| ↑ Immobility time in TST in mice | Whittle et al., 2009; Mlyniec and Nowak, 2012 |
| ↓ Saccharin preference in rat | Tassabehji et al., 2008 |
| Effect of zinc treatment/supplementation in depression—clinical trials | |
| Zinc supplementation (25 mg/12 weeks) significantly reduced scores in HDRS and BDI when compared with placebo treatment | Nowak et al., 2003a |
| Zinc supplementation (25 mg/12 weeks) augments the efficacy and speed of onset of therapeutic response to imipramine treatment, particularly in patients previously non-responsive to antidepressant pharmacotherapies | Siwek et al., 2009 |
| Zinc supplementation (25 mg/12 weeks) significantly reduced HDRS compared to placebo | Ranjbar et al., 2013 |
| Women who took multivitamins and zinc (7 mg/10 weeks) showed a significant reduction in anger-hostility score and depression-dejection score in the Profile of Moods State (POMS) | Sawada and Yokoi, 2010 |
| Zinc deficiency changes the brain function but zinc and macronutrient treatment improves altered brain functions | Sandstead, 2012 |
| Zinc supplementation (10 mg/6 months) did not induce differences in mental health outcomes between zinc and placebo groups, however, increases in serum zinc concentrations were associated with decreases in internalizing symptoms (depression and anxiety) | DiGirolamo et al., 2010 |
| No effect of zinc supplementation on the improvement of depressive symptoms | Nguyen et al., 2009 |
| Effect of zinc treatment—animal study | |
| ↓ In immobility time in both FST and TST | Kroczka et al., 2000, 2001; Nowak et al., 2003b; Rosa et al., 2003; Cunha et al., 2008; Franco et al., 2008 |
| ↓ Reduction in the number of trials in the passive-avoidance test in OB model; ↓ OB- induced hyperactivity in open field test in OB model | Nowak et al., 2003b |
| Zinc reversed the CMS-induced reduction in the consumption of sucrose | Sowa-Kucma et al., 2008 |
| Zinc prevented deficits in the fighting behavior in CUS model | Cieslik et al., 2007 |
| Zinc intensifies the effects of standard antidepressants in FST, TST, and CUS | Szewczyk et al., 2002, 2009; Rosa et al., 2003; Cieslik et al., 2007; Cunha et al., 2008 |
Zinc treatment/supplementation in depression
Some clinical studies have shown the beneficial effect of zinc supplementation in the treatment of depression (Table 1). One such study by Nowak et al. (2003a), was conducted in depressed patients, treated with tricyclic antidepressants and selective serotonin reuptake inhibitors supplemented with zinc or a placebo. Analysis of the Hamilton Depression Rating Scale (HDRS) and Beck Depression Inventory (BDI) scores revealed that patients who received the zinc supplementation of antidepressant treatment displayed much lower scores than patients treated with placebos and antidepressants. A beneficial effect of zinc as an adjunct agent was also found in treatment-resistant patients (Siwek et al., 2009). In this placebo-controlled, double blind study patients were randomized into two groups: the first were treated with imipramine and received one daily placebo and the second were treated with imipramine supplemented with zinc. It was found that zinc supplementation significantly reduced the depression scores [measured by Clinical Global Impression (CGI); Montgomery-Asberg Depression Rating Scale (MADRS); BDI and HDRS] and facilitated the effect of the treatment in antidepressant treatment resistant patients. No significant differences in the CGI, MADRS, BDI, and HDRS scores were demonstrated between zinc and placebo- supplemented antidepressant treatment non-resistant patients. The benefit of zinc supplementation in patients with major depression has been recently reported by Ranjbar et al. (2013). This randomized, double-blind, placebo-controlled trial is the next clinical study indicated that zinc supplementation in conjunction with antidepressants might be beneficial for reducing depressive symptoms.
The other study published by Sawada and Yokoi (2010) showed that young women taking multivitamins and zinc supplements exhibited a significant reduction in depression and anxiety symptoms than women taking only multivitamins. In 2012, Sandstead published the results from six randomized controlled comparative treatment experiments in Chinese and Mexican-American low-income children, aged 6–9 years; middle-income US premenopausal women; middle income US adolescents and middle- income US men, illustrating that subclinical zinc deficiency changes the brain function and that zinc and micronutrient treatment improves altered brain functions (Sandstead, 2012). Two studies have so far shown no effect of zinc supplementation on the improvement of depressive symptoms (Nguyen et al., 2009; DiGirolamo et al., 2010). However, these studies differ significantly from that previously described with respect to both the patients and the length and quality of applications. The first study by DiGirolamo et al. (2010) examined the effect of six months of zinc supplementation on the mental health of school-age children. The second study, investigated the impact of combinations of micronutrient supplements on symptoms of depression rather than effect of zinc supplementation as a stand-alone. Because of these methodological limitations in existing studies, further well-designed, adequately powered research is required.
The beneficial effects of zinc treatment have been also reported in preclinical studies (Table 1). Zinc administration induced an antidepressant-like effect (reduction in immobility time) in both the FST and TST (Kroczka et al., 2000, 2001; Nowak et al., 2003b; Rosa et al., 2003; Cunha et al., 2008; Franco et al., 2008). Zinc was also active in different models of depression. In the olfactory bulbectomy (OB) a reduction in the number of trials in the passive-avoidance test and a decreased OB-induced hyperactivity in rats after zinc treatment was observed (Nowak et al., 2003b). While in the chronic mild stress (CMS) model of depression; zinc reversed the CMS-induced reduction in the consumption of sucrose in rats (Sowa-Kucma et al., 2008). In chronic unpredictable stress (CUS) in turn, zinc treatment prevented deficits in the fighting behavior of chronically stressed rats (Cieslik et al., 2007). Moreover, zinc has been found to intensify the effects of standard antidepressants (IMI, fluoxetine, paroxetine, bupropion, or citalopram) in the FST, the TST, and CUS (Szewczyk et al., 2002, 2009; Rosa et al., 2003; Cieslik et al., 2007; Cunha et al., 2008).
Presented above data strongly indicated the importance of zinc deficiency in human depression and indicated the benefit of zinc supplementation in both the efficacy and the speed of the therapeutic response to antidepressants treatment. Thus, the understanding of the mechanisms involved in the antidepressant activity of zinc might contribute to the development of a new therapeutic strategy for the treatment of depression or depression-related diseases. Published so far data points out that the modulation of glutamatergic neurotransmission (via the NMDA or AMPA glutamate receptors), serotonergic transmission (especially via the 5-HT1A receptor) and regulation of BDNF level seems to be the most important interactions involved in the antidepressant-like activity of zinc (Nowak et al., 2004; Sowa-Kucma et al., 2008; Cichy et al., 2009; Szewczyk et al., 2009, 2010).
Zinc and alzheimer's disease
AD is a chronic neurodegenerative disorder and the most common cause of dementia. It is estimated that AD represents 60–80% of all dementia cases (Daviglus et al., 2010). The clinical features of AD vary from stable performance and cognitive health with only a gradual decline in the short-term memory to a serious state of cognitive impairment and into different forms of dementia (deterioration of memory, learning, orientation) (Daviglus et al., 2010). On the other hand the pathological features of AD is the accumulation of β-amyloid (Aβ) and the aggregation of Aβ is suggested as the cause of neurodegeneration observed in AD (Small and Cappai, 2006).
Although the key role of Aβ in the pathogenesis of AD is strongly established now, the mechanism by which Aβ induces toxicity or the causes and factors associated with the risk or progression of AD is still poorly understood. One of the several hypotheses proposed for the pathophysiology of AD is the trace elements hypothesis, with zinc taking the center stage. Zinc was first described as a possible factor leading to dementia by Burnet (1981) and, since then, the knowledge base regarding the role of zinc in the pathogenesis and therapy of AD has evolved rapidly.
Zinc levels in AD
Serum, cerebrospinal fluid (CSF) and brain zinc levels have been investigated in patients diagnosed with AD (Table 2). Several of these studies investigating serum zinc levels have shown either divergent data with no differences (Shore et al., 1984; Haines et al., 1991), a significant decrease (Jeandel et al., 1989; Baum et al., 2010; Brewer et al., 2010; Vural et al., 2010) or a significant increase (Gonzalez et al., 1999; Rulon et al., 2000) when compared to matched controls. The main problem associated with these clinical studies is that different methodologies and different selections of patients were used meaning that the end result could account for the various divergent data obtained in the studies. Studies looking at CSF zinc levels also showed some discrepancies. For instance, Hershey et al. (1983) and Sahu et al. (1988) found no differences in CSF levels of zinc in patients with dementia of the Alzheimer type relative to a matched group of healthy controls. In contrast, Molina et al. (1998) found a significant decrease in CSF zinc levels in AD patients than the control subjects.
Table 2.
Summary of the main clinical and preclinical findings supporting the involvement of zinc in AD.
| Serum/CSF/brain zinc status—human data | References |
|---|---|
| ↔ Serum zinc level in patients with and without cognitive impairment in the community | Haines et al., 1991 |
| ↔ Serum and hair zinc concentration in patients with AD | Shore et al., 1984 |
| ↑ Serum zinc level in AD epsilon 4 apoE allele carriers | Gonzalez et al., 1999 |
| ↑ Zinc serum in AD subjects compared with age-matched control subjects-postmortem study | Rulon et al., 2000 |
| ↓ Serum zinc level in AD patients | Baum et al., 2010 |
| ↓ Blood zinc in patients with AD than in controls | Brewer et al., 2010 |
| ↓ Serum zinc level in patients with senile dementia of the Alzheimer type (SDAT) when compared to control subjects | Jeandel et al., 1989 |
| ↓ Plasma zinc level in patients with AD compared with controls | Vural et al., 2010 |
| ↔ In CSF zinc level in patients with dementia of the Alzheimer type | Hershey et al., 1983; Sahu et al., 1988 |
| ↓ CSF zinc levels in AD patients as compared with controls | Molina et al., 1998 |
| ↓ Hippocampal zinc concentration in patients with AD—postmortem study | Corrigan et al., 1993 |
| ↓ Zinc level in both hemispheres of the superior frontal gyrus, the superior parietal gyrus, the medial temporal gyrus, the hippocampus and the thalamus in the AD patients—postmortem study | Panayi et al., 2002 |
| ↑ Zinc level in hippocampus and amygdala in AD patients—postmortem study | Danscher et al., 1997 |
| ↑ Tissue zinc in the AD-affected cortex compared with the control group | Religa et al., 2006 |
| ↑ Zinc in olfactory regions of AD patients as compared to control subjects | Samudralwar et al., 1995 |
| ↑ Zinc in amygdala and hippocampus in AD patients as compared to controls | Thompson et al., 1988 |
| Zinc dyshomeostasis as a new therapeutic target in AD—animal study | |
| Administration of DP-109 (the lipophilic metal chelator) reduced the aggregation of Aβ protein and deposition of amyloid plaques in aged female hAbetaPP-transgenic Tg2576 mice, compared to animals receiving vehicle treatment | Lee et al., 2004 |
| Clioqunol (metal chelator) reduced zinc accumulation in the neuritic plaques and inhibit amyloidogenic AβPP processing in the AβPP/PS1 mouse brain | Wang et al., 2012 |
| Carnosine supplementation in 3 × Tg-AD mice promotes a strong reduction in the hippocampal intraneuronal accumulation of Aβ and completely rescues AD and aging-related mitochondrial dysfunctions | Corona et al., 2011 |
| Selective intracellular release of zinc ions from bis(thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-beta peptide | Donnelly et al., 2008 |
| Presenilins are important for cellular zinc turnover and has the potential to indirectly impact β-amyloid aggregation through metal ion clearance | Greenough et al., 2011 |
| Zinc supplementation delays hippocampal-dependent memory deficits and reduces both Aβ and tau pathology in the hippocampus | Corona et al., 2010 |
| Zinc dyshomeostasis as a new therapeutic target in AD—human study | |
| PBT2 (copper/zinc ionophore) lowered CSF levels of Aβ and significantly improved cognition in AD patients | Lannfelt et al., 2008; Faux et al., 2010 |
Studies investigating zinc content in brain tissue suggests that an alteration in the zinc level seems to be fraction/region specific. Studies involving whole tissue samples have shown no differences in brain zinc levels between AD and the control subjects. Although, some alterations in the brain zinc levels were found when tissue was sub-fractionated (a decrease in nuclear but not in mitochondrial or microsomal fractions) or when different brain regions were analyzed separately (Wenstrup et al., 1990). Indeed, decreased zinc levels have been found in the neocortex, medial temporal gyrus, thalamus, and hippocampus (Corrigan et al., 1993; Panayi et al., 2002), whilst increased levels were found in the amygdala, hippocampus, cerebellum, olfactory areas and superior temporal gyrus (Thompson et al., 1988; Samudralwar et al., 1995; Danscher et al., 1997; Religa et al., 2006). The above mentioned data, even though inconsistent, strongly support the hypothesis that a deregulated zinc homeostasis is involved in the pathophysiology of AD.
Role of zinc in AD—possible molecular mechanisms
Amongst all the multiple roles of zinc in the pathogenesis of AD, the most widely studied is the involvement of zinc in the accumulation of Aβ. Post-mortem studies using different imaging techniques for zinc analysis have demonstrated significant increases in zinc levels in neuropil and plaques present in the brain of AD patients when compared to normal age-matched controls (Lovell et al., 1998; Suh et al., 2000; Dong et al., 2003; Miller et al., 2006). On the other hand, lack of synaptic zinc prevents Aβ deposition (Lee et al., 2002).
Aβ is the product of proteolytic cleavage from the amyloid precursor protein (APP) by the enzyme known as β-secretase or β-site APP cleaving enzyme-1 (BACE-1) (Masters et al., 1985). Several pathways for the involvement of zinc in APP processing or Aβ aggregation has been suggested. It was found that APP synthesis is regulated by zinc-containing transcription factors NF-κB and sp1 (Grilli et al., 1996). Zinc is also involved in processing of APP protein (Lee et al., 2009). The processing of APP relies on a number of activities by enzymes secretases (α-,β-, and γ-). The predominant route by which APP is processed in the brain is cleavage by the α-secretase, within the Aβ region, producing sAPP (soluble amyloid precursor peptide) (Ling et al., 2003). Further processing by the β-secretase and γ-secretase leads to the formation of Aβ peptide (Wilquet and De, 2004). It was found that APP contains a specific zinc binding site localized in the cysteine-rich region of the APP ectodomain (spanning the α-secretase position) (Bush et al., 1994b) and it is suggested that changes in the intracellular concentration of zinc may influence the relative activities of APP secretases (Bush et al., 1994a,b). However, is worth mentioning that zinc is clearly not the only factor influencing APP processing and its role has not been fully determined.
Recent evidence suggests that oxidative stress is an additional factor contributing to the progression of AD (Butterfield et al., 2001; Jomova et al., 2010) and that ROS or exogenous oxidants are able to promote a harmful zinc release from MTs (Aizenman et al., 2000; Bossy-Wetzel et al., 2004). Zinc accumulation can in turn, induce mitochondrial dysfunction and further ROS generation (Sensi et al., 2003). Results presented by Sensi et al. (2008) indicate that such ROS-dependent intraneuronal zinc rises are particularly high in AD neurons expressing mutant APP, presenilin-1 (PS-1) and tau (Sensi et al., 2008).
Zinc dyshomeostasis as a new therapeutic target in AD
Considering the fact that zinc contributes to the aggregation of the Aβ protein and deposition of amyloid plaques in AD, research has been focused on the use of metal complexation ability as therapeutic agents in AD (Table 2). Indeed, the metal chelator, clioquinol (CQ) and zinc modulator—DP-109 were found to significantly decrease the formation of amyloid plaques in the brains of APP/PS1 double transgenic mice and aged female hAbetaPP-transgenic Tg2576 mice, respectively (Lee et al., 2004; Wang et al., 2012). Faux et al. (2010) describes a successful phase 2 clinical trial of the quinoline derivative, PBT2 in AD (Faux et al., 2010). This randomized, placebo controlled trial found that this metal-protein attenuating compound (MPAC) that affects the Cu2(+)-mediated and Zn2(+)-mediated toxic oligomerization of Abeta seen in AD significantly lowered the CSF levels of Aβ and significantly improved cognition in AD patients (Lannfelt et al., 2008; Faux et al., 2010). The other possible therapeutic compound for AD, suggested recently is carnosine—a peptide with cooper/zinc chelating properties (Trombley et al., 1998). Corona et al. (2011) found that dietary supplementation of carnosine reduces hippocampal intraneuronal accumulation of Aβ and rescues mitochondrial dysfunctions in triple-transgenic AD mice (3 × Tg-AD) but does not affect the development of the tau pathology and only slightly reduces cognitive deficits (Corona et al., 2011). Furthermore, the paper of Donnelly et al. (2008) demonstrated the beneficial effect of the selective intracellular delivery of zinc using bis(thiosemicarbazonato) complexes in the reduction of the extracellular levels of Aβ and suggested the role of these metal-loaded compounds as potential therapeutic agents for AD (Donnelly et al., 2008). In turn Greenough et al. (2011) reported that presenilin, which mediates the proteolytic cleavage of the β-amyloid precursor protein to release β-amyloid, is important for cellular cooper/zinc turnover and has the potential to indirectly impact on amyloid aggregation through zinc ion clearance (Greenough et al., 2011).
Interestingly, a delay in hippocampal-dependent memory deficits and reduction of both the Aβ and tau pathology in the hippocampus in 3 × Tg-AD mice was also observed after zinc supplementation (Corona et al., 2010). This study also indicated the involvement of the BDNF-tyrosine kinase type B (TrkB) receptor pathway in the mechanism of the beneficial effect of zinc supplementation in this AD model (Corona et al., 2010).
All of these data further emphasizes the integral role of zinc in the mechanism of AD and support the hypothesis that restoring zinc homeostasis might be beneficial in the treatment of AD, although it also indicated the complex interactions between AD and zinc.
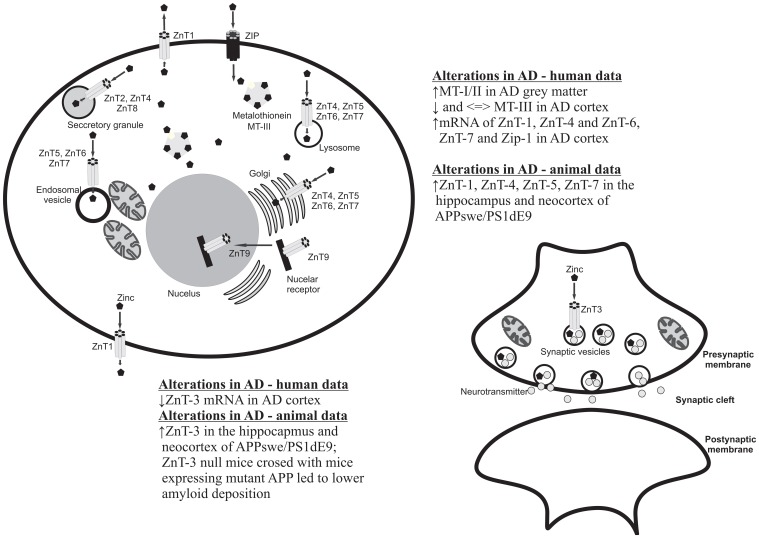
Role of metallothioneins and zinc transporters in AD
There are several proteins/pathways that interact with zinc and that are also relevant to AD (Figure 1). One of these is MT. As noted earlier, MTs are zinc- binding proteins involved in the regulation of the transport, storage and transfer of zinc to various enzymes and transcription factors (Liuzzi and Cousins, 2004; DiGirolamo et al., 2010). The involvement of MTs in the regulation of zinc homeostasis makes it important in the context of the Zinc hypothesis of AD. Indeed, there are a number of studies that have reported increases, decreases and no change in MT isoforms in the brain. The study published by Adlard et al. (1998) showed a significant increase in MT I/II in the gray matter of preclinical AD cases when compared to non-AD cases. The authors suggested that the increase in MT I/II might be associated with the initial stages of AD processes due to the oxidative stress or alterations in the metabolism of heavy metals (Adlard et al., 1998). MT-III, also known as the growth inhibitory factor (GIF) was found to be down-regulated in the AD cortex (Uchida et al., 1991; Tsuji et al., 1992; Cuajungco and Lees, 1997; Yu et al., 2001), although no changes in the MT-III level in AD was also observed (Erickson et al., 1994; Amoureux et al., 1997). These discrepancies across different studies may result from the stage of the disease or cellular zinc status. Another important group of proteins involved in the homeostasis of zinc and the pathogenesis of AD are zinc transporter (ZnTs) proteins. Zhang et al. (2008) showed that six ZnTs such as: 1–7 are extensively present in the Aβ, being therefore positive plaques in the cortex of human AD brains. Recent studies showed alterations in levels of ZnTs proteins in the brain of subjects diagnosed with the preclinical stage of AD (PCAD), mild cognitive impairment (MCI), early (EAD), and the late (LAD) stage of AD when compared to the control subjects (Lovell et al., 2005, 2006; Smith et al., 2006; Lovell, 2009; Lyubartseva et al., 2010; Lyubartseva and Lovell, 2012). Human postmortem brain tissue from Braak-staged individuals with AD displayed a reduced expression of ZnT-3 mRNA (Beyer et al., 2009) and increased mRNA levels of the other more established zinc transporters, such as LIV1, ZIP1, ZnT1, ZnT6 in the AD cortex (Beyer et al., 2012). Also animal studies have linked dyshomeostasis in the brain zinc level to the pathogenesis and progression of AD. Lee et al. (2002) using a ZnT-3 null mice crossed with mice expressing mutant APP showed that the absence of synaptic zinc reduces the plaque load and increases the ratio of soluble/insoluble Aβ species. As such, this data suggested that synaptic zinc plays a key role in Aβ aggregation and plaque accumulation. Other studies also reported that with aging, female mice exhibit higher levels of synaptic, insoluble Aβ and plaques than males and that these sex differences disappeared in ZnT-3 knockout mice, correlating with the well described age-adjusted increase incidence for AD in females rather than males (Katzman et al., 1989). Recent studies published by Zhang et al. (2010) showed significant increases of ZnT-1, ZnT-3, ZnT-4, ZnT-6, and ZnT-7 in the hippocampus and neocortex of APPswe/PS1dE9 transgenic mice which corresponding to a form of early onset AD. Lang et al. (2012) in turn demonstrated that over-expression of Drosophila homolog of human Zip1 results in zinc accumulation in Aβ42–expressing fly brains and that inhibition of Zip1 expression induces a reduction of Aβ42 fibril deposits and improves cognition (Lang et al., 2012).
Figure 1.
Cellular localization of zinc transporters and metallothioneins and summary of the main clinical and preclinical findings supporting the involvement of these proteins in AD.
Zinc in brain aging
Aging is an inevitable process associated with progressive pathological features such as: oxidative stress, altered cell metabolism, damaged of nucleic acid, or deposition of abnormal forms of proteins. In the brain aging is characterized by neuronal loss, cognitive impairment, and susceptibility to neurological disorders (Mocchegiani et al., 2005).
Recent progress in studies involving age related processes provide evidence that changes occurring in the brain during aging are related to zinc homeostasis and that zinc deficiency is a common cause of morbidity among the elderly (Mocchegiani et al., 2005). In aging, zinc deficiency is usually the result of an inadequate zinc dietary intake. It has been reported that only 40% of elderly people have a sufficient intake of zinc (Andriollo-Sanchez et al., 2005; Mocchegiani et al., 2008). Studies comparing old and young mice fed with low dietary zinc indicated that zinc is an important nutritional factor for a proper inflammatory/immune response (Kelly et al., 1996). Accordingly, zinc has anti-inflammatory properties and a low zinc status is associated with increased susceptibility to infection plus intracellular zinc has been found to play a key role in signaling in immune cells (Haase and Rink, 2009; Hasan et al., 2012). On the other hand aging is characterized by the progressive dysregulation of immune responses. Therefore, zinc has been suggested as a good factor in providing the remodeling of some age-associated changes and also as leading to healthy ageing through the reduction of inflammation (Kahmann et al., 2008). The study by Wong et al. (2013) suggests that age-related epigenetic dysregulation in ZnT expression may change cellular zinc levels and increase inflammation with age. They found that reduced Zip6 expression enhanced proinflammatory responses and that this age-induced Zip6 dysregulation correlated with an increased Zip6 promoter methylation. Interestingly, dietary supplementation reduced aged-associated inflammation (Wong et al., 2013). The other mechanism linking age, zinc and inflammation is associated with MTs. It was found that ageing is associated with a higher MT expression and consequently, low availability of intracellular zinc for normal immune responses. On the other hand, the supplementation of zinc in aging improves immune function and leads to decreased mortality from infections (Mocchegiani et al., 2010). In another study, Mocchegiani et al. (2011) showed evidence that zinc deficiency and an altered immune response is more evident in people with a polymorphism in IL-6 and metal-response element binding transcription factor-1 (MT1A) and that these individuals will benefit more from zinc supplementation.
Conclusions
From the foregoing results, it is obvious that zinc homeostasis may play a major role in the initiation and propagation of the pathological features of psychiatric and neurodegenerative disorders. However, more studies are needed to explain the exact mechanisms linking zinc and processes related to these diseases.
First, since zinc deficiency is prevalent in patients with psychiatric and neurodegenerative disorders, the appropriate preventive measures should be considered especially in the elderly. Conversely, even if the beneficial effects of zinc supplementation were reported either in treatment or in the prevention of depressive or aging symptoms, zinc supplement users should be overly cautious and avoid overdosing.
Some of the studies presented above suggest that zinc can be useful not only by itself but in combination with other drugs used in treatment. Other important aspects in the context of zinc and treatment of patients are metal chelating drugs, for which the positive effect was particularly emphasized in AD. The weakness in most of these drugs, however, are the side effects caused by the chelation of other important divalent metal ions in the brain. Chelation should thus be used only when the brain zinc level is expected to have neurotoxic effects.
Recently, the zinc-homeostasis regulating proteins such as transporters and MTs have been gaining more prominence in related literature indicating they may be very important players in the pathophysiology of neurodegenerative disorders. Therefore, more studies are needed to fully understand the influence of peripheral zinc deficiency or an overdose on these proteins.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author would like to thank the Reviewers for their helpful review of this manuscript.
References
- Adlard P. A., West A. K., Vickers J. C. (1998). Increased density of metallothionein I/II-immunopositive cortical glial cells in the early stages of Alzheimer's disease. Neurobiol. Dis. 5, 349–356 10.1006/nbdi.1998.0203 [DOI] [PubMed] [Google Scholar]
- Aimo L., Cherr G. N., Oteiza P. I. (2010). Low extracellular zinc increases neuronal oxidant production through nadph oxidase and nitric oxide synthase activation. Free Radic. Biol. Med. 48, 1577–1587 10.1016/j.freeradbiomed.2010.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E., Stout A. K., Hartnett K. A., Dineley K. E., McLaughlin B., Reynolds I. J. (2000). Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J. Neurochem. 75, 1878–1888 10.1046/j.1471-4159.2000.0751878.x [DOI] [PubMed] [Google Scholar]
- Amani R., Saeidi S., Nazari Z., Nematpour S. (2010). Correlation between dietary zinc intakes and its serum levels with depression scales in young female students. Biol. Trace Elem. Res. 137, 150–158 10.1007/s12011-009-8572-x [DOI] [PubMed] [Google Scholar]
- Amoureux M. C., Van G. D., Herrero M. T., Dom R., Colpaert F. C., Pauwels P. J. (1997). Regulation of metallothionein-III (GIF) mRNA in the brain of patients with Alzheimer disease is not impaired. Mol. Chem. Neuropathol. 32, 101–121 10.1007/BF02815170 [DOI] [PubMed] [Google Scholar]
- Andriollo-Sanchez M., Hininger-Favier I., Meunier N., Venneria E., O'Connor J. M., Maiani G., et al. (2005). Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur. J. Clin. Nutr. 59(Suppl. 2), S58–S62 [DOI] [PubMed] [Google Scholar]
- Baum L., Chan I. H., Cheung S. K., Goggins W. B., Mok V., Lam L., et al. (2010). Serum zinc is decreased in Alzheimer's disease and serum arsenic correlates positively with cognitive ability. Biometals 23, 173–179 10.1007/s10534-009-9277-5 [DOI] [PubMed] [Google Scholar]
- Besser L., Chorin E., Sekler I., Silverman W. F., Atkin S., Russell J. T., et al. (2009). Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 29, 2890–2901 10.1523/JNEUROSCI.5093-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer N., Coulson D. T., Heggarty S., Ravid R., Hellemans J., Irvine G. B., et al. (2012). Zinc transporter mRNA levels in Alzheimer's disease postmortem brain. J. Alzheimers Dis. 29, 863–873 10.3233/JAD-2012-112105 [DOI] [PubMed] [Google Scholar]
- Beyer N., Coulson D. T., Heggarty S., Ravid R., Irvine G. B., Hellemans J., et al. (2009). ZnT3 mRNA levels are reduced in Alzheimer's disease post-mortem brain. Mol. Neurodegener. 4:53 10.1186/1750-1326-4-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Talantova M. V., Lee W. D., Scholzke M. N., Harrop A., Mathews E., et al. (2004). Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 41, 351–365 10.1016/S0896-6273(04)00015-7 [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Kanzer S. H., Zimmerman E. A., Molho E. S., Celmins D. F., Heckman S. M., et al. (2010). Subclinical zinc deficiency in Alzheimer's disease and Parkinson's disease. Am. J. Alzheimers. Dis. Other Demen. 25, 572–575 10.1177/1533317510382283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet F. M. (1981). A possible role of zinc in the pathology of dementia. Lancet 1, 186–188 10.1016/S0140-6736(81)90062-3 [DOI] [PubMed] [Google Scholar]
- Bush A. I., Pettingell W. H., Multhaup G., Paradis M., Vonsattel J. P., Gusella J. F., et al. (1994a). Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265, 1464–1467 10.1126/science.8073293 [DOI] [PubMed] [Google Scholar]
- Bush A. I., Pettingell W. H., Jr., Paradis M. D., Tanzi R. E. (1994b). Modulation of A beta adhesiveness and secretase site cleavage by zinc. J. Biol. Chem. 269, 12152–12158 [PubMed] [Google Scholar]
- Butterfield D. A., Drake J., Pocernich C., Castegna A. (2001). Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 7, 548–554 10.1016/S1471-4914(01)02173-6 [DOI] [PubMed] [Google Scholar]
- Choi D. W., Yokoyama M., Koh J. (1988). Zinc neurotoxicity in cortical cell culture. Neuroscience 24, 67–79 10.1016/0306-4522(88)90312-0 [DOI] [PubMed] [Google Scholar]
- Cichy A., Sowa-Kucma M., Legutko B., Pomierny-Chamiolo L., Siwek A., Piotrowska A., et al. (2009). Zinc-induced adaptive changes in NMDA/glutamatergic and serotonergic receptors. Pharmacol. Rep. 61, 1184–1191 [DOI] [PubMed] [Google Scholar]
- Cieslik K., Klenk-Majewska B., Danilczuk Z., Wrobel A., Lupina T., Ossowska G. (2007). Influence of zinc supplementation on imipramine effect in a chronic unpredictable stress (CUS) model in rats. Pharmacol. Rep. 59, 46–52 [PubMed] [Google Scholar]
- Corona C., Frazzini V., Silvestri E., Lattanzio R., La S. R., Piantelli M., et al. (2011). Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS ONE 6:e17971 10.1371/journal.pone.0017971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona C., Masciopinto F., Silvestri E., Viscovo A. D., Lattanzio R., Sorda R. L., et al. (2010). Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 1, e91 10.1038/cddis.2010.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan F. M., Reynolds G. P., Ward N. I. (1993). Hippocampal tin, aluminum and zinc in Alzheimer's disease. Biometals 6, 149–154 10.1007/BF00205853 [DOI] [PubMed] [Google Scholar]
- Cote A., Chiasson M., Peralta M. R., III., Lafortune K., Pellegrini L., Toth K. (2005). Cell type-specific action of seizure-induced intracellular zinc accumulation in the rat hippocampus. J. Physiol. 566, 821–837 10.1113/jphysiol.2005.089458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J., Liuzzi J. P., Lichten L. A. (2006). Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 281, 24085–24089 10.1074/jbc.R600011200 [DOI] [PubMed] [Google Scholar]
- Cuajungco M. P., Lees G. J. (1997). Zinc and Alzheimer's disease: is there a direct link? Brain Res. Brain Res. Rev. 23, 219–236 10.1016/S0165-0173(97)00002-7 [DOI] [PubMed] [Google Scholar]
- Cunha M. P., Machado D. G., Bettio L. E., Capra J. C., Rodrigues A. L. (2008). Interaction of zinc with antidepressants in the tail suspension test. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1913–1920 10.1016/j.pnpbp.2008.09.006 [DOI] [PubMed] [Google Scholar]
- Danscher G., Jensen K. B., Frederickson C. J., Kemp K., Andreasen A., Juhl S., et al. (1997). Increased amount of zinc in the hippocampus and amygdala of Alzheimer's diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J. Neurosci. Methods 76, 53–59 10.1016/S0165-0270(97)00079-4 [DOI] [PubMed] [Google Scholar]
- Daviglus M. L., Bell C. C., Berrettini W., Bowen P. E., Connolly E. S., Jr., Cox N. J., et al. (2010). National institutes of health state-of-the-science conference statement: preventing alzheimer disease and cognitive decline. Ann. Intern. Med. 153, 176–181 10.7326/0003-4819-153-3-201008030-00260 [DOI] [PubMed] [Google Scholar]
- DiGirolamo A. M., Ramirez-Zea M., Wang M., Flores-Ayala R., Martorell R., Neufeld L. M., et al. (2010). Randomized trial of the effect of zinc supplementation on the mental health of school-age children in Guatemala. Am. J. Clin. Nutr. 92, 1241–1250 10.3945/ajcn.2010.29686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Atwood C. S., Anderson V. E., Siedlak S. L., Smith M. A., Perry G., et al. (2003). Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 42, 2768–2773 10.1021/bi0272151 [DOI] [PubMed] [Google Scholar]
- Donnelly P. S., Caragounis A., Du T., Laughton K. M., Volitakis I., Cherny R. A., et al. (2008). Selective intracellular release of copper and zinc ions from bis(thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-beta peptide. J. Biol. Chem. 283, 4568–4577 10.1074/jbc.M705957200 [DOI] [PubMed] [Google Scholar]
- Erickson J. C., Sewell A. K., Jensen L. T., Winge D. R., Palmiter R. D. (1994). Enhanced neurotrophic activity in Alzheimer's disease cortex is not associated with down-regulation of metallothionein-III (GIF). Brain Res. 649, 297–304 10.1016/0006-8993(94)91076-6 [DOI] [PubMed] [Google Scholar]
- Faux N. G., Ritchie C. W., Gunn A., Rembach A., Tsatsanis A., Bedo J., et al. (2010). PBT2 rapidly improves cognition in Alzheimer's Disease: additional phase II analyses. J. Alzheimers Dis. 20, 509–516 [DOI] [PubMed] [Google Scholar]
- Flinn J. M., Hunter D., Linkous D. H., Lanzirotti A., Smith L. N., Brightwell J., et al. (2005). Enhanced zinc consumption causes memory deficits and increased brain levels of zinc. Physiol. Behav. 83, 793–803 10.1016/j.physbeh.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Franco J. L., Posser T., Brocardo P. S., Trevisan R., Uliano-Silva M., Gabilan N. H., et al. (2008). Involvement of glutathione, ERK1/2 phosphorylation and BDNF expression in the antidepressant-like effect of zinc in rats. Behav. Brain Res. 188, 316–323 10.1016/j.bbr.2007.11.012 [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Suh S. W., Silva D., Frederickson C. J., Thompson R. B. (2000). Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 130, 1471S–1483S [DOI] [PubMed] [Google Scholar]
- Gao H. L., Xu H., Xin N., Zheng W., Chi Z. H., Wang Z. Y. (2011). Disruption of the CaMKII/CREB signaling is associated with zinc deficiency-induced learning and memory impairments. Neurotox. Res. 19, 584–591 10.1007/s12640-010-9206-y [DOI] [PubMed] [Google Scholar]
- Gao H. L., Zheng W., Xin N., Chi Z. H., Wang Z. Y., Chen J., et al. (2009). Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and -independent signaling pathways. Neurotox. Res. 16, 416–425 10.1007/s12640-009-9072-7 [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Martin T., Cacho J., Brenas M. T., Arroyo T., Garcia-Berrocal B., et al. (1999). Serum zinc, copper, insulin and lipids in Alzheimer's disease epsilon 4 apolipoprotein E allele carriers. Eur. J. Clin. Invest. 29, 637–642 10.1046/j.1365-2362.1999.00471.x [DOI] [PubMed] [Google Scholar]
- Greenough M. A., Volitakis I., Li Q. X., Laughton K., Evin G., Ho M., et al. (2011). Presenilins promote the cellular uptake of copper and zinc and maintain copper chaperone of SOD1-dependent copper/zinc superoxide dismutase activity. J. Biol. Chem. 286, 9776–9786 10.1074/jbc.M110.163964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M., Goffi F., Memo M., Spano P. (1996). Interleukin-1beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J. Biol. Chem. 271, 15002–15007 10.1074/jbc.271.25.15002 [DOI] [PubMed] [Google Scholar]
- Haase H., Rink L. (2009). Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 29, 133–152 10.1146/annurev-nutr-080508-141119 [DOI] [PubMed] [Google Scholar]
- Haines A., Iliffe S., Morgan P., Dormandy T., Wood B. (1991). Serum aluminium and zinc and other variables in patients with and without cognitive impairment in the community. Clin. Chim. Acta 198, 261–266 10.1016/0009-8981(91)90360-O [DOI] [PubMed] [Google Scholar]
- Hasan R., Rink L., Haase H. (2012). Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 19, 253–264 10.1177/1753425912458815 [DOI] [PubMed] [Google Scholar]
- Hershey C. O., Hershey L. A., Varnes A., Vibhakar S. D., Lavin P., Strain W. H. (1983). Cerebrospinal fluid trace element content in dementia: clinical, radiologic, and pathologic correlations. Neurology 33, 1350–1353 10.1212/WNL.33.10.1350 [DOI] [PubMed] [Google Scholar]
- Huang L., Tepaamorndech S. (2013). The SLC30 family of zinc transporters - a review of current understanding of their biological and pathophysiological roles. Mol. Aspects Med. 34, 548–560 10.1016/j.mam.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Huang Y. Z., Pan E., Xiong Z. Q., McNamara J. O. (2008). Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57, 546–558 10.1016/j.neuron.2007.11.026 [DOI] [PubMed] [Google Scholar]
- Hwang J. J., Park M. H., Choi S. Y., Koh J. Y. (2005). Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J. Biol. Chem. 280, 11995–12001 10.1074/jbc.M403172200 [DOI] [PubMed] [Google Scholar]
- Irmisch G., Schlaefke D., Richter J. (2010). Zinc and fatty acids in depression. Neurochem. Res. 35, 1376–1383 10.1007/s11064-010-0194-3 [DOI] [PubMed] [Google Scholar]
- Jeandel C., Nicolas M. B., Dubois F., Nabet-Belleville F., Penin F., Cuny G. (1989). Lipid peroxidation and free radical scavengers in Alzheimer's disease. Gerontology 35, 275–282 10.1159/000213037 [DOI] [PubMed] [Google Scholar]
- Jomova K., Vondrakova D., Lawson M., Valko M. (2010). Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem. 345, 91–104 10.1007/s11010-010-0563-x [DOI] [PubMed] [Google Scholar]
- Kahmann L., Uciechowski P., Warmuth S., Plumakers B., Gressner A. M., Malavolta M., et al. (2008). Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 11, 227–237 10.1089/rej.2007.0613 [DOI] [PubMed] [Google Scholar]
- Katzman R., Aronson M., Fuld P., Kawas C., Brown T., Morgenstern H., et al. (1989). Development of dementing illnesses in an 80-year-old volunteer cohort. Ann. Neurol. 25, 317–324 10.1002/ana.410250402 [DOI] [PubMed] [Google Scholar]
- Keller K. A., Grider A., Coffield J. A. (2001). Age-dependent influence of dietary zinc restriction on short-term memory in male rats. Physiol. Behav. 72, 339–348 10.1016/S0031-9384(00)00421-2 [DOI] [PubMed] [Google Scholar]
- Kelly E. J., Quaife C. J., Froelick G. J., Palmiter R. D. (1996). Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J. Nutr. 126, 1782–1790 [DOI] [PubMed] [Google Scholar]
- Krezel A., Hao Q., Maret W. (2007). The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 463, 188–200 10.1016/j.abb.2007.02.017 [DOI] [PubMed] [Google Scholar]
- Kroczka B., Branski P., Palucha A., Pilc A., Nowak G. (2001). Antidepressant-like properties of zinc in rodent forced swim test. Brain Res. Bull. 55, 297–300 10.1016/S0361-9230(01)00473-7 [DOI] [PubMed] [Google Scholar]
- Kroczka B., Zieba A., Dudek D., Pilc A., Nowak G. (2000). Zinc exhibits an antidepressant-like effect in the forced swimming test in mice. Pol. J. Pharmacol. 52, 403–406 [PubMed] [Google Scholar]
- Lang M., Wang L., Fan Q., Xiao G., Wang X., Zhong Y., et al. (2012). Genetic inhibition of solute-linked carrier 39 family transporter 1 ameliorates abeta pathology in a Drosophila model of Alzheimer's disease. PLoS Genet. 8:e1002683 10.1371/journal.pgen.1002683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannfelt L., Blennow K., Zetterberg H., Batsman S., Ames D., Harrison J., et al. (2008). Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 7, 779–786 10.1016/S1474-4422(08)70167-4 [DOI] [PubMed] [Google Scholar]
- Lee J., Kim C. H., Kim D. G., Ahn Y. S. (2009). Zinc inhibits amyloid beta production from Alzheimer's amyloid precursor protein in SH-SY5Y Cells. Korean J. Physiol. Pharmacol. 13, 195–200 10.4196/kjpp.2009.13.3.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Cole T. B., Palmiter R. D., Suh S. W., Koh J. Y. (2002). Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 99, 7705–7710 10.1073/pnas.092034699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Friedman J. E., Angel I., Kozak A., Koh J. Y. (2004). The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol. Aging 25, 1315–1321 10.1016/j.neurobiolaging.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Lichten L. A., Cousins R. J. (2009). Mammalian zinc transporters: nutritional and physiologic regulation. Annu. Rev. Nutr. 29, 153–176 10.1146/annurev-nutr-033009-083312 [DOI] [PubMed] [Google Scholar]
- Ling Y., Morgan K., Kalsheker N. (2003). Amyloid precursor protein (APP) and the biology of proteolytic processing: relevance to Alzheimer's disease. Int. J. Biochem. Cell Biol. 35, 1505–1535 10.1016/S1357-2725(03)00133-X [DOI] [PubMed] [Google Scholar]
- Liuzzi J. P., Cousins R. J. (2004). Mammalian zinc transporters. Annu. Rev. Nutr. 24, 151–172 10.1146/annurev.nutr.24.012003.132402 [DOI] [PubMed] [Google Scholar]
- Lovell M. A. (2009). A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer's disease. J. Alzheimers Dis. 16, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell M. A., Robertson J. D., Teesdale W. J., Campbell J. L., Markesbery W. R. (1998). Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 158, 47–52 10.1016/S0022-510X(98)00092-6 [DOI] [PubMed] [Google Scholar]
- Lovell M. A., Smith J. L., Markesbery W. R. (2006). Elevated zinc transporter-6 in mild cognitive impairment, Alzheimer disease, and pick disease. J. Neuropathol. Exp. Neurol. 65, 489–498 10.1097/01.jnen.0000229237.98124.91 [DOI] [PubMed] [Google Scholar]
- Lovell M. A., Smith J. L., Xiong S., Markesbery W. R. (2005). Alterations in zinc transporter protein-1 (ZnT-1) in the brain of subjects with mild cognitive impairment, early, and late-stage Alzheimer's disease. Neurotox. Res. 7, 265–271 10.1007/BF03033884 [DOI] [PubMed] [Google Scholar]
- Lyubartseva G., Lovell M. A. (2012). A potential role for zinc alterations in the pathogenesis of Alzheimer's disease. Biofactors 38, 98–106 10.1002/biof.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubartseva G., Smith J. L., Markesbery W. R., Lovell M. A. (2010). Alterations of zinc transporter proteins ZnT-1, ZnT-4 and ZnT-6 in preclinical Alzheimer's disease brain. Brain Pathol. 20, 343–350 10.1111/j.1750-3639.2009.00283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Bosmans E., De Jongh R., Kenis G., Vandoolaeghe E., Neels H. (1997). Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9, 853–858 10.1006/cyto.1997.0238 [DOI] [PubMed] [Google Scholar]
- Maes M., D'Haese P. C., Scharpe S., D'Hondt P., Cosyns P., De Broe M. E. (1994). Hypozincemia in depression. J. Affect. Disord. 31, 135–140 10.1016/0165-0327(94)90117-1 [DOI] [PubMed] [Google Scholar]
- Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., et al. (2009). The inflammatory and neurodegenerative (IandND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 24, 27–53 10.1007/s11011-008-9118-1 [DOI] [PubMed] [Google Scholar]
- Masters B. A., Quaife C. J., Erickson J. C., Kelly E. J., Froelick G. J., Zambrowicz B. P., et al. (1994). Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J. Neurosci. 14, 5844–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 82, 4245–4249 10.1073/pnas.82.12.4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin I. J., Hodge J. S. (1990). Zinc in depressive disorder. Acta Psychiatr. Scand. 82, 451–453 10.1111/j.1600-0447.1990.tb03077.x [DOI] [PubMed] [Google Scholar]
- Miller L. M., Wang Q., Telivala T. P., Smith R. J., Lanzirotti A., Miklossy J. (2006). Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer's disease. J. Struct. Biol. 155, 30–37 10.1016/j.jsb.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Mlyniec K., Budziszewska B., Reczynski W., Sowa-Kucma M., Nowak G. (2013). The role of the GPR39 receptor in zinc deficient-animal model of depression. Behav. Brain Res. 238, 30–35 10.1016/j.bbr.2012.10.020 [DOI] [PubMed] [Google Scholar]
- Mlyniec K., Davies C. L., Budziszewska B., Opoka W., Reczynski W., Sowa-Kucma M., et al. (2012). Time course of zinc deprivation-induced alterations of mice behavior in the forced swim test. Pharmacol. Rep. 64, 567–575 [DOI] [PubMed] [Google Scholar]
- Mlyniec K., Nowak G. (2012). Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol. Rep. 64, 249–255 [DOI] [PubMed] [Google Scholar]
- Mlyniec K., Nowak G. (2013). GPR39 up-regulation after selective antidepressants. Neurochem. Int. 62, 936–939 10.1016/j.neuint.2013.02.024 [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Bertoni-Freddari C., Marcellini F., Malavolta M. (2005). Brain, aging and neurodegeneration: role of zinc ion availability. Prog. Neurobiol. 75, 367–390 10.1016/j.pneurobio.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Burkle A., Fulop T. (2008). Zinc and ageing (ZINCAGE Project). Exp. Gerontol. 43, 361–362 10.1016/j.exger.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Costarelli L., Giacconi R., Piacenza F., Basso A., Malavolta M. (2011). Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenomic approach. Biogerontology 12, 455–465 10.1007/s10522-011-9337-4 [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Malavolta M., Costarelli L., Giacconi R., Cipriano C., Piacenza F., et al. (2010). Zinc, metallothioneins and immunosenescence. Proc. Nutr. Soc. 69, 290–299 10.1017/S0029665110001862 [DOI] [PubMed] [Google Scholar]
- Molina J. A., Jimenez-Jimenez F. J., Aguilar M. V., Meseguer I., Mateos-Vega C. J., Gonzalez-Munoz M. J., et al. (1998). Cerebrospinal fluid levels of transition metals in patients with Alzheimer's disease. J. Neural Transm. 105, 479–488 10.1007/s007020050071 [DOI] [PubMed] [Google Scholar]
- Narang R. L., Gupta K. R., Narang A. P., Singh R. (1991). Levels of copper and zinc in depression. Indian J. Physiol. Pharmacol. 35, 272–274 [PubMed] [Google Scholar]
- Nguyen P. H., Grajeda R., Melgar P., Marcinkevage J., DiGirolamo A. M., Flores R., et al. (2009). Micronutrient supplementation may reduce symptoms of depression in Guatemalan women. Arch. Latinoam. Nutr. 59, 278–286 [PubMed] [Google Scholar]
- Noh K. M., Koh J. Y. (2000). Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J. Neurosci. 20, RC111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G., Siwek M., Dudek D., Zieba A., Pilc A. (2003a). Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol. J. Pharmacol. 55, 1143–1147 [PubMed] [Google Scholar]
- Nowak G., Szewczyk B., Wieronska J. M., Branski P., Palucha A., Pilc A., et al. (2003b). Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res. Bull. 61, 159–164 10.1016/S0361-9230(03)00104-7 [DOI] [PubMed] [Google Scholar]
- Nowak G., Zieba A., Dudek D., Krosniak M., Szymaczek M., Schlegel-Zawadzka M. (1999). Serum trace elements in animal models and human depression. Part I. Zinc. Hum. Psychopharmacol. Clin. Exp. 14, 83–86 [DOI] [PubMed] [Google Scholar]
- Panayi A. E., Spyrou N. M., Iversen B. S., White M. A., Part P. (2002). Determination of cadmium and zinc in Alzheimer's brain tissue using inductively coupled plasma mass spectrometry. J. Neurol. Sci. 195, 1–10 10.1016/S0022-510X(01)00672-4 [DOI] [PubMed] [Google Scholar]
- Paoletti P., Vergnano A. M., Barbour B., Casado M. (2009). Zinc at glutamatergic synapses. Neuroscience 158, 126–136 10.1016/j.neuroscience.2008.01.061 [DOI] [PubMed] [Google Scholar]
- Perry D. K., Smyth M. J., Stennicke H. R., Salvesen G. S., Duriez P., Poirier G. G., et al. (1997). Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 272, 18530–18533 10.1074/jbc.272.30.18530 [DOI] [PubMed] [Google Scholar]
- Piechal A., Blecharz-Klin K., Pyrzanowska J., Widy-Tyszkiewicz E. (2012). Maternal zinc supplementation improves spatial memory in rat pups. Biol. Trace Elem. Res. 147, 299–308 10.1007/s12011-012-9323-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L. M., Rink L., Haase H. (2010). The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health 7, 1342–1365 10.3390/ijerph7041342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife C. J., Findley S. D., Erickson J. C., Froelick G. J., Kelly E. J., Zambrowicz B. P., et al. (1994). Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry 33, 7250–7259 10.1021/bi00189a029 [DOI] [PubMed] [Google Scholar]
- Railey A. M., Micheli T. L., Wanschura P. B., Flinn J. M. (2010). Alterations in fear response and spatial memory in pre- and post-natal zinc supplemented rats: remediation by copper. Physiol. Behav. 100, 95–100 10.1016/j.physbeh.2010.01.040 [DOI] [PubMed] [Google Scholar]
- Ranjbar E., Shams J., Sabetkasaei M., Shirazi M., Rashidkhani B., Mostafavi A., et al. (2013). Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr. Neurosci. [Epub ahead of print]. 10.1179/1476830513Y.0000000066 [DOI] [PubMed] [Google Scholar]
- Religa D., Strozyk D., Cherny R. A., Volitakis I., Haroutunian V., Winblad B., et al. (2006). Elevated cortical zinc in Alzheimer disease. Neurology 67, 69–75 10.1212/01.wnl.0000223644.08653.b5 [DOI] [PubMed] [Google Scholar]
- Roozbeh J., Sharifian M., Ghanizadeh A., Sahraian A., Sagheb M. M., Shabani S., et al. (2011). Association of zinc deficiency and depression in the patients with end-stage renal disease on hemodialysis. J. Ren. Nutr. 21, 184–187 10.1053/j.jrn.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Rosa A. O., Lin J., Calixto J. B., Santos A. R., Rodrigues A. L. (2003). Involvement of NMDA receptors and L-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav. Brain Res. 144, 87–93 10.1016/S0166-4328(03)00069-X [DOI] [PubMed] [Google Scholar]
- Roy A., Evers S. E., Avison W. R., Campbell M. K. (2010). Higher zinc intake buffers the impact of stress on depressive symptoms in pregnancy. Nutr. Res. 30, 695–704 10.1016/j.nutres.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Rulon L. L., Robertson J. D., Lovell M. A., Deibel M. A., Ehmann W. D., Markesber W. R. (2000). Serum zinc levels and Alzheimer's disease. Biol. Trace Elem. Res. 75, 79–85 10.1385/BTER:75:1-3:79 [DOI] [PubMed] [Google Scholar]
- Sahu R. N., Pandey R. S., Subhash M. N., Arya B. Y., Padmashree T. S., Srinivas K. N. (1988). CSF zinc in Alzheimer's type dementia. Biol. Psychiatry 24, 480–482 10.1016/0006-3223(88)90190-4 [DOI] [PubMed] [Google Scholar]
- Samudralwar D. L., Diprete C. C., Ni B. F., Ehmann W. D., Markesbery W. R. (1995). Elemental imbalances in the olfactory pathway in Alzheimer's disease. J. Neurol. Sci. 130, 139–145 10.1016/0022-510X(95)00018-W [DOI] [PubMed] [Google Scholar]
- Sandstead H. H. (2012). Subclinical zinc deficiency impairs human brain function. J. Trace Elem. Med. Biol. 26, 70–73 10.1016/j.jtemb.2012.04.018 [DOI] [PubMed] [Google Scholar]
- Sato M., Kondoh M. (2002). Recent studies on metallothionein: protection against toxicity of heavy metals and oxygen free radicals. Tohoku J. Exp. Med. 196, 9–22 10.1620/tjem.196.9 [DOI] [PubMed] [Google Scholar]
- Sawada T., Yokoi K. (2010). Effect of zinc supplementation on mood states in young women: a pilot study. Eur. J. Clin. Nutr. 64, 331–333 10.1038/ejcn.2009.158 [DOI] [PubMed] [Google Scholar]
- Sensi S. L., Paoletti P., Koh J. Y., Aizenman E., Bush A. I., Hershfinkel M. (2011). The neurophysiology and pathology of brain zinc. J. Neurosci. 31, 16076–16085 10.1523/JNEUROSCI.3454-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi S. L., Rapposelli I. G., Frazzini V., Mascetra N. (2008). Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer's disease. Exp. Gerontol. 43, 488–492 10.1016/j.exger.2007.10.018 [DOI] [PubMed] [Google Scholar]
- Sensi S. L., Ton-That D., Sullivan P. G., Jonas E. A., Gee K. R., Kaczmarek L. K., et al. (2003). Modulation of mitochondrial function by endogenous Zn2+ pools. Proc. Natl. Acad. Sci. U.S.A. 100, 6157–6162 10.1073/pnas.1031598100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Henkin R. I., Nelson N. R., Agarwal R. P., Wyatt R. J. (1984). Hair and serum copper, zinc, calcium, and magnesium concentrations in Alzheimer-type dementia. J. Am. Geriatr. Soc. 32, 892–895 [DOI] [PubMed] [Google Scholar]
- Siwek M., Dudek D., Paul I. A., Sowa-Kucma M., Zieba A., Popik P., et al. (2009). Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J. Affect. Disord. 118, 187–195 10.1016/j.jad.2009.02.014 [DOI] [PubMed] [Google Scholar]
- Siwek M., Dudek D., Schlegel-Zawadzka M., Morawska A., Piekoszewski W., Opoka W., et al. (2010). Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J. Affect. Disord. 126, 447–452 10.1016/j.jad.2010.04.024 [DOI] [PubMed] [Google Scholar]
- Small D. H., Cappai R. (2006). Alois Alzheimer and Alzheimer's disease: a centennial perspective. J. Neurochem. 99, 708–710 10.1111/j.1471-4159.2006.04212.x [DOI] [PubMed] [Google Scholar]
- Smart T. G., Hosie A. M., Miller P. S. (2004). Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist 10, 432–442 10.1177/1073858404263463 [DOI] [PubMed] [Google Scholar]
- Smith J. L., Xiong S., Markesbery W. R., Lovell M. A. (2006). Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer's disease brain. Neuroscience 140, 879–888 10.1016/j.neuroscience.2006.02.049 [DOI] [PubMed] [Google Scholar]
- Sowa-Kucma M., Legutko B., Szewczyk B., Novak K., Znojek P., Poleszak E., et al. (2008). Antidepressant-like activity of zinc: further behavioral and molecular evidence. J. Neural Transm. 115, 1621–1628 10.1007/s00702-008-0115-7 [DOI] [PubMed] [Google Scholar]
- Suh S. W., Jensen K. B., Jensen M. S., Silva D. S., Kesslak P. J., Danscher G., et al. (2000). Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer's diseased brains. Brain Res. 852, 274–278 10.1016/S0006-8993(99)02096-X [DOI] [PubMed] [Google Scholar]
- Suh S. W., Won S. J., Hamby A. M., Yoo B. H., Fan Y., Sheline C. T., et al. (2009). Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 29, 1579–1588 10.1038/jcbfm.2009.80 [DOI] [PubMed] [Google Scholar]
- Szewczyk B., Branski P., Wieronska J. M., Palucha A., Pilc A., Nowak G. (2002). Interaction of zinc with antidepressants in the forced swimming test in mice. Pol. J. Pharmacol. 54, 681–685 [PubMed] [Google Scholar]
- Szewczyk B., Poleszak E., Sowa-Kucma M., Wrobel A., Slotwinski S., Listos J., et al. (2010). The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test. Amino Acids 39, 205–217 10.1007/s00726-009-0412-y [DOI] [PubMed] [Google Scholar]
- Szewczyk B., Poleszak E., Wlaz P., Wrobel A., Blicharska E., Cichy A., et al. (2009). The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 323–329 10.1016/j.pnpbp.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Takeda A. (2000). Movement of zinc and its functional significance in the brain. Brain Res. Brain Res. Rev. 34, 137–148 10.1016/S0165-0173(00)00044-8 [DOI] [PubMed] [Google Scholar]
- Takeda A., Tamano H., Tochigi M., Oku N. (2005). Zinc homeostasis in the hippocampus of zinc-deficient young adult rats. Neurochem. Int. 46, 221–225 10.1016/j.neuint.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Tamano H., Kan F., Kawamura M., Oku N., Takeda A. (2009). Behavior in the forced swim test and neurochemical changes in the hippocampus in young rats after 2-week zinc deprivation. Neurochem. Int. 55, 536–541 10.1016/j.neuint.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Tassabehji N. M., Corniola R. S., Alshingiti A., Levenson C. W. (2008). Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav. 95, 365–369 10.1016/j.physbeh.2008.06.017 [DOI] [PubMed] [Google Scholar]
- Thompson C. M., Markesbery W. R., Ehmann W. D., Mao Y. X., Vance D. E. (1988). Regional brain trace-element studies in Alzheimer's disease. Neurotoxicology 9, 1–7 [PubMed] [Google Scholar]
- Trombley P. Q., Horning M. S., Blakemore L. J. (1998). Carnosine modulates zinc and copper effects on amino acid receptors and synaptic transmission. Neuroreport 9, 3503–3507 10.1097/00001756-199810260-00031 [DOI] [PubMed] [Google Scholar]
- Tsuji S., Kobayashi H., Uchida Y., Ihara Y., Miyatake T. (1992). Molecular cloning of human growth inhibitory factor cDNA and its down-regulation in Alzheimer's disease. EMBO J. 11, 4843–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Takio K., Titani K., Ihara Y., Tomonaga M. (1991). The growth inhibitory factor that is deficient in the Alzheimer's disease brain is a 68 amino acid metallothionein-like protein. Neuron 7, 337–347 10.1016/0896-6273(91)90272-2 [DOI] [PubMed] [Google Scholar]
- Vural H., Demirin H., Kara Y., Eren I., Delibas N. (2010). Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer's disease. J.Trace Elem. Med. Biol. 24, 169–173 10.1016/j.jtemb.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Wang T., Wang C. Y., Shan Z. Y., Teng W. P., Wang Z. Y. (2012). Clioquinol reduces zinc accumulation in neuritic plaques and inhibits the amyloidogenic pathway in AbetaPP/PS1 transgenic mouse brain. J. Alzheimers Dis. 29, 549–559 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Tamano H., Kikuchi T., Takeda A. (2010). Susceptibility to stress in young rats after 2-week zinc deprivation. Neurochem. Int. 56, 410–416 10.1016/j.neuint.2009.11.014 [DOI] [PubMed] [Google Scholar]
- Wenstrup D., Ehmann W. D., Markesbery W. R. (1990). Trace element imbalances in isolated subcellular fractions of Alzheimer's disease brains. Brain Res. 533, 125–131 10.1016/0006-8993(90)91804-P [DOI] [PubMed] [Google Scholar]
- Whittle N., Lubec G., Singewald N. (2009). Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids 36, 147–158 10.1007/s00726-008-0195-6 [DOI] [PubMed] [Google Scholar]
- Wilquet V., De S. B. (2004). Amyloid-beta precursor protein processing in neurodegeneration. Curr. Opin. Neurobiol. 14, 582–588 10.1016/j.conb.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Wojcik J., Dudek D., Schlegel-Zawadzka M., Grabowska M., Marcinek A., Florek E., et al. (2006). Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol. Rep. 58, 571–576 [PubMed] [Google Scholar]
- Wong C. P., Magnusson K. R., Ho E. (2013). Increased inflammatory response in aged mice is associated with age-related zinc deficiency and zinc transporter dysregulation. J. Nutr. Biochem. 24, 353–359 10.1016/j.jnutbio.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Jing X. P., Zhang S. P., Gu R. X., Tang F. X., Wang X. L., et al. (2013). High dose zinc supplementation induces hippocampal zinc deficiency and memory impairment with inhibition of BDNF signaling. PLoS ONE 8:e55384 10.1371/journal.pone.0055384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. H., Lukiw W. J., Bergeron C., Niznik H. B., Fraser P. E. (2001). Metallothionein III is reduced in Alzheimer's disease. Brain Res. 894, 37–45 10.1016/S0006-8993(00)03196-6 [DOI] [PubMed] [Google Scholar]
- Zhang L. H., Wang X., Stoltenberg M., Danscher G., Huang L., Wang Z. Y. (2008). Abundant expression of zinc transporters in the amyloid plaques of Alzheimer's disease brain. Brain Res. Bull. 77, 55–60 10.1016/j.brainresbull.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Zhang L. H., Wang X., Zheng Z. H., Ren H., Stoltenberg M., Danscher G., et al. (2010). Altered expression and distribution of zinc transporters in APP/PS1 transgenic mouse brain. Neurobiol. Aging 31, 74–87 10.1016/j.neurobiolaging.2008.02.018 [DOI] [PubMed] [Google Scholar]