Abstract
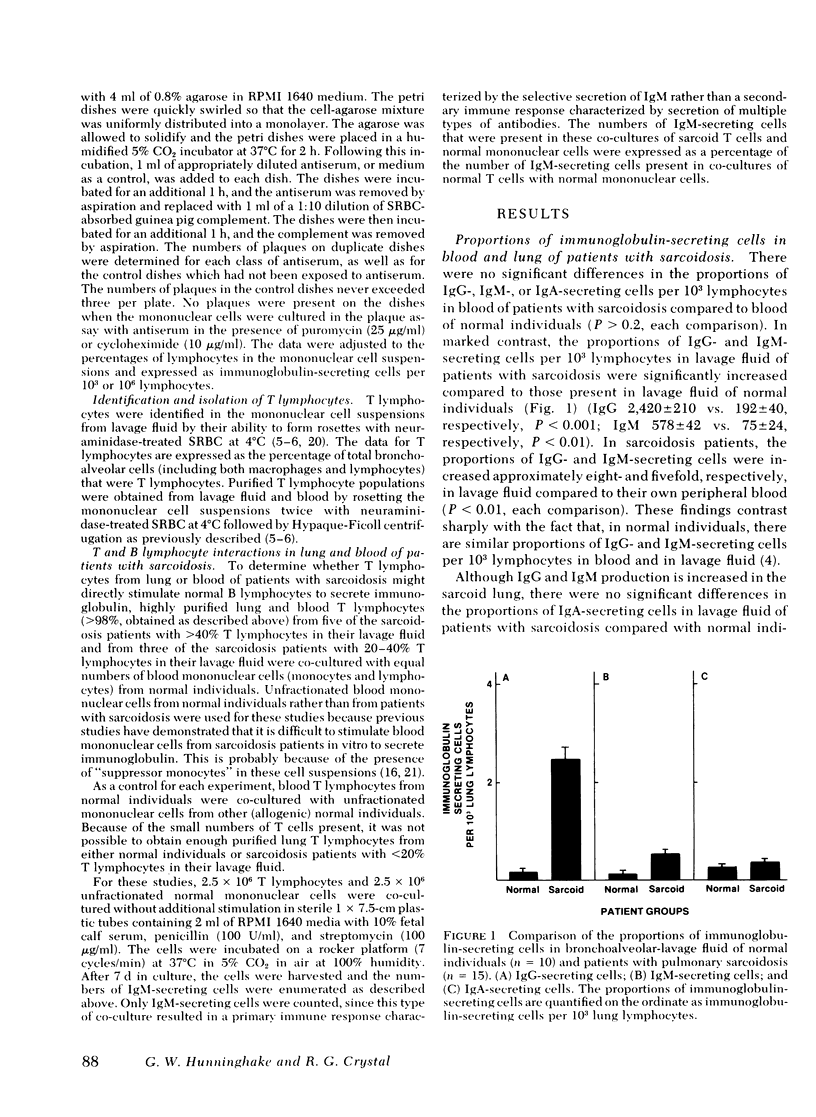
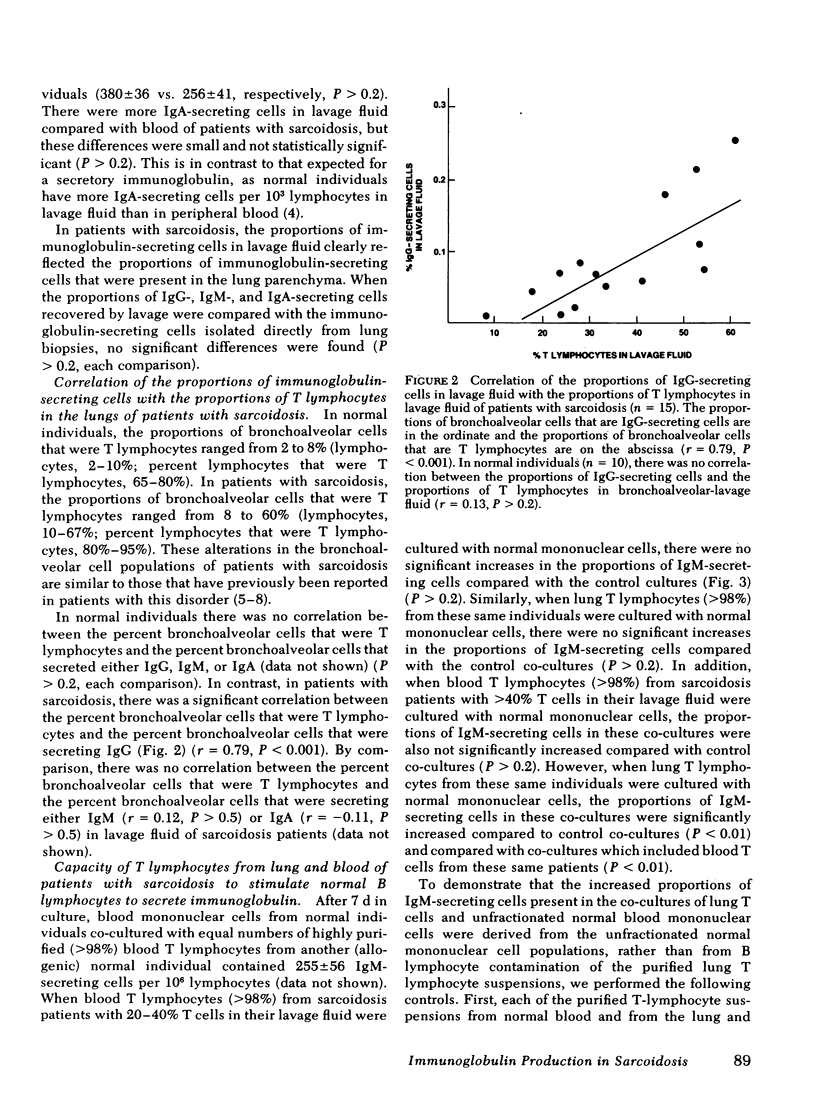
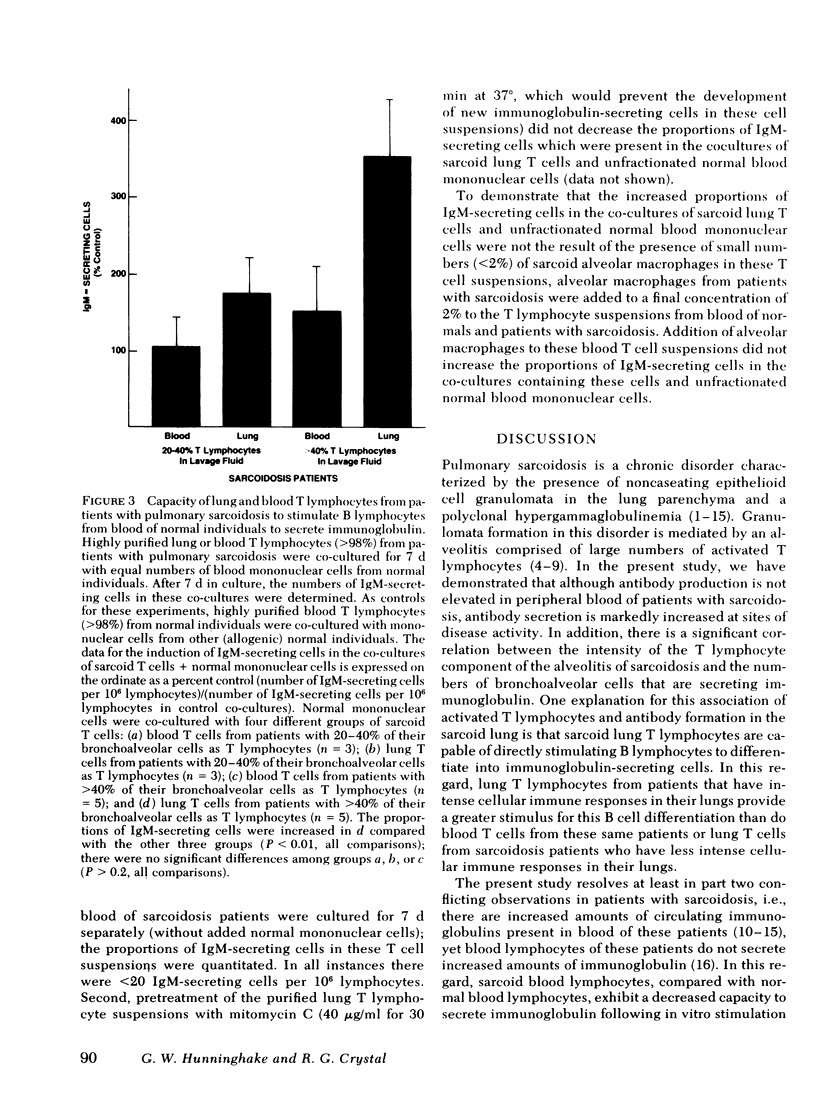
Pulmonary sarcoidosis is a disorder in which local granuloma formation is perpetuated by activated lung T lymphocytes. The present study suggests that lung T lymphocytes may also play a critical role in modulating local production of antibodies in this disorder. In untreated patients with pulmonary sarcoidosis, the numbers of IgG- and IgM-secreting cells per 103 lung lymphocytes are markedly increased compared with those in normal individuals (P < 0.001 and P < 0.01, respectively); the numbers of IgA-secreting cells in lavage fluid of these patients are not increased (P > 0.2). In contrast to lungs, the numbers of IgG-, IgM-, and IgA-secreting cells in blood of patients with this disorder are similar to those in normal individuals (P > 0.2, each comparison). In patients with pulmonary sarcoidosis, there is a direct correlation between the percentage of bronchoalveolar cells that are T lymphocytes and the percentage of bronchoalveolar cells that secrete IgG (r = 0.79; P < 0.001); in normal individuals there is no such relationship (P > 0.2). When purified sarcoid lung T cells from patients with high proportions of T lymphocytes in their lavage fluid were co-cultured with blood mononuclear cells from normal individuals (without added antigens or mitogens), the B lymphocytes in these normal mononuclear cell suspensions were induced to differentiate into immunoglobulin-secreting cells (P < 0.01). In contrast, blood T lymphocytes from these same patients and lung T lymphocytes from sarcoidosis patients with low proportions of T lymphocytes in their lavage fluid did not stimulate normal B cells to produce immunoglobulin (P > 0.2, all comparisons). These findings suggest that in pulmonary sarcoidosis (a) the lung is an important site of immunoglobulin production; (b) activated lung T lymphocytes play an important role in modulating this local production of antibody, and thus are likely to modulate the polyclonal hyperglobulinemia observed in these individuals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carrington C. B. Structure and function in sarcoidosis. Ann N Y Acad Sci. 1976;278:265–283. doi: 10.1111/j.1749-6632.1976.tb47038.x. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Rossman M. D. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980 Mar;92(3):406–416. doi: 10.7326/0003-4819-92-3-406. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., McMillan L. J., Dauber J. H., Rossman M. D. Immune complexes in sarcoidosis: a correlation with activity and duration of disease. Chest. 1978 Sep;74(3):261–264. doi: 10.1378/chest.74.3.261. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Rowlands D. T. Antibodies to T cells in sarcoidosis. Ann N Y Acad Sci. 1976;278:88–100. doi: 10.1111/j.1749-6632.1976.tb47019.x. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., DeHoratius R., Israel H., Peake G. T., Messner R. P. Suppressor cell function in sarcoidosis. Ann Intern Med. 1979 Feb;90(2):169–173. doi: 10.7326/0003-4819-90-2-169. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Kueppers F., DeRemee R. A., Huston K. A., McDuffie F. C. Pulmonary and extrapulmonary sarcoidosis in relation to circulating immune complexes: a quantification of immune complexes by two radioimmunoassays. Am Rev Respir Dis. 1977 Aug;116(2):261–266. doi: 10.1164/arrd.1977.116.2.261. [DOI] [PubMed] [Google Scholar]
- Hedfors E., Norberg R. Evidence for circulating immune complexes in sarcoidosis. Clin Exp Immunol. 1974 Mar;16(3):493–496. [PMC free article] [PubMed] [Google Scholar]
- Hirshaut Y., Glade P., Vieira B. D., Ainbender E., Dvorak B., Siltzbach L. E. Sarcoidosis, another disease associated with serologic evidence for herpes-like virus infection. N Engl J Med. 1970 Sep 3;283(10):502–506. doi: 10.1056/NEJM197009032831002. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fulmer J. D., Young R. C., Jr, Gadek J. E., Crystal R. G. Localization of the immune response in sarcoidosis. Am Rev Respir Dis. 1979 Jul;120(1):49–57. doi: 10.1164/arrd.1979.120.1.49. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Katz P., Fauci A. S. Inhibition of polyclonal B-cell activation by suppressor monocytes in patients with sarcoidosis. Clin Exp Immunol. 1978 Jun;32(3):554–562. [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. N., Scadding J. G. Sarcoidosis. Am Rev Respir Dis. 1974 Dec;110(6):774–802. doi: 10.1164/arrd.1974.110.6P1.774. [DOI] [PubMed] [Google Scholar]
- Oreskes I., Siltzbach L. E. Changes in rheumatoid factor activity during the course of sarcoidosis. Am J Med. 1968 Jan;44(1):60–67. doi: 10.1016/0002-9343(68)90237-4. [DOI] [PubMed] [Google Scholar]
- Rosen Y., Athanassiades T. J., Moon S., Lyons H. A. Nongranulomatous interstitial pneumonitis in sarcoidosis. Relationship to development of epithelioid granulomas. Chest. 1978 Aug;74(2):122–125. doi: 10.1378/chest.74.2.122. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Heesom N. The production of granulomata by antigen-antibody complexes. J Pathol. 1969 May;98(1):31–39. doi: 10.1002/path.1710980105. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]



