Abstract
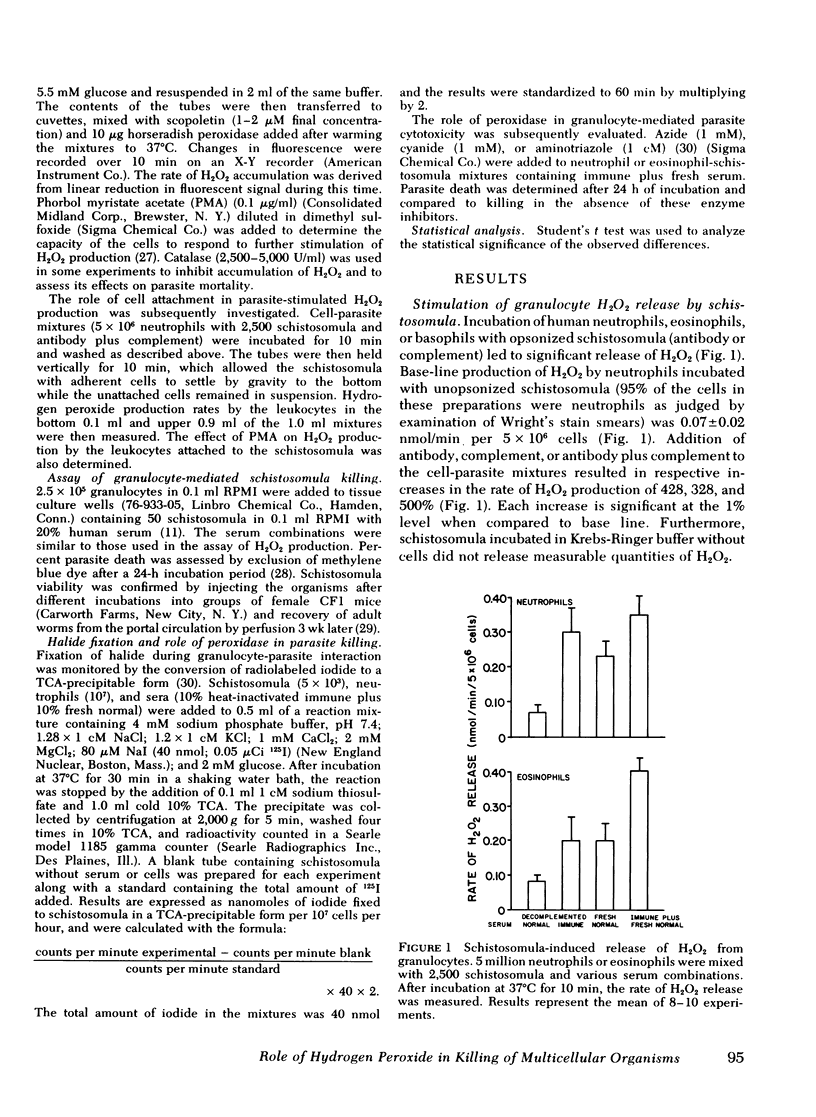
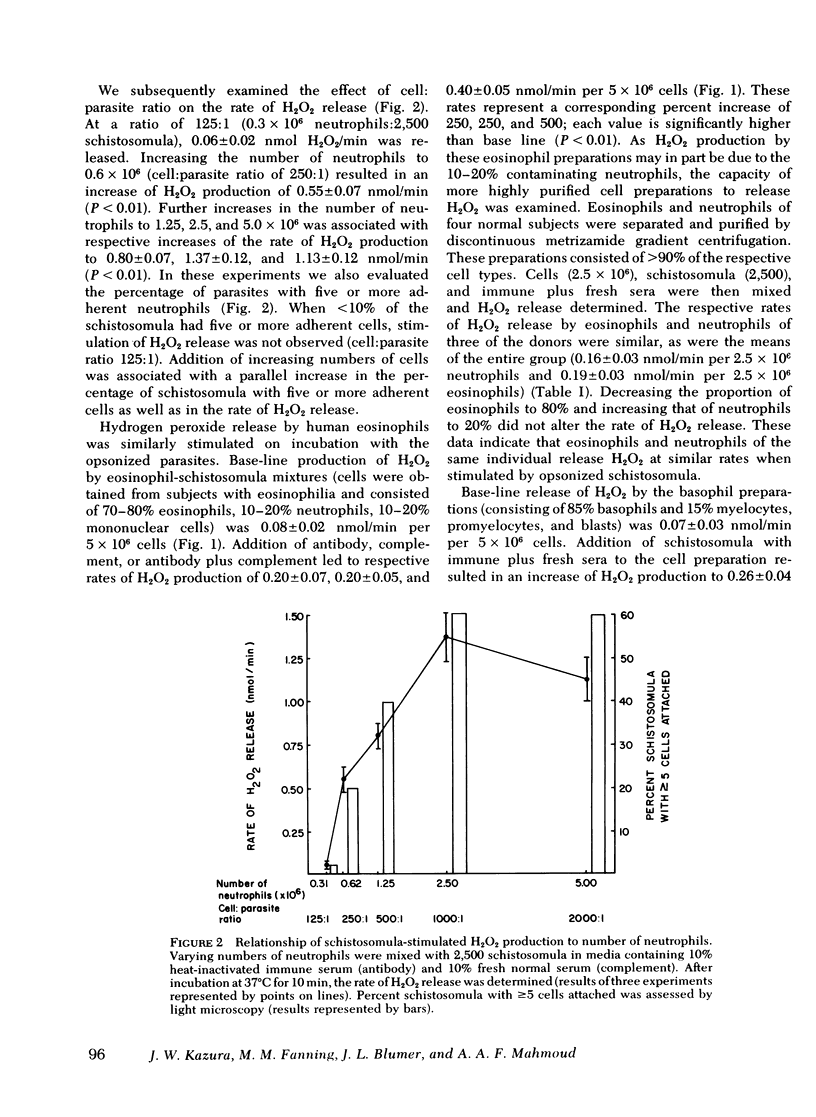
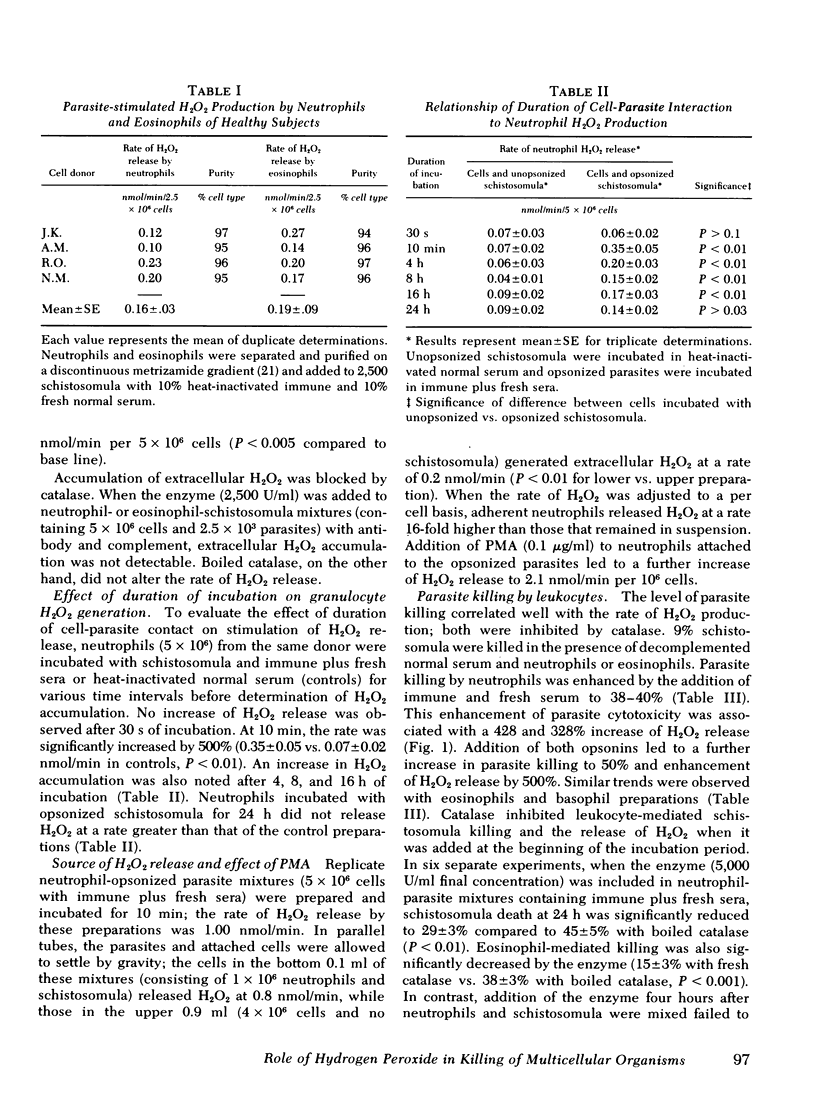
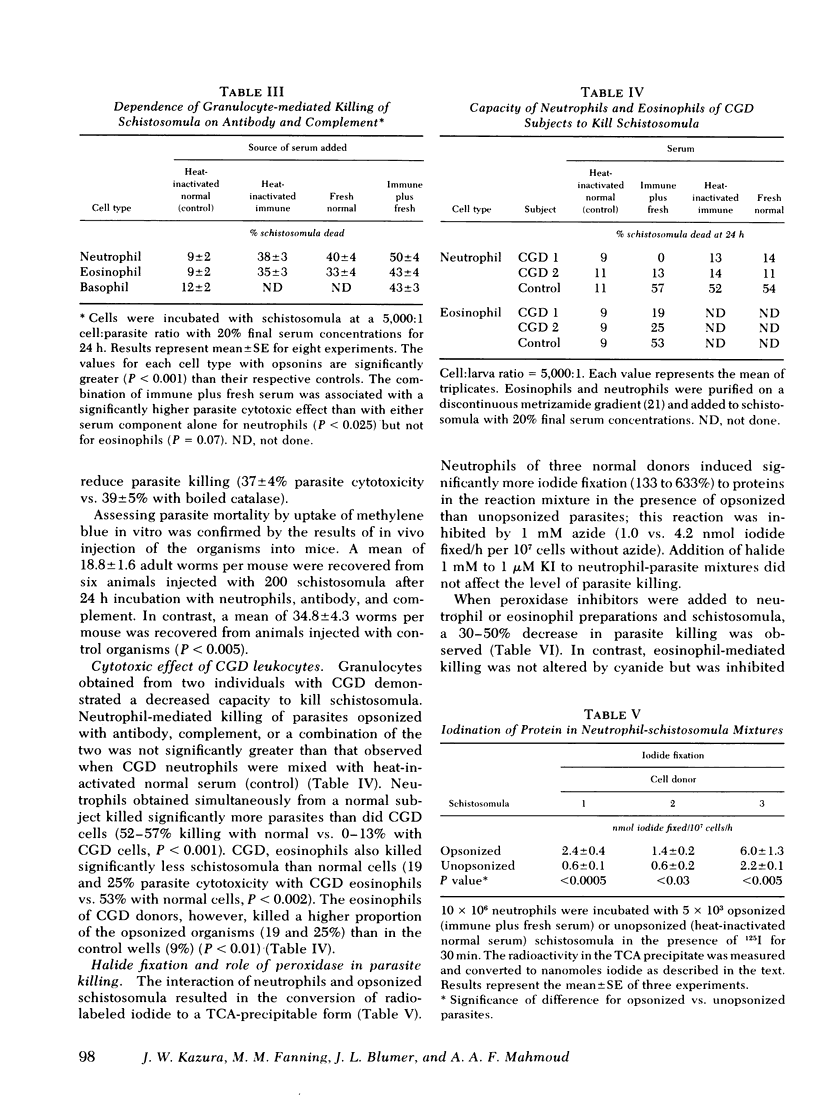
Human as well as murine granulocytes have been shown to kill the larval stages of helminth parasites; the mechanism of this cell-mediated cytotoxicity is, however, poorly understood. The present study was designed to assess the role of peroxidative processes in killing of schistosomula of Schistosoma mansoni by human granulocytes in vitro. The rate of H2O2 production by human neutrophils, eosinophils, and basophils was measured upon incubation with schistosomula alone or in the presence of specific antibody or complement. Opsonized parasites (antibody and/or complement) increased the rate of H2O2 production by neutrophils, eosinophils, and basophils by respective percentages of 500, 500, and 371. The rate of H2O2 release was directly related to the number of granulocytes and to the proportion of cells attached to the surface of the schistosomula. Increased hydrogen peroxide release occurred by 10 min of incubation and was demonstrable up to 16 h after addition of leukocytes to schistosomula. The primary source of this oxygen product was found to be the granulocytes adherent to the schistosomula and not those that remained unattached. Hydrogen peroxide production by neutrophils and eosinophils was quantitatively similar (schistosomula coated with antibody plus complement stimulated 5 × 106 neutrophils and eosinophils to release H2O2 at respective rates of 0.35 and 0.40 nmol/min). Granulocyte-mediated parasite killing correlated with rate of H2O2 generation; both processes were inhibited by catalase. To define further the role of oxidative metabolites, neutrophils and eosinophils of two subjects with chronic granulomatous disease were used; marked reduction of granulocyte-mediated parasite mortality was observed.
Peroxidase was required for H2O2-mediated killing. Addition of the peroxidase inhibitors azide (1 mM), cyanide (1 mM), or aminotriazole (1 cM) to neutrophilschistosomula mixtures significantly reduced parasite cytotoxicity (P < 0.01); similar reduction was observed when eosinophils were used (P < 0.01). Fixation of halide (iodide) to trichloroacetic acid-precipitable protein (2.4-6.0 nmol/h per 107 neutrophils) was demonstrated in the presence of granulocytes, opsonins, and parasites; this process was completely inhibited by 1 mM azide.
These data indicate that contact between the surfaces of human granulocytes and schistosomula results in release of cellular hydrogen peroxide and iodination. The generation of H2O2 and its interaction with peroxidase appear to be crucial in effecting in vitro granulocyte-mediated parasite cytotoxicity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar A. R., Smithers S. R., Kay A. B. Killing of schistosomula of Schistosoma mansoni coated with antibody and/or complement by human leukocytes in vitro: requirement for complement in preferential killing by eosinophils. J Immunol. 1979 Feb;122(2):628–637. [PubMed] [Google Scholar]
- Arap Siongok T. K., Mahmoud A. A., Ouma J. H., Warren K. S., Muller A. S., Handa A. K., Houser H. B. Morbidity in Schistosomiasis mansoni in relation to intensity of infection: study of a community in Machakos, Kenya. Am J Trop Med Hyg. 1976 Mar;25(2):273–284. doi: 10.4269/ajtmh.1976.25.273. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Metabolic and bactericidal activities of human eosinophils. Br J Haematol. 1971 Mar;20(3):277–285. doi: 10.1111/j.1365-2141.1971.tb07038.x. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Szejda P. Eosinophils versus neutrophils in host defense. Killing of newborn larvae of Trichinella spiralis by human granulocytes in vitro. J Clin Invest. 1979 Nov;64(5):1415–1422. doi: 10.1172/JCI109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A., Szejda P. Mechanisms of killing of newborn larvae of Trichinella spiralis by neutrophils and eosinophils. Killing by generators of hydrogen peroxide in vitro. J Clin Invest. 1979 Dec;64(6):1558–1564. doi: 10.1172/JCI109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E. Effector mechanisms against schistosomes in vitro. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):29–38. doi: 10.4269/ajtmh.1977.26.29. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Wassom D. L., Gleich G. J., Loegering D. A., David J. R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979 Jan;122(1):221–229. [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Joseph M., Torpier G., Capron M., Rousseaux R., Santoro F., Bazin H. IgE and cells in schistosomiasis. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):39–47. doi: 10.4269/ajtmh.1977.26.39. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. A., Smithers S. R. The effects of immune rhesus monkey serum on schistosomula of Schistosoma mansoni during cultivation in vitro. Int J Parasitol. 1972 Mar;2(1):79–98. doi: 10.1016/0020-7519(72)90036-7. [DOI] [PubMed] [Google Scholar]
- Coles G. C. Carbohydrate metabolism of larval Schistosoma mansoni. Int J Parasitol. 1972 Sep;2(3):341–352. doi: 10.1016/0020-7519(72)90072-0. [DOI] [PubMed] [Google Scholar]
- Conrad M. E. Hematologic manifestations of parasitic infections. Semin Hematol. 1971 Jul;8(3):267–303. [PubMed] [Google Scholar]
- David J. R., Butterworth A. E., Remold H. G., David P. H., Houba V., Sturrock R. F. Antibody-dependent, eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: effect of metabolic inhibitors and other agents which alter cell function. J Immunol. 1977 Jun;118(6):2221–2229. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., McPhail L. C., Huntley C. C., Muss H. B., Bass D. A. Oxidative metabolism of the human eosinophil. Blood. 1977 Sep;50(3):525–535. [PubMed] [Google Scholar]
- Dean D. A., Wistar R., Chen P. Immune response of guinea pigs to Schistosoma mansoni. I. In vitro effects of antibody and neutrophils, eosinophils and macrophages on schistosomula. Am J Trop Med Hyg. 1975 Jan;24(1):74–82. doi: 10.4269/ajtmh.1975.24.74. [DOI] [PubMed] [Google Scholar]
- Dean D. A., Wistar R., Murrell K. D. Combined in vitro effects of rat antibody and neutrophilic leukocytes on schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1974 May;23(3):420–428. doi: 10.4269/ajtmh.1974.23.420. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Mahmoud A. A. Killing of schistosomula of Schistosoma mansoni by normal human monocytes. J Immunol. 1979 Aug;123(2):949–951. [PubMed] [Google Scholar]
- Glauert A. M., Butterworth A. E., Sturrock R. F., Houba V. The mechansim of antibody-dependent, eosinophil-mediated damage to schistosomula of Schistosoma mansoni in vitro: a study by phase-contrast and electron microscopy. J Cell Sci. 1978 Dec;34:173–192. doi: 10.1242/jcs.34.1.173. [DOI] [PubMed] [Google Scholar]
- Goldring O. L., Clegg J. A., Smithers S. R., Terry R. J. Acquisition of human blood group antigens by Schistosoma mansoni. Clin Exp Immunol. 1976 Oct;26(1):181–187. [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong E. C., Henderson W. R., Klebanoff S. J. Bactericidal activity of eosinophil peroxidase. J Immunol. 1980 Mar;124(3):1378–1382. [PubMed] [Google Scholar]
- Kassis A. I., Aikawa M., Mahmoud A. F. Mouse antibody-dependent eosinophil and macrophage adherence and damage to schistosomula of Schistosoma mansoni. J Immunol. 1979 Feb;122(2):398–405. [PubMed] [Google Scholar]
- Kazura J. W., Aikawa M. Host defense mechanisms against Trichinella spiralis infection in the mouse: eosinophil-mediated destruction of newborn larvae in vitro. J Immunol. 1980 Jan;124(1):355–361. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Mahmoud A. A., Kellermeyer R. W., Warren K. S. Production of monospecific rabbit antihuman eosinophil serums and demonstration of a blocking phenomenon. N Engl J Med. 1974 Feb 21;290(8):417–420. doi: 10.1056/NEJM197402212900801. [DOI] [PubMed] [Google Scholar]
- McLaren D. J., Clegg J. A., Smithers S. R. Acquisition of host antigens by young Schistosoma mansoni in mice: correlation with failure to bind antibody in vitro. Parasitology. 1975 Feb;70(1):67–75. doi: 10.1017/s0031182000048873. [DOI] [PubMed] [Google Scholar]
- McLaren D. J., Mackenzie C. D., Ramalho-Pinto F. J. Ultrastructural observations on the in vitro interaction between rat eosinophils and some parasitic helminths (Schistosoma mansoni, Trichinella spiralis and Nippostrongylus brasiliensis). Clin Exp Immunol. 1977 Oct;30(1):105–118. [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., McLaren D. J., Smithers S. R. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J Exp Med. 1978 Jan 1;147(1):147–156. doi: 10.1084/jem.147.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson J. C., Sher A., Caulfield J. P. Newly transformed schistosomula spontaneously lose surface antigens and C3 acceptor sites during culture. J Immunol. 1980 Apr;124(4):2055–2057. [PubMed] [Google Scholar]
- Smithers S. R., Gammage K. Recovery of Schistosoma mansoni from the skin, lungs and hepatic portal system of naive mice and mice previously exposed to S. mansoni: evidence for two phases of parasite attrition in immune mice. Parasitology. 1980 Apr;80(2):289–300. doi: 10.1017/s0031182000000755. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Tai P. C. Studies on blood eosinophils. II. Patients with Löffler's cardiomyopathy. Clin Exp Immunol. 1976 Jun;24(3):423–434. [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Douglass K. H., McIntyre P. A. Hydrogen peroxide production and killing of Staphylococcus aureus by human polymorphonuclear leukocytes. Blood. 1977 Mar;49(3):437–444. [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]



