Abstract
The main culprit in the pathogenesis of ischemia/reperfusion (I/R) injury is the overproduction of reactive oxygen species (ROS). Hydrogen peroxide (H2O2), the most abundant form of ROS produced during I/R, causes inflammation, apoptosis and subsequent tissue damages. Here, we report H2O2-responsive antioxidant nanoparticles formulated from copolyoxalate containing vanillyl alcohol (VA) (PVAX) as a novel I/R-targeted nanotherapeutic agent. PVAX was designed to incorporate VA and H2O2-responsive peroxalate ester linkages covalently in its backbone. PVAX nanoparticles therefore degrade and release VA, which is able to reduce the generation of ROS, and exert anti-inflammatory and anti-apoptotic activity. In hind-limb I/R and liver I/R models in mice, PVAX nanoparticles specifically reacted with overproduced H2O2 and exerted highly potent anti-inflammatory and anti-apoptotic activities that reduced cellular damages. Therefore, PVAX nanoparticles have tremendous potential as nanotherapeutic agents for I/R injury and H2O2-associated diseases.
Ischemia/reperfusion (I/R) injury is cellular damage after reperfusion of previously ischemic tissues, and has been associated with several pathophysiological conditions including coronary arterial disease and stroke1,2,3,4,5. Reperfusion of blood flow to the ischemic tissues results in a large generation of toxic reactive oxygen species (ROS) and exacerbates initial tissue damages, which is the main culprit in the pathogenesis of I/R injury. In particular, hydrogen peroxide (H2O2) induces release of pro-inflammatory cytokines and triggers apoptosis, leading to the oxidative damage to tissues6,7. Therefore, targeting H2O2 as a diagnostic marker and therapeutic agent has tremendous potential.
Nanomaterials are being explored for many clinical applications in medicine8. Because nanomaterials possess greater permeability than other materials and could be formulated to respond to specific environmental factors, such as pH or temperature9,10, they hold great potential to be utilized in diagnosing and treating various medical conditions. In this study, we developed H2O2-responsive nanoparticles as I/R-targeted diagnostic and therapeutic agents and characterized their potential in animal models.
Results
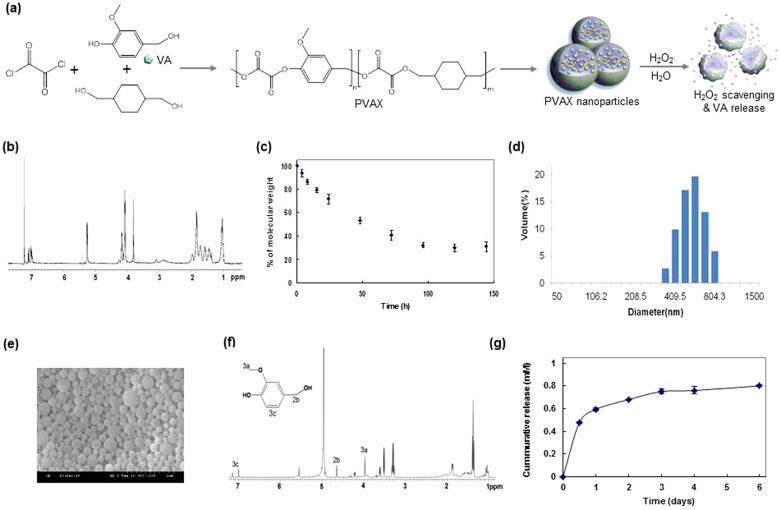
We molecularly engineered PVAX to exploit the therapeutic activity of bioactive VA and the ability of peroxalate ester bonds to rapidly react with H2O2. VA is an active pharmaceutical ingredient in Gastrodia elata Blume, an herbal agent for brain ischemic injury and coronary heart diseases, and it exerts antioxidant, anti-inflammatory and anti-nociceptive activity11,12. PVAX was synthesized from a one-step polymerization of oxalyl chloride, VA and 1,4-cyclohexanedimethanol (Fig. 1a). PVAX possesses peroxalate ester bonds and VA covalently incorporated in its backbone. The chemical structure of PVAX was confirmed by 1H NMR and its molecular weight was determined to be ~12,000 Da with polydispersity of 1.6 (Fig. 1b). Despite its rapid hydrolysis kinetics with a half-life of ~36 h at pH 7.4 (Fig. 1c), PVAX was formulated into the solid nanoparticles under aqueous conditions because of its intrinsic hydrophobicity. PVAX nanoparticles were round spheres and their hydrodynamic diameter was determined to be ~500 nm (Fig. 1d and 1e).
Figure 1. Chemical characterization of H2O2-activatable PVAX nanoparticles.
(a) A schematic diagram of PVAX nanoparticle + synthesis and therapy for I/R. (b) 1H NMR spectrum of PVAX in CDCl3. 1H NMR in deuterated chloroform on a 400 MHz spectrometer: 7.0 ~ 7.3 (m, 3H, Ar), 5.3 (m, 2H OCH2-PhO-CH3), 4.1 ~ 4.2 (m, 4H, COOCH2CH), 3,8 (m, 3H, OCH3), 2.2 (m, 2H, C(CH2)3HO), 1.0 ~ 1.8 (m, 8H, Cyclic CH2). (c) Hydrolysis kinetics of PVAX under physiological conditions, N = 4/group. (d) A representative dynamic light scattering of PVAX nanoparticles suspended in PBS. (e) A representative SEM image of empty PVAX nanoparticles. (f) 1H NMR spectrum of PVAX after hydrolysis of 2 days in D2O. (g) Release kinetics of VA from PVAX.
PVAX was designed to release VA during its hydrolytic degradation under physiological conditions. In order to confirm the VA release from PVAX, PVAX was incubated in H2O at 37°C for 3 days and the supernatant was collected for 1H NMR. As shown in Fig. 1f, PVAX underwent hydrolytic degradation to release VA. We then investigated the release kinetics of VA from the PVAX nanoparticles under the physiological conditions. PVAX nanoparticles (1 mg/mL) released ~120 μg of VA during their hydrolytic degradation and more than half of the VA was released within 24 h (Fig. 1g). The rapid hydrolysis and VA release may provide considerable benefits for the treatment of diseases that require the fast onset of therapeutic action, such as acute liver injury and vascular diseases.
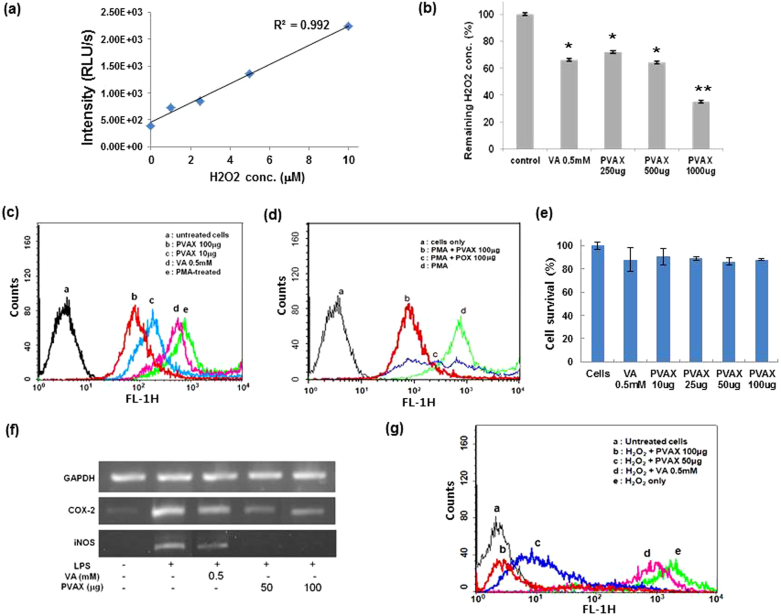
PVAX contains peroxalate ester bonds in its backbone, which are able to perform peroxalate chemiluminescence reaction in the presence of H2O2 and fluorophore. We therefore formulated chemiluminescent PVAX nanoparticles which encapsulate fluorophore rubrene (Rb) and investigated whether chemiluminescent PVAX nanoparticles could detect H2O2 by performing a three-component peroxalate chemiluminescence reaction. PVAX nanoparticles encapsulated with rubrene luminesced in the presence of H2O2, with a linear relationship between the chemiluminescence intensity and H2O2 concentration (Fig. 2a). PVAX nanoparticles should also scavenge H2O2 because peroxalate ester bonds in PVAX will react with H2O2 to generate dioxetanedione intermediates, which then should instantaneously decompose into CO2.
Figure 2. Anti-oxidant and anti-inflammatory properties of PVAX nanoparticles in vitro.
(a) Sensitivity of chemiluminescent PVAX nanoparticles to H2O2. (b) Scavenging of H2O2 by PVAX nanoparticles. *, P < 0.01 versus Control; **, P < 0.01 versus VA, N = 4/group. (c) Inhibition of ROS generation by PVAX in PMA-stimulated macrophages. (d) Comparison in antioxidant activity of PVAX and POX. (e) Cytotoxicity of PVAX nanoparticles determined by MTT assay. VA = vanillyl alcohol. N = 4/group. (f) Anti-inflammatory activity of PVAX in LPS-stimulated macrophages. (g) Anti-apoptotic property of PVAX in H2O2-stimulated macrophages.
We also found that PVAX nanoparticles dramatically reduced the H2O2 concentration after 24 h in a dose-dependent manner, demonstrating that peroxalate ester bonds in PVAX undergo H2O2-mediated oxidation (Fig. 2b). Interestingly, VA also showed moderate H2O2 scavenging activity. Therefore, the highly potent H2O2 scavenging activity of PVAX nanoparticles is attributed to the combined effects of peroxalate ester bonds and VA released. The antioxidant activity of PVAX nanoparticles was investigated by measuring the level of intracellular ROS in RAW 264.7 macrophages stimulated with phorbol-12-myristate-13-acetate (PMA) using dichlorofluorescein-diacetate (DCFH-DA) as a marker of intracellular oxidative stress13,14,15. PMA treatment resulted in strong dichlorofluorescein (DCF) fluorescence in cells, which is indicative of oxidative stress in cells (Fig. 2c). VA (0.5 mM) slightly suppressed ROS generation, but PVAX nanoparticles remarkably inhibited the intracellular ROS generation. In order to further confirm the inhibitory effects of VA on ROS generation, we also prepared polyoxalate (POX) which has only aliphatic peroxalate ester bonds, but does not release VA. PVAX nanoparticles exhibited significantly more reduction in PMA-induced ROS generation than POX nanoparticles (Fig. 2d). These results demonstrate that the PVAX nanoparticles exert strong antioxidant activities by first scavenging intracellular H2O2 and then releasing VA that inhibits the further generation of ROS. In addition, cell toxicity study using MTT assay revealed that PVAX nanoparticles showed excellent biocompatibility (Fig. 2e).
We then evaluated the anti-inflammatory activities of PVAX nanoparticles in cells stimulated with lipopolysaccharide (LPS) by measuring the level of mRNA of genes related to inflammation. LPS stimulation induced remarkable expression of mRNA of pro-inflammatory mediators, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (Fig. 2f). VA (0.5 mM) suppressed the expression of these pro-inflammatory mediators without changes in the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), an internal control. However, PVAX nanoparticles exhibited the stronger inhibitory effect on the mRNA expression of iNOS and COX-2 in the LPS-stimulated cells than free VA.
We then investigated anti-apoptotic activities of PVAX nanoparticles in H2O2-stimulated cells (Fig. 2g). H2O2-stimulation activated the apoptotic cascade in cells, as evidenced in rightward shift in FITC-Annexin V fluorescence by flow cytometry, in good agreement with the literature16,17. In contrast, PVAX nanoparticles exerted inhibitory effects on H2O2-induced apoptosis in a dose-dependent manner. A dose of 100 μg of PVAX nanoparticles significantly (~71%) inhibited H2O2-induced apoptosis. Highly potent anti-inflammatory and anti-apoptotic activities of PVAX nanoparticles may be attributed to the combined effects of their antioxidant property and VA release. Taken together, PVAX nanoparticles demonstrate great synergistic therapeutic effects as a polymeric prodrug of VA as well as a highly potent H2O2-scavenging agent.
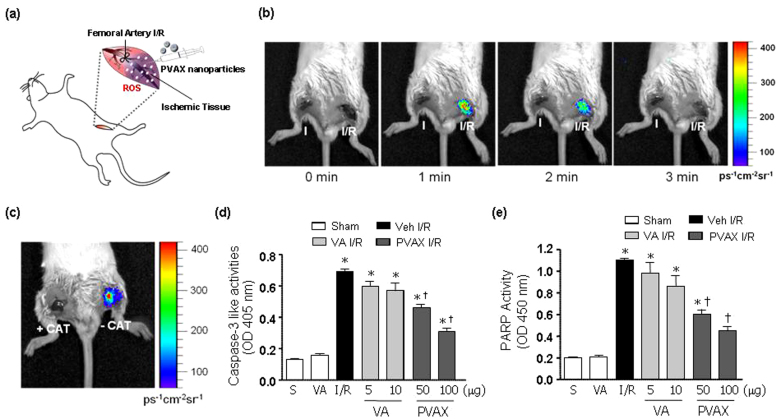
To further extrapolate our in vitro findings, we investigated potential of H2O2-responsive PVAX nanoparticles as I/R-targeted therapeutic agents using a mouse model of hind-limb I/R injury (Fig. 3a). Initially, we tested H2O2-responsiveness of PVAX nanoparticles by determining whether chemiluminescent PVAX nanoparticles could image H2O2 generated endogenously during I/R. Ischemia was induced for 45 minutes in both limbs and chemiluminescent PVAX nanoparticles (PVAX/Rb) were directly injected just distal to the ligation sites (50 μg PVAX/Rb per site). Left hind limb was reperfused (I/R) but right hind-limb remained ligated (I). Chemiluminescent images were then captured at different time points. The site of I/R injury exhibited an intense chemiluminescence light emission lasting about 2 min after reperfusion demonstrating that chemiluminescent PVAX nanoparticles are capable of imaging endogenously generated H2O2 (Fig. 3b). Negligible chemiluminescent emission was observed at the site of ischemia only. To confirm whether PVAX nanoparticles are specific for H2O2, H2O2-degrading enzyme catalase was injected prior to the injection of PVAX nanoparticles. Pre-administration of catalase almost completely inhibited chemiluminescence emission at the site of I/R injury (Fig. 3c), further demonstrating that PVAX nanoparticles specifically detect H2O2. To our best understanding, this is the first study to report the imaging of endogenously generated H2O2 in I/R injury.
Figure 3. Bioimaging and apoptosis in hind-limb I/R model after PVAX in vivo.
(a) A schematic diagram of hind-limb ischemia/reperfusion protocol. (b) In vivo imaging of PVAX/Rb after I/R in mouse hind-limbs. Reperfusion at different time points as indicated. Acquisition time = 30 sec/image. (c) In vivo imaging of PVAX/Rb with and without catalase. (d - e) Quantification of caspase-3 activities (d) and PARP-1 activity (e) after I/R with different concentrations of PVAX in gastrocnemius muscles. *, P < 0.05 vs Sham; †, P < 0.05 vs Veh + IR. N = 4–6/group.
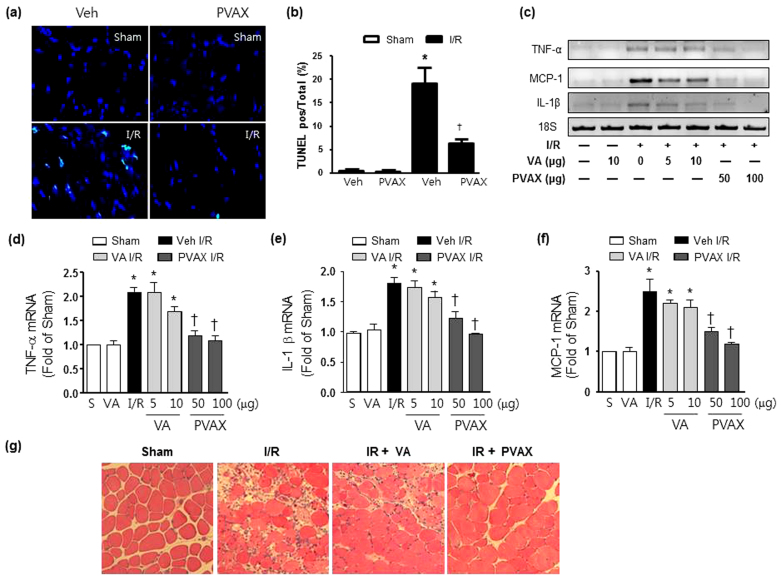
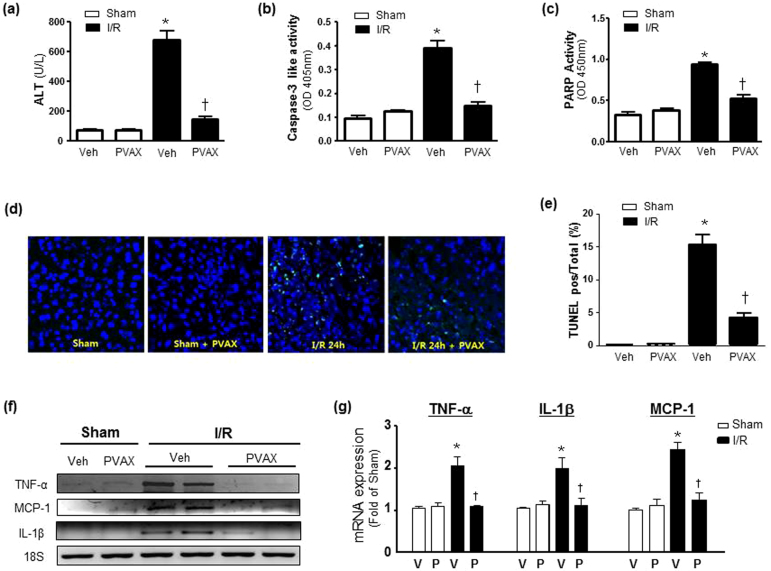
To test the therapeutic potential of PVAX nanoparticles for I/R injury, we injected PVAX nanoparticles in the gastrocnemius muscle after hind-limb I/R. Since I/R is known to induce apoptosis and cellular damage, we examined the ability of PVAX nanoparticles to inhibit the activation of polyADP ribose polymerase-1 (PARP-1) and caspase-3, both critical enzymes involved in apoptosis18,19,20. Since VA has been shown to have antioxidant and anti-inflammatory effect by itself, we injected the equivalent amount of VA (by weight, 1 VA = 10 PVAX) in a contralateral leg for comparison with PVAX. After I/R, there was significant activation of PARP-1 and caspase-3 (Fig. 3d and 3e). Treatment of PVAX nanoparticles showed significant inhibition of PARP-1 and caspase-3 activation by I/R in a dose dependent manner. In comparison, VA alone at the equivalent amounts contained in PVAX was able to modestly inhibit PARP-1 and caspase-3 activation only at the highest dose. Furthermore, treatment of PVAX nanoparticles demonstrated significant decrease in apoptotic myocytes after I/R compared to VA alone (Fig. 4a and 4b). There was also significant attenuation of various inflammation markers, such as tumor necrosis factor-alpha (TNF-α), monocyte chemotactic protein-1 (MCP-1), and interleukin -1β (IL-1β), and, after I/R in PVAX group compared to the I/R + vehicle group (Fig. 4c–4f). VA alone showed no significant suppression of these inflammatory markers. In addition, histological analysis showed that significant muscle damage induced by I/R injury was effectively blocked by PVAX nanoparticles (Fig. 4g). The higher therapeutic effects of PVAX than VA can be explained by the synergistic effects of H2O2-scavening peroxalate ester bonds and antioxidant and anti-inflammatory effect of VA. This study provides proof-of-concept that molecularly engineered PVAX nanoparticles are able to scavenge and image overproduced H2O2 and serve as I/R targeted therapeutic agents.
Figure 4. Cell death and inflammation in hind-limb I/R model after PVAX in vivo.
(a) Representative TUNEL staining of gastrocnemius muscles after I/R with and without PVAX. (b) Quantification of apoptosis rate in gastrocnemus muscles after I/R with and without PVAX. *, P < 0.05 versus Sham; †, P < 0.05 versus Veh + I/R. N = 4–6/group. (c) mRNA levels of factors associated with inflammation after I/R with different concentrations of PVAX. (d–f) Quantification of TNF-α (d), IL-1β (e), and MCP-1 (f) mRNA levels after I/R with different concentrations of PVAX in HL I/R model. *, P < 0.05 vs Sham; †, P < 0.05 vs Veh + IR. N = 4-6/group. (g) Haematoxylin and eosin-stained histological sections of gastrocnemius muscle showed less damage and less apparent leucocyte infiltration in muscles in PVAX treated mice after I/R.
In order to further demonstrate the therapeutic potential of PVAX nanoparticles in another clinically relevant setting, we used a mouse model of hepatic I/R. Hepatic I/R is a feature of many clinically important scenarios including liver transplantation21,22. In this model, PVAX (3 mg/kg) nanoparticles were injected intraperitoneally (i.p.) one hour prior to performing I/R in liver and again given just after reperfusion to evaluate their therapeutic potential. After 1 h of ischemia and 6 h of reperfusion, a dramatic increase in the serum alanine transaminase (ALT) activity, a marker of liver damage, was observed in a vehicle-treated group, compared with sham-operated controls (Fig. 5a). PVAX therapy significantly attenuated the serum ALT elevations induced by I/R. In addition, I/R markedly increased the PARP-1 and caspase activities in the liver, which was prevented by PVAX (Fig. 5b and 5c). Treatment with PVAX nanoparticles was also effective in decreasing apoptosis in hepatocytes after I/R compared to the saline I/R group (Fig. 5d and 5e). Furthermore, I/R significantly increased the mRNA expression of the proinflammatory cytokine TNF-α, MCP-1 and IL-1β (Fig. 5f and 5g). The I/R-induced acute hepatic proinflammatory responses, likely orchestrated by activated Kupffer and endothelial cells, were significantly attenuated by PVAX nanoparticles. Based on these findings, we conclude that PVAX nanoparticles have potential to be used as effective I/R-targeted nano-therapeutic agents and intrinsic antioxidant and anti-inflammatory properties of PVAX may further contribute to their overall beneficial effect during I/R injury.
Figure 5. I/R-specific therapy using PVAX in hepatic I/R model in vivo.
(a) Serum ALT level after 60 min ischemia and 6 h reperfusion with or without i.p. administration of PVAX. *, P < 0.05 versus Sham; †, P < 0.05 versus Veh + I/R. N = 4–6/group. (b–c) Quantification of caspase-3 activities (b) and PARP-1 activity (c) of the liver after I/R with and without PVAX. *, P < 0.05 versus Sham; †, P < 0.05 versus Veh + I/R. N = 4–6/group. (d) Representative TUNEL staining of liver after I/R with and without PVAX. (e) Quantification of apoptosis rate in liver after I/R with and without PVAX. *, P < 0.05 versus Sham; †, P < 0.05 versus Veh + I/R. N = 4–6/group. (f) Semi-quantitative PCR of mRNA levels of factors associated with inflammation after i.p. administration of PVAX after hepatic I/R. (g) Quantification of TNF-α, MCP-1 and IL-1β mRNA levels after I/R with or without PVAX in hepatic I/R model. *, P < 0.05 versus Sham; †, P < 0.05 versus Veh + I/R. N = 4–6/group.
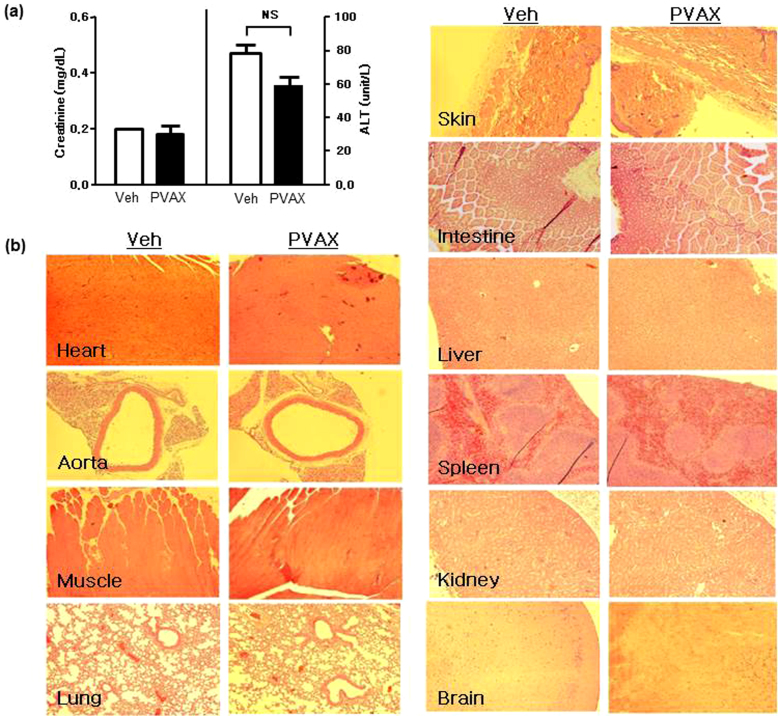
Finally, to test the safety profile of PVAX, we administered 100 μg of PVAX nanoparticles daily for 7 days in mice. Serum tests for renal and hepatic function showed no significant abnormalities after 7 days (Fig. 6a). In addition, there was no obvious histological evidence of accumulated toxicity in the different organs associated with administration of PVAX nanoparticles for 7 days (Fig. 6b), demonstrating the excellent in vivo biocompatibility of PVAX.
Figure 6. Safety profiles of PVAX.
(a) Creatinine and ALT level after daily i.p. administration of PVAX (100 μg/day) for 7 days. NS = not significant. N = 4/group. (b) Representative Hematoxylin and Eosin (H&E)-stained tissue sections of various organs after PVAX. PVAX was administered i.p. at 100 mg/day for 7 days. N = 3/group.
Discussion
Tissue damage is the most important determinant of morbidity and mortality after conditions associated with I/R injuries, such as myocardial infarction, vascular thromboembolic events, post cardiovascular surgery, transplant surgery, and post traumatic injuries2,5,23,24. Therefore, limiting cellular death is paramount for favorable outcomes in these conditions. Particularly, suppression of ROS overproduction during I/R using various antioxidants have been shown to effectively block the deleterious effects of ROS, such as apoptosis, in experimental settings in vitro and in vivo4,25. However, the beneficial effects of antioxidant therapy in human clinical studies have been disappointing due mainly to non- specific suppression of ROS in the body26,27. I/R-specific drug formulations would allow targeted release of drugs into specific areas or tissues that are undergoing a pathological process, leading to the enhanced therapeutic efficacy as well as decrease in related side effects. Therefore, H2O2-responsive PVAX nanoparticles may be able to serve as I/R targeted nanotherapeutic agents.
Ideal targeted drug delivery system would have combined target specificity with stimuli responsiveness to enhance the effects of the system10. Several such drug delivery systems have been generated that are responsive to pH, temperature, magnetic field, and concentrations of electrolytes or glucose9,10. PVAX nanoparticles are the first nanotherapeutic system that is shown in animal models to effectively treat tissues undergoing I/R injury by targeting endogenously generated H2O2 with high sensitivity and specificity.
In summary, we present novel I/R targeted nano-therapeutic agents based on molecularly engineered PVAX nanoparticles, which are sensitive and specific to H2O2. PVAX nanoparticles exhibit significant intrinsic antioxidant, anti-inflammatory, and anti-apoptotic activities both in vitro and in vivo models of I/R injury. We anticipate enormous potential of multifunctional PVAX nanoparticles for the H2O2-associated diseases, such as cardiovascular and neurovascular diseases.
Methods
PVAX synthesis
1,4-Cyclohexanedimethanol (21.96 mmol) and 4-vanillyl alcohol (5.49 mmol) were dissolved in 20 mL of dry tetrahydrofuran (THF), under nitrogen, to which triethylamine (60 mmol) was added dropwise at 4°C. Oxalyl chloride (27.45 mmol) in 25 mL of dry THF was added to the mixture dropwise at 4°C. The reaction was continued at room temperature for 6 h under nitrogen atmosphere and the resulting polymers were obtained through the extraction using dichloromethane and isolation by precipitating in cold hexane. The chemical structure of polymers was identified with a 400 MHz 1H NMR spectrometer and their molecular weight was determined using a gel permeation chromatography (GPC).
Particle preparation and characterization
Fifty milligrams of PVAX dissolved in 500 μL of DCM was added to 5 mL of 10% poly-vinyl alcohol (PVA) solution. The mixture was sonicated using a sonicator (Fisher Scientific, Sonic Dismembrator 500) for 30 sec and a homogenizer (PRO Scientific, PRO 200) for 2 min to form a fine oil/water emulsion. The emulsion was added into 20 mL PVA 1% solution and further homogenized for 1 min. The remaining solvent was removed using a rotary evaporator. PVAX nanoparticles were obtained by centrifuging at 11,000 g for 5 min at 4°C, washing with deionized water twice and lyophilizing the recovered pellet. The morphology and size of PVAX nanoparticles were observed by a scanning electron microscopy (SEM, S-3000N, Hitachi, Japan) with accelerating voltage of 10 Kv. The hydrodynamic size of PVAX nanoparticles was determined using a particle analyzer (ELS-8000, Photal Otsuka Electronics, Japan).
Release kinetics of vanillyl alcohol from PVAX nanoparticles
PVAX nanoparticles (5 mg) were added into 5 mL of phosphate buffer solution (pH 7.4) and the particles suspension was incubated at 37°C with mechanical stirring. At appropriate intervals, the solution was centrifuged at 2000×g for 20 sec and the 1 mL aliquot of supernatant was taken and replaced with an equal volume of fresh phosphate buffer solution. The concentration of vanillyl alcohol in the supernatant was measured using a UV spectrometer (S-3100, Scinco, Korea) and the release kinetics was determined by comparing the concentrations of vanillyl alcohol standard solutions.
Cytotoxicity assay and detection of H2O2
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the cytotoxicity of PVAX nanoparticles. Mouse macrophage RAW 264.7 cells were cultured at a density of 1 × 106 cells/well in a 24 well plate containing 1 mL of culture medium for 24 h. Cells were treated with various amounts of nanoparticles and incubated for 24 h. Each well was given 20 μL of MTT solution and were incubated for 4 h. Two hundred microlitters of dimethyl sulfoxide (DMSO) was added to each well to dissolve the resulting formazan crystals. After 30 min of incubation, the absorbance at 570 nm was measured using a microplate reader (E-Max, Molecular Device Co. US). The cell viability was obtained by comparing the absorbance of nanoparticles-treated cells to that of control cells.
Flow cytometry
RAW 264.7 cells (1 × 106 cells) were cultured for 24 h. Cells were treated with vanillyl alcohol and PVAX nanoparticles for 4 h. Phorbol-12-myristate-13-acetate (PMA, 0.5 mg) was given to cells for the generation of reactive oxygen species and 20 h later cells were treated with DCFH-DA. Cells were also treated with 100 μM of H2O2 for 4 h to induce apoptosis and treated with Annexin-V labeled with FITC for 15 min. Flow cytometry was performed with a Flow Cytometry Caliber (Becton Dickinson, US).
Reverse transcription-polymerase chain reaction (RT-PCR)
The RAW 264.7 cells were plated at a density of 2 × 105 in 24 well tissue culture plates. The cells were pretreated with 0.5 mM vanillyl alcohol and a various amount of PVAX nanoparticles for 24 h and then treated with 1 μL of LPS (1 mg/mL) for 12 h. Total cellular RNA was isolated using 1 mL of TRIzol (Invitrogen, Life Technologies Co, Groningen, Netherlands) according to the manufacturer's instructions. One microgram of total RNA was reverse-transcribed into cDNA using oligo (dT) primer (Invitrogen), 5X First Strand buffer (Invitrogen), dNTP (Gibco), RNase inhibitor (Invitrogen), SuperScript II (Invitrogen), and RNase H reverse transcriptase (Invitrogen). PCR was performed on aliquots of the cDNA preparations to detect iNOS, COX-2, IL-1β and GAPDH (the internal standard) gene expressions. The PCR primers used in this study are listed below: sense iNOS, 5′-AAT GGC AAC ATC AGG TCG GCC ATC ACT-3′, anti-sense iNOS, 5′-GCT GTG TGT CAC AGA AGT CTC GAA CTC-3′; sense COX-2, 5′-GGA GAG ACT ATC AAG ATA GT-3′, anti-sense COX-2, 5′-ATG GTC AGT-AGA CTT TTA CA-3′; sense GAPDH, 5′-TGA ACG GGA AGC TCA CTG G-3′, anti-sense GAPDH, 5′-TCC ACC ACC CTG TTG CTG TA-3′. The PCR primers used for animal tissues are listed below: sense TNF-α, 5′-CCT CAG CCT CTT CTC CTT CCT-3′, anti-sense TNF-α, 5′-GGT GTG GGT GAG GAG CA-3; sense IL-1 β, 5′-CTG AAA GCT CTC CAC CTC-3′, anti-sense IL-1 β reverse, 5′-TGC TGA TGT ACC AGT TGG GG-3′; sense MCP-1 forward, 5′-CCC CAC TCA CCT GCT GCT ACT-3′, anti-sense MCP-1 reverse, 5′-GGC ATC ACA GTC CGA GTC ACA -3′; sense 18S forward, 5′-GTT ATG GTT CCT TTG TCG CTC GCT C-3′, anti-sense 18S reverse, 5′- TCG GCC CGA GGT TAT CTA GAG TCA C-3′. After amplification, portions of the PCR reactions were electrophoresed on 2% agarose gel, and visualized under UV after ethidium bromide staining.
Animal surgeries
Hind limb I/R surgeries were performed in 15–16 week old male mice (Charles River Laboratory, Wilmington, MA). After mice were anaesthetized, femoral artery was identified and tied around a specialized 30G-catheter with a 7-0 silk suture. The animal remained under anesthesia for a specified duration of ischemia. Reperfusion was achieved by cutting the suture and re-establishing arterial blood flow. Sham operated mice underwent the same procedure without femoral artery occlusion/reperfusion. Mice were sacrificed and analyzed at 2 days for biochemical/molecular studies, and at 2 weeks for histological analysis.
Hepatic I/R surgeries were done in 10–12 week-old male mice (Charles River Laboratory, Wilmington, MA). One hour prior to anesthesia, PVAX group mice received 50 μl of PVAX nanoparticle through intraperitoneal route. Mice in saline group received same volume of normal saline. After one hour, all mice were anaesthetized with intraperitoneal injection of mixed solution of Ketamine and Xylazine (8:1 ratio). Midline incision was performed for laparotomy. After identifying the portal triad and biliary tree, the main trunk of hepatic artery and portal vein were clamped with vascular clip except for the vasculatures to the right lower lobe to achieve ischemic injury to approximately 70% of the liver. 60 minutes of ischemic time was allowed in I/R group mice. No vascular clamp was done for Sham group mice. After one hour, reperfusion was achieved by releasing the vascular clip. At that moment, additional 50 μl of PVAX nanoparticle was given to PVAX group, and same amount of saline was administered for Saline group. Then the midline incision was closed with 5-0 black silk suture. Half of the mice in each group were sacrificed at 6 hours for inflammatory activities (TNF-α, IL-1β, and MCP-1) and serum ALT measurements, and the rest were sacrificed at 24 hours for apoptotic activities (caspase-3, PARP-1). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
Chemiluminescence imaging
In vivo bioluminescence imaging was carried out with a Xenogen IVIS 200 imaging system (Caliper LS, Hopkinton, MA). Images and measurements of luminescent signals were acquired and analyzed using Living Image software. Balb/c mice (Orient Bio, Korea) were anesthetized using 1–3% isoflurane, and placed onto the warmed stage inside the camera box. The animals received continuous exposure to 1–2% isoflurane to sustain sedation during imaging. All experiment procedures were performed with the approval of Chonbuk National University Animal Care Committee.
Statistical analyses
Calculations and statistics were performed using GraphPad 5.0 software (GraphPad Software Inc., La Jolla, CA). Statistical analysis was carried out using the one-way analysis of variance (ANOVA) and Bonferroni's tests for post hoc differences between group means. Statistical significance was defined as P < 0.05. Results are presented as mean ± the standard error of the mean (SEM).
Author Contributions
D.L., S.B. and P.M.K. designed and performed experiments, analyzed data and wrote the paper; D.H., H.L., J.H.Y., O.H., S.P., Q.K. performed experiments and analyzed data; G.K. supervised experiments and analyzed data.
Acknowledgments
This work was supported by the World Class University Program (R31-20029) funded by the Ministry of Education, Science and Technology, Korea (DL and PMK), Basic Science Research Program (2010-0021903) of National Research Foundation of Korea (DL) and National Institutes of Health RO1 HL091998 (PMK).
References
- Eltzschig H. K. & Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med 17, 1391–401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell F. W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 10, 620–30 (2002). [DOI] [PubMed] [Google Scholar]
- Collard C. D. & Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 94, 1133–8 (2001). [DOI] [PubMed] [Google Scholar]
- Gottlieb R. A., Burleson K. O., Kloner R. A., Babior B. M. & Engler R. L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94, 1621–8 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeroudi M. O., Hartley C. J. & Bolli R. Myocardial reperfusion injury: role of oxygen radicals and potential therapy with antioxidants. Am J Cardiol 73, 2B–7B (1994). [DOI] [PubMed] [Google Scholar]
- Chang M. C., Pralle A., Isacoff E. Y. & Chang C. J. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc 126, 15392–3 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. W., Albers A. E., Pralle A., Isacoff E. Y. & Chang C. J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc 127, 16652–9 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Khang G. & Lee D. Application of nanomedicine in cardiovascular diseases and stroke. Curr Pharm Des 17, 1825–33 (2011). [DOI] [PubMed] [Google Scholar]
- Lee S., Yang S. C., Kao C. Y., Pierce R. H. & Murthy N. Solid polymeric microparticles enhance the delivery of siRNA to macrophages in vivo. Nucleic Acids Res 37, e145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Zhong Z. & Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules 10, 197–209 (2009). [DOI] [PubMed] [Google Scholar]
- Jung H. J., Song Y. S., Lim C. J. & Park E. H. Anti-angiogenic, anti-inflammatory and anti-nociceptive activities of vanillyl alcohol. Arch Pharm Res 31, 1275–9 (2008). [DOI] [PubMed] [Google Scholar]
- Lee J. Y. et al. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch Pharm Res 29, 849–58 (2006). [DOI] [PubMed] [Google Scholar]
- Carter W. O., Narayanan P. K. & Robinson J. P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol 55, 253–8 (1994). [DOI] [PubMed] [Google Scholar]
- Soh N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal Bioanal Chem 386, 532–43 (2006). [DOI] [PubMed] [Google Scholar]
- Wattamwar P. P. et al. Antioxidant Activity of Degradable Polymer Poly(trolox ester) to Suppress Oxidative Stress Injury in the Cells. Advanced Functional Materials 20, 147–154 (2010). [Google Scholar]
- Sawyer R. T. et al. Beryllium-stimulated reactive oxygen species and macrophage apoptosis. Free Radic Biol Med 38, 928–37 (2005). [DOI] [PubMed] [Google Scholar]
- Mao Y. et al. Hydrogen peroxide-induced apoptosis in human gastric carcinoma MGC803 cells. Cell Biol Int 30, 332–7 (2006). [DOI] [PubMed] [Google Scholar]
- Slee E. A. et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9- dependent manner [In Process Citation]. J Cell Biol 144, 281–92 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L. & Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54, 375–429 (2002). [DOI] [PubMed] [Google Scholar]
- Yu S. W. et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297, 259–63 (2002). [DOI] [PubMed] [Google Scholar]
- Reid K. M. et al. Liver I/R injury is improved by the arginase inhibitor, N(omega)-hydroxy-nor-L-arginine (nor-NOHA). Am J Physiol Gastrointest Liver Physiol 292, G512–7 (2007). [DOI] [PubMed] [Google Scholar]
- Ushitora M. et al. Prevention of hepatic ischemia-reperfusion injury by pre-administration of catalase-expressing adenovirus vectors. J Control Release 142, 431–7 (2010). [DOI] [PubMed] [Google Scholar]
- Schlag M. G., Harris K. A. & Potter R. F. Role of leukocyte accumulation and oxygen radicals in ischemia-reperfusion-induced injury in skeletal muscle. Am J Physiol Heart Circ Physiol 280, H1716–21 (2001). [DOI] [PubMed] [Google Scholar]
- Woodruff T. M. et al. Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J Surg Res 116, 81–90 (2004). [DOI] [PubMed] [Google Scholar]
- Kang P. M., Haunstetter A., Aoki H., Usheva A. & Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 87, 118–25 (2000). [DOI] [PubMed] [Google Scholar]
- Cook N. R. et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med 167, 1610–8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekananthan D. P., Penn M. S., Sapp S. K., Hsu A. & Topol E. J. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361, 2017–23 (2003). [DOI] [PubMed] [Google Scholar]