Abstract
Our objective was to determine if the presence of metabolic complications (MC) conveyed an additional risk for left ventricular (LV) dysfunction in people with HIV. HIV+ and HIV− men and women were categorized into four groups: (1) HIV+ with MC (43±7 years, n=64), (2) HIV+ without MC (42±7 years, n=59), (3) HIV− with MC (44±8 years, n=37), or (4) HIV− controls without MC (42±8 years, n=41). All participants underwent two-dimensional (2-D), Doppler, and tissue Doppler echocardiography. Overall, the prevalence of systolic dysfunction (15 vs. 4%, p=0.02) and LV hypertrophy (9 vs. 1%, p=0.03) was greater in HIV+ than in HIV− participants. Participants with MC had a greater prevalence of LV hypertrophy (10% vs. 1%). Early mitral annular velocity during diastole was significantly (p<0.005) lower in groups with MC (HIV+/MC+: 11.6±2.3, HIV−/MC+: 12.0±2.3 vs. HIV+/MC−: 12.4±2.3, HIV−/MC−: 13.1±2.4 cm/s) and tended to be lower in groups with HIV (p=0.10). However, there was no interaction effect of HIV and MC for any systolic or diastolic variable. Regardless of HIV status, participants with MC had reduced LV diastolic function. Although both the presence of MC and HIV infection were associated with lower diastolic function, there was no additive negative effect of HIV on diastolic function beyond the effect of MC. Also, HIV was independently associated with lower systolic function. Clinical monitoring of LV function in individuals with metabolic risk factors, regardless of HIV status, is warranted.
Introduction
Left ventricular (LV) dysfunction is common in late-stage acquired immune deficiency syndrome (AIDS),1,2 but since the advent of combination antiretroviral therapy (cART) the incidence of AIDS-related cardiomyopathy has significantly declined.3,4 Despite these improvements, evidence from both industrialized5–12 and developing countries9 indicates that the prevalence of subclinical LV dysfunction in individuals with well-controlled HIV infection may approach 50%12,13 and may represent a newly recognized comorbid condition.14,15
Mechanisms for subclinical LV function abnormalities in HIV are unclear. The HIV virus and its related proteins16,17 may play a role in LV dysfunction; however, other factors also likely contribute to impaired contractility.15 HIV infection and cART are associated with adverse metabolic risk factors or complications, frequently referred to as HIV-related metabolic syndrome, that are related to increased risk for myocardial infarction in individuals with HIV.18 This syndrome includes insulin resistance,19,20 dyslipidemia,21,22 and central fat accumulation,23,24 all characteristics of non-HIV-associated metabolic syndrome, a condition associated with higher cardiovascular (CV) disease risk.25–27 In HIV−people, the metabolic syndrome is associated with an increased risk for CV morbidity (including coronary artery disease and congestive heart failure) and mortality.25 Recent evidence has also linked metabolic risk factors to subclinical LV dysfunction in HIV− individuals.25,26,28,29 LV dysfunction is an important predictor of overall mortality in the general population.30
The objective of this study was to determine if the presence of metabolic risk factors/complications similar to the metabolic syndrome in the general population conveys an additional risk for LV dysfunction in men and women infected with HIV. We hypothesized that in participants with HIV infection, metabolic risk factors present an additive risk for LV systolic and diastolic dysfunction over HIV infection alone.
Materials and Methods
Participants
HIV-infected men and women were recruited from the AIDS Clinical Trials Unit, the Infectious Diseases Clinics at Washington University School of Medicine (WUSM), and the surrounding metro St. Louis community. HIV− men and women were recruited from the Division of Cardiology, the Department of Radiology, and through a volunteer recruitment service at WUSM. Participants were categorized to one of four groups: (1) HIV-positive participants currently taking cART with three or more metabolic risk factors/metabolic complications (MC), (HIV+/MC+; n=64), (2) HIV-positive participants taking cART having one or no metabolic risk factors/complications (HIV+/MC−; n=59), (3) HIV-negative participants with three or more metabolic risk factors/complications (HIV−/MC+; n=37), or (4) HIV-negative healthy controls with similar age, gender, and activity level with one or no metabolic risk factors/complications (HIV−/MC−; n=41). For both HIV+ and HIV− groups, metabolic complications were defined according to a modification of the amended National Cholesterol Education Program's Adult Treatment Panel III (ATP-III) guidelines and a definition of HIV metabolic syndrome.
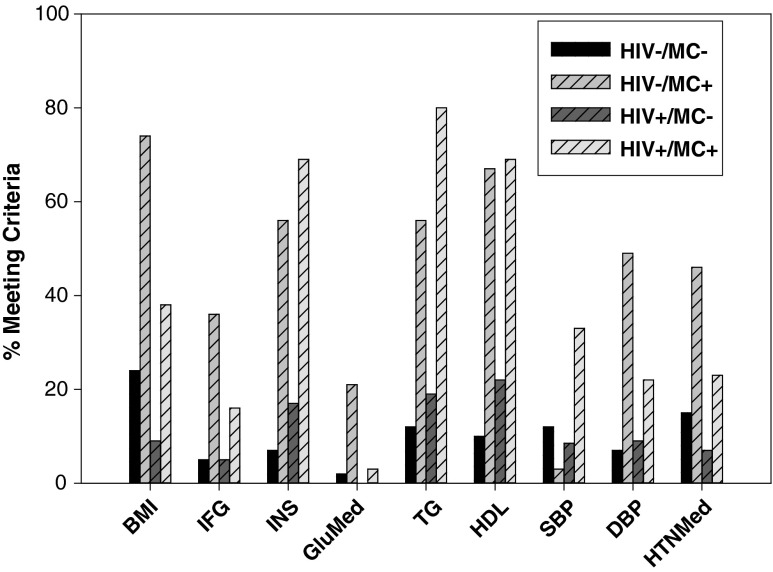
All participants with MC met three or more of the following criteria: (1) body mass index (BMI) ≥30 kg/m2; (2) fasting serum glucose ≥100 mg/dl OR fasting serum insulin ≥13 μU/ml OR use of glucose-lowering medication; (3) fasting serum triglyceride ≥150 mg/dl; (4) resting blood pressure: systolic ≥130 OR diastolic ≥85 mm Hg OR antihypertensive therapy; and (5) low high-density lipoprotein (HDL) (<40 mg/dl in men or <50 mg/dl in women) (Fig. 1).31 Most of the prior research examined the prevalence of the metabolic syndrome in HIV+ individuals using the traditional ATP-III criteria of impaired fasting glucose32–35; however, others have used additional criteria36,37 to define impaired fasting glucose. We included fasting insulin as a criterion for abnormal glucose metabolism because HIV infection is associated with fasting hyperinsulinemia but normal glycemia, while typical metabolic syndrome (HIV−) is associated with fasting hyperinsulinemia and hyperglycemia.24 In addition, due to the known relationship between insulin resistance and diastolic dysfunction,38 we believed it was important to include a measure of fasting insulin in order not to exclude participants who had insulin resistance but normal fasting glucose.
FIG. 1.
Percent meeting individual component of metabolic complication criteria. BMI, body mass index; IFG, impaired fasting glucose; INS, plasma insulin; GluMed, glucose-lowering medication; TG, triglyceride; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; HTNMed, hypertension medication.
BMI was used as a criterion for abnormal body composition as waist circumference (WC) was not available for all participants. The World Health Organization defines abdominal adiposity as BMI>30 kg/m2 in the definition of the metabolic syndrome and abdominal adiposity is common in HIV+ adults, so it is likely a valid surrogate for WC. In addition, although unknown in HIV, the prevalence of the metabolic syndrome in the general population is essentially identical whether BMI or WC is used.39 While differences in the prevalence of abdominal adiposity between HIV+ and HIV− individuals with the metabolic syndrome are not clear,32,37 increased BMI is associated with a higher prevalence of ATP-III-defined metabolic syndrome in HIV+ people suggesting that abdominal adiposity is an important component of HIV-associated metabolic syndrome. Regardless, abdominal obesity was recently determined not to be an obligatory criterion for the definition of the metabolic syndrome.40
HIV-infected participants had been taking their current cART regimen for ≥6 months (Table 1). For HIV+/MC+, 30 (47%) were on a protease inhibitor (PI)-based regimen, 24 (38%) were on a nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen, one (2%) was on both a PI- and NNRTI-based regimen, one (2%) was on triple NRTI therapy, two (3%) were not on therapy, and six (9%) were unknown due to loss of contact. For HIV+/MC−, 27 (46%) were on a PI-based regimen, 29 (49%) were on an NNRTI-based regimen, one (2%) was on triple NRTI therapy, one (2%) was not on therapy, and one (2%) was unknown due to loss of contact. There were no differences in the major types of NRTI between HIV+/MC+ and HIV+/MC− (zidovudine: 20 vs. 24%, lamivudine: 34 vs. 30%, tenofovir: 39 vs. 51%). Other types of NRTI occurred in small percentages in each group. All HIV-infected participants had plasma HIV RNA that was undetectable (<400 copies/ml) except for nine HIV+/MC+ and eight HIV+/MC− participants who had viral loads of 401–50,000 copies RNA/ml (Table 1). No participants had a current opportunistic infection or CD4 count below 200 cells/μl. The duration of HIV infection and time on cART were similar between groups (Table 1).
Table 1.
Demographic Characteristics of Study Participants
| |
HIV− |
HIV+ |
|
||
|---|---|---|---|---|---|
| Variable | MC− (n=41) | MC+ (n=37) | MC− (n=59) | MC+ (n=64) | p |
| Age (years) | 42±6 | 44±8 | 42±7 | 43±7 | 0.739 |
| Male, n (%) | 35 (85) | 33 (89) | 45 (76) | 53 (83) | 0.433 |
| African-American, n (%) | 14 (34) | 7 (19) | 31 (53) | 29 (45) | 0.005 |
| Other nonwhite, n (%) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | |
| CD4 (cells/μl) | N/A | N/A | 515±206 | 537±260 | — |
| Median viral load (copies/ml) | N/A | N/A | UD (0–33,872) | UD (0–48,600) | — |
| Median HIV duration (months) | N/A | N/A | 133 (14–262) | 121 (23–272) | — |
| Median cART use (months) | N/A | N/A | 71 (3–246) | 73 (4–237) | — |
| Current tobacco use, n (%) | 10 (24) | 10 (27) | 25 (43) | 14 (22) | 0.064 |
| Antiretroviral therapy, n (%) | |||||
| NRTI | N/A | N/A | 56 (95) | 60 (94) | — |
| NNRTI | N/A | N/A | 26 (44) | 29 (45) | — |
| PI | N/A | N/A | 32 (54) | 39 (61) | — |
| Fusion | NA | NA | 0 (0) | 2 (3) | — |
| Medical therapy, n (%) | |||||
| HTN | 6 (15) | 16 (43) | 4 (7) | 15 (23) | <0.001 |
| Lipid lowering | 3 (7) | 10 (27) | 2 (3) | 16 (25) | <0.001 |
HIV, human immunodeficiency virus; MC, metabolic complications; cART, combined antiretroviral therapy; UD, undetectable<400 copies/ml; BMI, body mass index; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; Fusion, fusion inhibitor; HTN, hypertension.
Participants were excluded if they consumed more than three alcohol-containing beverages per week, were coinfected with hepatitis C or B, reported using recreational drugs for 6 months prior to enrollment, had documented type 2 diabetes or coronary artery disease, and were not weight stable (less than 2% weight change in the 3 months prior to the study). None of the participants participated regularly (more than two times/week) in physical activities that would constitute exercise training. HIV+/MC− had a higher percentage of current tobacco users than the other groups (Table 1). The Human Studies Committee at Washington University approved the study and all participants provided informed consent prior to participation.
Fasting blood chemistry, glucose tolerance, and body composition
Upon enrollment, participants provided a comprehensive medical history and underwent a physical examination, fasting blood chemistry, complete blood cell count, plasma HIV RNA quantification (Roche Amplicor HIV-1 Monitor, Indianapolis, IN), and serum lipid, lipoprotein, and endocrine profiles [insulin, triglycerides (TG), total-, LDL-, and HDL-cholesterol]. Plasma analyses were performed as previously described.41
Resting echocardiography
Indices of LV structure and function were measured by two-dimensional (2-D), Doppler, and tissue Doppler imaging (TDI) by a blinded echocardiographer. 2-D measurements included LV volumes and end diastolic and end systolic volumes from the apical four- and two-chamber views (method of disks). LV ejection fractions were determined by the biplane method and by the area-length method, respectively, normalized to body surface area.42 LV mass was measured by the 2-D-directed M-mode-derived cubed method.43 Pulsed wave Doppler (PWD) transmitral velocities were obtained at the mitral leaflet tips according to established guidelines.44 Indices of LV diastolic function include the peak transmitral early diastolic (E) and late diastolic atrial (A) inflow velocities, the ratio of the peak mitral E/A velocities, and the E-wave deceleration time.45 Isovolumic relaxation time was determined by measuring the time from the closure of the aortic valve to the onset of mitral flow. Resting heart rate, systolic and diastolic blood pressures, and mean arterial pressure were measured at the time of echocardiography.
TDI was determined by the placement of a 3-mm sample volume at the lateral and septal mitral annulus in the four-chamber view. Systolic (S′) and early diastolic (E′) myocardial wall velocities from the two sites were averaged to derive S′ and E′.46 Measurement at the lateral and septal annulus has been shown to have high specificity for indicating relatively load-independent impaired relaxation.47,48 Measurements represent the average of three to five cycles. We chose these diastolic measures because the E/A ratio is a load-dependent measure of the pressure gradient between the left atrium and LV (influenced by alterations in relaxation); mitral valve annular velocity (E′) is a relatively load-independent measure of LV relaxation; isovolumic relaxation time is a load-dependent measure of LV relaxation; E-wave deceleration time is an indirect measure of LV stiffness; and E/E′ is an estimate of LV filling pressures.47
Systolic dysfunction was defined by the LV ejection fraction categorized as normal (≥55%), mildly decreased (45–54%), moderately decreased (35–44%), and severely depressed (<35%) as defined by Mondy et al.14 Diastolic dysfunction was defined on the basis of Doppler peak early (E) and late (A) diastolic mitral inflow velocity, mitral inflow deceleration time, and mitral annular velocity (E′) averaged from the lateral wall and septum.49,50 Left ventricular hypertrophy was determined by M-mode echocardiography values for LV mass index ≥127 for men and ≥100 for women.43
Statistics
Differences in demographic variables among the four groups (HIV+/MC+, HIV+/MC−, HIV−/MC+, and HIV−/MC−) were tested using Fisher's exact tests for categorical variables and one-way analysis of variance (ANOVA) for continuous variables. Differences in dependent variables of interest between MC+/MC− and HIV+/HIV− were tested first using ANOVA, including the HIV, MC, and the interaction of the two, with Tukey Honestly Significant Difference (HSD) testing for pairwise comparisons. Groups differed slightly by race; thus, all dependent variables were also assessed among the groups using analysis of covariance (ANCOVA), adjusting for race. Again, the models included HIV, MC, and their interaction, with Tukey HSD testing for pairwise comparisons. Groups also differed by BMI, but this was expected as BMI is reflected in MC status. Therefore, BMI was not included in the ANCOVAs. A p-value<0.05 was considered statistically significant.
For variables where MC was a significant predictor, multivariable regression was used to explore which of the metabolic criteria that define MC status (i.e., BMI, fasting glucose, fasting insulin, systolic blood pressure, diastolic blood pressure, and triglycerides) was the strongest predictor of LV dysfunction. Models were built in a manual backward step-wise fashion, retaining variables with p<0.05 in the final model. Interactions of final predictors with HIV status were also assessed to determine whether the effect of the predictors differed by HIV status. All data are expressed as means±SD unless specified otherwise. SAS version 9.2 (SAS, Cary, NC) was used for all analyses.
Results
Demographics
The groups were similar in age but differed significantly by race (Table 1). Mean CD4 count and median viral load, time since HIV diagnosis, and time on cART were similar in the HIV+ groups (Table 1). HIV+ groups tended to have a higher percentage of tobacco users than groups without HIV (Table 1). A greater percentage of HIV−/MC+ were taking hypertension medications than other groups and a greater percentage of participants in the MC+ groups were taking a lipid-lowering medication than participants in the MC− groups (Table 1).
Body composition and metabolic characteristics
BMI and DBP were greater in HIV−/MC+ than all other groups (Table 2). Fasting plasma insulin, HOMA, triglycerides, and systolic blood pressure were greater in MC+ groups than MC− groups, but did not differ by HIV status. High-density lipoprotein (HDL) cholesterol was lower in MC+ groups than in MC− groups. Fasting plasma glucose was lower in HIV−/MC− than all other groups, and was lower in HIV+/MC− than in HIV−/MC+. There were no differences in fasting plasma total- and low-density lipoprotein cholesterol levels among groups (Table 2).
Table 2.
Body Composition and Metabolic Characteristics
| |
HIV− |
HIV+ |
ANCOVA resultsa |
||||
|---|---|---|---|---|---|---|---|
| Variable | MC− (n=41) | MC+ (n=37) | MC− (n=59) | MC+ (n=64) | HIV p | MC p | HIV *MC p |
| BMIb | 27.3±4.2 | 33.8±4.7 | 25.7±3.6 | 28.7±5.4 | <0.001 | <0.001 | 0.005 |
| Glucose (mg/dl)c | 81.4±10.4 | 96.3±19.2 | 88.7±6.9 | 92.1±8.1 | 0.315 | <0.001 | 0.001 |
| Insulin (μU/ml) | 6.8±4.8 | 19.4±13.4 | 8.1±5.0 | 17.4±13.4 | 0.766 | <0.001 | 0.248 |
| HOMA | 1.4±1.0 | 4.9±3.6 | 1.8±1.2 | 4.1±3.2 | 0.555 | <0.001 | 0.081 |
| TG (mg/dl) | 116.7±106.5 | 213.4±134.1 | 116.2±54.9 | 242.3±169.2 | 0.251 | <0.001 | 0.366 |
| HDL (mg/dl) | 53.8±13.4 | 39.3±7.4 | 49.4±12.9 | 37.5±8.4 | 0.009 | <0.001 | 0.489 |
| Total cholesterol (mg/dl) | 183.7±31.0 | 184.8±30.8 | 170.2±31.9 | 186.5±34.1 | 0.185 | 0.059 | 0.113 |
| LDL (mg/dl) | 106.0±26.5 | 105.7±29.8 | 97.7±27.8 | 104.0±32.0 | 0.195 | 0.441 | 0.463 |
| SBP (mm Hg) | 117.3±10.9 | 125.8±11.2 | 115.8±12.4 | 125.3±13.5 | 0.411 | <0.001 | 0.812 |
| DBP (mm Hg)d | 75.3±7.8 | 84.2±7.0 | 75.3±7.5 | 77.6±9.7 | 0.002 | <0.001 | 0.005 |
Adjusted for race.
Significant pairwise comparisons for BMI: HIV−/MC− vs. HIV−/MC+ p<0.001; HIV−/MC+ vs. HIV+/MC− p<0.001; HIV−/MC+ vs. HIV+/MC+ p<0.001; HIV+/MC− vs. HIV+/MC+ p=0.001.
Significant pairwise comparisons for glucose: HIV−/MC− vs. HIV−/MC+, p<0.001; HIV−/MC− vs. HIV+/MC− p=0.008; HIV−/MC− vs. HIV+/MC+ p<0.001; HIV−/MC+ vs. HIV+/MC−p=0.013.
Significant pairwise comparisons for DBP: HIV−/MC− vs. HIV−/MC+ p<0.001; HIV−/MC+ vs. HIV+/MC− p<0.001; HIV−/MC+ vs. HIV+/MC+ p<0.001.
HIV, human immunodeficiency virus; MC, metabolic complications; BMI, body mass index; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Prevalence of clinical echocardiographic abnormalities
Overall, the prevalence of systolic dysfunction was greater in HIV+ participants (15%) than in HIV− participants (4%) (p=0.02). The prevalence of systolic dysfunction was similar in those who had MC (10%) than in those who did not (11%), irrespective of HIV status (p=0.82). The prevalence of systolic dysfunction tended to be greater in HIV+/MC+ (14%) and HIV+/MC− (15%) compared to HIV−/MC+ (3%) and HIV−/MC− (5%) (p=0.11). Overall, there was no significant difference in prevalence of diastolic dysfunction between HIV+ participants (33%) compared to HIV− participants (23%), regardless of MC status (p=0.20), or between those with (33%) and without (25%) metabolic complications, irrespective of HIV status (p=0.28). The prevalence of diastolic dysfunction was similar among HIV+/MC+ (38%), HIV+/MC− (27%), HIV−/MC+ (24%), and HIV−/MC− (22%) (p=0.32). Those with HIV had a higher prevalence of left ventricular hypertrophy (HIV+: 9% vs. HIV−: 1%, p=0.03) and those with MC (10%) had a higher prevalence of left ventricular hypertrophy (LVH) than those without MC (2%) (p=0.03). The prevalence of LVH was 14% in HIV+/MC+, 3% in HIV+/MC−, 3% in HIV−/MC+, and 0% in HIV−/MC− and tended to be higher in HIV+/MC+ than HIV+/MC− (p=0.06), HIV−/MC+ (p=0.09), and HIV−/MC− (p=0.01).
LV structure and function
There were significant independent effects of HIV and MC on LV mass index. LV mass index was higher in HIV+ participants and for MC+ participants (Table 3). HIV had a significant or trended toward a significant independent negative effect on several systolic function variables including ejection fraction, fractional shortening, LV ejection time, and annular velocity measured during systole (Table 3). Both HIV infection and the presence of MC had significant or trended toward having an independent negative effect on several indices reflective of diastolic function, e.g., lower E/A ratio (Table 3) and lower early diastolic mitral annular velocity during diastole (E′) in HIV+ groups and in MC+ groups. However, there was no interaction effect of HIV and MC for any systolic or diastolic variable (i.e., effects of HIV did not significantly differ by MC status).
Table 3.
Echocardiographic Parameters
| |
HIV− |
HIV+ |
ANCOVA resultsa |
||||
|---|---|---|---|---|---|---|---|
| Variable | MC− (n=41) | MC+ (n=37) | MC− (n=59) | MC+ (n=64) | HIV p | MC p | HIV*MC p |
| BSA (m2) | 2.0±0.3 | 2.2±0.2 | 2.0±0.3 | 2.1±0.4 | 0.039 | <0.001 | 0.203 |
| HR (bpm) | 63.1±9.7 | 67.7±11.5 | 64.6±12.2 | 65.9±12.3 | 0.656 | 0.059 | 0.288 |
| LV structure | |||||||
| LVM-mm (g) | 166.4±38.0 | 206.9±49.6 | 183.2±38.8 | 200.2±47.8 | 0.615 | <0.001 | 0.058 |
| LVMI (g/m2) | 83.3±12.8 | 91.7±17.3 | 93.4±16.4 | 95.8±21.7 | 0.007 | 0.045 | 0.262 |
| LV systolic function | |||||||
| EF (%) | 62±6 | 62±6 | 59±6 | 61±7 | 0.022 | 0.405 | 0.535 |
| FS (%) | 31.3±6.5 | 33.1±4.8 | 33.7±6.2 | 34.7±6.0 | 0.093 | 0.161 | 0.532 |
| LVET (ms) | 300.8±21.8 | 291.2±28.7 | 291.1±32.9 | 283.5±33.9 | 0.056 | 0.060 | 0.827 |
| S′ (cm/s) | 8.9±1.3 | 8.4±1.5 | 8.1±1.0 | 8.1±0.9 | 0.003 | 0.133 | 0.174 |
| LV diastolic function | |||||||
| E-wave (cm/s) | 71.6±11.5 | 72.0±17.2 | 68.9±15.6 | 67.4±15.4 | 0.035 | 0.950 | 0.583 |
| E/A ratio | 1.59±0.41 | 1.51±0.48 | 1.52±0.52 | 1.29±0.44 | 0.026 | 0.027 | 0.230 |
| E′ (cm/s) | 13.1±2.4 | 12.0±2.3 | 12.4±2.3 | 11.6±2.3 | 0.105 | 0.005 | 0.795 |
| E/E′ | 5.6±1.0 | 6.1±1.3 | 5.6±1.3 | 5.9±1.4 | 0.533 | 0.020 | 0.520 |
| DT (ms) | 199.2±36.2 | 199.3±38.0 | 199.7±36.9 | 202.9±35.6 | 0.556 | 0.832 | 0.739 |
| IVRT (ms) | 82.1±16.0 | 88.4±16.5 | 87.8±15.7 | 87.7±13.0 | 0.237 | 0.173 | 0.149 |
Adjusted for race.
HIV, human immunodeficiency virus; MC, metabolic complications; BSA, body surface area; HR, heart rate; LVM-mm, left ventricular mass measured by M-mode; LVMI, left ventricular mass index (LVM/BSA); EF, ejection fraction; LVET, left ventricular ejection time; FS, fractional shortening; S′, systolic myocardial velocity averaged from the lateral wall and septum; E′, myocardial velocity during early diastole averaged from the lateral wall and septum; DT, deceleration time; IVRT, isovolumic contraction time.
Components of MC as predictors of LV function
In backward step-wise multivariable regression models of LV structure and function, HIV status and components of MC were used as predictors (i.e., BMI, fasting glucose, fasting insulin, systolic blood pressure, diastolic blood pressure, triglyceride) (Table 4). For prediction of reduced annular velocity during systole (S′), higher fasting glucose and HIV infection were independent predictors; interactions with HIV were not significant. For prediction of annular velocity during diastole (E′), higher fasting insulin and HIV infection independently predicted reduced E′. In a similar model, higher fasting insulin, diastolic blood pressure, and HIV infection remained independently associated with a reduced E/A ratio. The interactions of fasting glucose and diastolic blood pressure with HIV were not significant. Finally, in the model predicting LV mass index, systolic blood pressure, BMI, HIV infection, and the interaction of HIV infection and BMI remained in the final model. The interaction of BMI and HIV suggested that a negative association of BMI with LV mass index was largely confined in HIV+ participants, but not in HIV− participants. There were no significant associations with cART class (PI vs. no-PI) and LV outcome variables in HIV+ participants.
Table 4.
Regression Results for Components of Metabolic Complications as Predictors of Left Ventricular Function
| Dependent variable | Independent variables | β | SE | p |
|---|---|---|---|---|
| Annular velocity during systole (S′) | Fasting glucose | −0.0001 | 0.0001 | 0.037 |
| HIV infection (positive versus negative) | −0.006 | 0.002 | 0.001 | |
| Annular velocity during diastole (E′) | Fasting insulin | −0.0004 | 0.0001 | 0.005 |
| DBP | −0.0008 | 0.0002 | <0.001 | |
| HIV infection (positive versus negative) | −0.008 | 0.003 | 0.015 | |
| E/A ratio | Fasting insulin | −0.0077 | 0.0029 | 0.009 |
| DBP | −0.0137 | 0.0038 | <0.001 | |
| HIV infection (positive versus negative) | −0.186 | 0.068 | 0.007 | |
| LVMI | SBP | 0.3187 | 0.0972 | 0.001 |
| BMI | 0.4369 | 0.3691 | 0.238 | |
| HIV infection (positive versus negative) | 51.167 | 14.152 | <0.001 | |
| HIV infection * BMI | −1.549 | 0.479 | 0.001 |
Components of metabolic complications used as predictors were BMI, fasting glucose, fasting insulin, SBP, DBP, and TG. Results shown are from backward step-wise multivariable regression, retaining variables with p<0.05 in the final model.
S′, systolic myocardial velocity averaged from the lateral wall and septum; HIV, human immunodeficiency virus; E′, myocardial velocity during early diastole averaged from the lateral wall and septum; DBP, diastolic blood pressure; LVMI, left ventricular mass index; SBP, systolic blood pressure; BMI, body mass index.
Discussion
This is the first study to examine the potential relationships among well-controlled HIV infection and metabolic risk factors for cardiovascular disease and LV function, and compare these to HIV-negative men and women with and without similar metabolic risk factors. The main finding was that HIV infection and metabolic risk factors have independent negative associations with diastolic function. However, well-controlled HIV infection does not act additively or synergistically with indices of metabolic risk that adversely affect diastolic function. In addition, it appears that HIV also has an independent negative association with systolic function and LV mass. An adverse relationship of HIV infection with LV function and mass has been previously reported in primate models of simian immunodeficiency (SIV) and in humans with HIV.9,16,17 Furthermore, other studies have shown that metabolic risk factors have an independent negative relationship with LV function and mass in HIV-negative individuals.27–29 However, no previous study has examined the potential additive effect of these variables on the relationship with LV function in people living with well-controlled HIV. Our data confirm previous reports from SIV-infected nonhuman primates and HIV-infected humans that demonstrated the adverse relationship among these chronic infections and LV function.
The finding of no additional negative effect of HIV infection on the relationship with diastolic function and LV mass indexed for body surface area over the detrimental effects of metabolic complications was surprising and contrary to our hypothesis. It is unclear how the presence of metabolic complications mediates impaired diastolic function. One metabolic complication, obesity, is significantly related to diastolic dysfunction and increased LV mass.46 Specifically, there is impaired LV relaxation and increased LV end-diastolic pressure with increased BMI.46 Although the mechanisms are not completely clear, increased levels of reactive oxygen species51,52 as well as lipid-induced apoptosis53 have been implicated in the development of obesity-related impaired LV relaxation. In the current study, HIV− participants with metabolic risk factors had greater BMI than HIV+ participants with metabolic complications. Perhaps if the degree of obesity was identically matched between metabolically complicated groups, then greater impairments in LV function and mass may have been detected in HIV+ participants. Also, in the current study, we found a relationship between higher plasma insulin (along with diastolic blood pressure) and lower diastolic function among all participants (and in separate HIV+ and HIV− only analyses, data not shown) indicating that insulin resistance may play a role in diastolic dysfunction regardless of HIV infection. We previously reported relationships between myocardial insulin resistance and reduced diastolic function in a smaller study of HIV+ men.54 A positive association between insulin resistance and diastolic dysfunction has also been shown in HIV− individuals.38 However, the mechanism for insulin resistance-mediated diastolic dysfunction in humans with HIV remains to be elucidated.
In the present study, HIV, and less commonly peripheral metabolic risk factors, were associated with greater abnormalities in systolic and diastolic function. With the exception of the E/A ratio, none of the measured parameters of systolic and diastolic function was significantly predicted by both HIV and metabolic risk factors; therefore, there is little evidence for an additive or synergistic effect of HIV with the presence of metabolic risk factors on LV function. Although not fully understood, it appears that HIV replication16 may mediate impaired diastolic dysfunction through the viral glycoprotein 12055,56 and the CXCR4-iPLA2-p38 mitogen-activated (MAP) kinase-troponin I inflammatory signaling pathway.56–58 It is possible that peripheral metabolic complications and HIV may mediate diastolic dysfunction through a similar inflammatory pathway and therefore that is the reason we did not see an additive effect of metabolic complications with HIV in the current study. Metabolic syndrome is a proinflammatory state59 and the MAP kinase pathway60 and other G-protein-associated pathways61 have been implicated in playing a role in non-HIV metabolic syndrome and CVD. However, we did not measure serum inflammatory cytokines or obtain myocardial biopsies to examine the effect of these inflammatory pathways on LV function in the current study. In addition, 86% of the current participants were virologically suppressed with cART.
There is some previous evidence that HIV mediates systolic dysfunction. Systolic dysfunction was found in animal models that expressed increased amounts of HIV proteins62–64 and in patients with advanced disease (i.e., increased plasma viremia)65–67; however, it is unclear if HIV-associated systolic dysfunction is mediated through a pathway similar to HIV-associated diastolic dysfunction. Additional research in this area, especially in HIV-infected individuals with HIV suppression, is warranted.
LV mass was also independently associated with both HIV infection and the presence of metabolic complications. The association of LV mass with metabolic complications is in agreement with previous studies in HIV− individuals.68 However, the presence of both HIV and metabolic complications did not result in greater increases in LV mass. The finding of an independent effect of HIV is in agreement with Hsue et al.9 who reported that HIV infection was associated with both greater LV mass and diastolic dysfunction. However, the authors did not examine the relationship between individual metabolic risk factors and LV mass and function. There is a well-established relationship between obesity and increased LV mass.69
It is possible that no differences in LV mass index were found between HIV+ and HIV− groups with metabolic complications because HIV− participants with metabolic complications were significantly more obese than HIV+ participants with metabolic complications. However, in the current study, the relationship between higher BMI and increased LV mass index appeared to be present only in HIV+ individuals; therefore the presence of increased obesity in the HIV− group may not have obscured potential differences in LV mass between groups. The presence of increased LV mass (i.e., LV hypertrophy) is an important clinical problem as it increases the risk for cardiovascular morbidity and mortality, including the development of both systolic and diastolic dysfunction, and progression to heart failure.70 Therefore, it appears that LV mass should be monitored in HIV+ individuals with well-controlled HIV infection with a potential focus on modulation of obesity and elevated blood pressure, two well-established risk factors for increased LV mass.71
Although moderate and severe LV systolic dysfunction was not present in these participants, diastolic abnormalities are clinically important. They play a fundamental role in the development of heart failure, as they precede systolic failure in as many as 40% of cases.72,73 Diastolic dysfunction has a marked effect on exercise tolerance and quality of life,74 and when it progresses to heart failure with preserved ejection fraction (HFpEF, also known as diastolic heart failure), it has a mortality similar to systolic heart failure.74 HFpEF is defined by clinical signs or symptoms of heart failure, evidence of normal left ventricular function, and evidence of diastolic dysfunction.75 Although mortality is lower in HFpEF than in those with heart failure and reduced ejection fraction, the overall absolute number of deaths in the general population attributable to HFpEF is likely higher due to the aging population.76 In addition, the presence of insulin resistance is associated with a worse prognosis and increased risk for cardiovascular-related death in those with HFpEF.77 Therefore, since 33% of HIV-infected people with well-controlled viremia (as in the current study) have evidence of diastolic dysfunction, close monitoring of LV function appears to be warranted in HIV+ men and women with well-controlled HIV infection.
Women were underrepresented in this study and future studies should examine differences in LV function and mass between genders. Because this was a cross-sectional study causality cannot be confirmed. Our conclusions cannot be extended to HIV+ individuals not taking cART or those with greater plasma viremia. Groups were not perfectly matched on metabolic risk factors; HIV− individuals with metabolic complications had greater BMI, plasma glucose, and diastolic blood pressure than HIV+ participants with metabolic complications.
Conclusions
Regardless of HIV status, men and women with metabolic risk factors had worse indices of LV diastolic function in comparison to those without risk factors. Although both the presence of metabolic risk factors and HIV infection were associated with lower diastolic function, there was no additive negative effect of HIV on diastolic function beyond the effect of metabolic risk factors. Also, HIV was independently associated with lower systolic function. Clinical monitoring of LV function and implementation of interventions to improve metabolic health and cardiovascular risk in individuals with metabolic risk factors, regardless of HIV status, are warranted.
Acknowledgments
We thank the participants for their altruism and patience, the nursing staff of the Clinical Research Unit at Washington University for their help in performing these studies, and the nursing staff of the AIDS Clinical Trials Unit for recruitment of the participants. This project was supported by National Institutes of Health grants (DK074343 to W.T.C., AT003083, DK049393, and DK059531 to K.E.Y., RR019508 to D.N.R., and HL073120 to L.R.P.) and P30DK056341, DK020579, AI069495, RR000954, UL1RR024992, and KL2RR024994 from the National Center for Research Resources (NCRR) and NIH Roadmap for Medical Research. Additional support was provided by NIH grant S10RR024532 and a grant from the Barnes-Jewish Hospital Foundation to the Cardiovascular Imaging and Clinical Research Core Laboratory.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Himelman RB. Chung WS. Chernoff DN. Schiller NB. Hollander H. Cardiac manifestations of human immunodeficiency virus infection: A two-dimensional echocardiographic study. J Am Coll Cardiol. 1989;13:1030–1036. doi: 10.1016/0735-1097(89)90256-8. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese LH. Proffitt MR. Yen-Lieberman B. Hobbs RE. Ratliff NB. Congestive cardiomyopathy and illness related to the acquired immunodeficiency syndrome (AIDS) associated with isolation of retrovirus from myocardium. Ann Intern Med. 1987;107:691–692. doi: 10.7326/0003-4819-107-5-691. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ., Jr Delaney KM. Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Pugliese A. Isnardi D. Saini A. Scarabelli T. Raddino R. Torre D. Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Infect. 2000;40:282–284. doi: 10.1053/jinf.2000.0672. [DOI] [PubMed] [Google Scholar]
- 5.Coudray N. de Zuttere D. Force G, et al. Left ventricular diastolic function in asymptomatic and symptomatic human immunodeficiency virus carriers: An echocardiographic study. Eur Heart J. 1995;16:61–67. doi: 10.1093/eurheartj/16.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Garcia T. Sobrino JM. Pujol E. Galvez J. Benitez E. Giron-Gonzalez JA. Ventricular mass and diastolic function in patients infected by the human immunodeficiency virus. Heart. 2000;84:620–624. doi: 10.1136/heart.84.6.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso JS. Moura B. Martins L. Mota-Miranda A. Rocha Goncalves F. Lecour H. Left ventricular dysfunction in human immunodeficiency virus (HIV)-infected patients. Int J Cardiol. 1998;63:37–45. doi: 10.1016/s0167-5273(97)00276-3. [DOI] [PubMed] [Google Scholar]
- 8.Meng Q. Lima JA. Lai H, et al. Use of HIV protease inhibitors is associated with left ventricular morphologic changes and diastolic dysfunction. J Acquir Immune Defic Syndr. 2002;30:306–310. doi: 10.1097/00126334-200207010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hsue PY. Hunt PW. Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster I. Thoni GJ. Ederhy S, et al. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol. 2008;101:1213–1217. doi: 10.1016/j.amjcard.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 11.Nayak G. Ferguson M. Tribble DR, et al. Cardiac diastolic dysfunction is prevalent in HIV-infected patients. AIDS Patient Care STDS. 2009;23:231–238. doi: 10.1089/apc.2008.0142. [DOI] [PubMed] [Google Scholar]
- 12.Reinsch N. Kahlert P. Esser S, et al. Echocardiographic findings and abnormalities in HIV-infected patients: Results from a large, prospective, multicenter HIV-heart study. Am J Cardiovasc Dis. 2011;1:176–184. [PMC free article] [PubMed] [Google Scholar]
- 13.Blaylock JM. Byers DK. Gibbs BT, et al. Longitudinal assessment of cardiac diastolic function in HIV-infected patients. Int J STD AIDS. 2012;23:105–110. doi: 10.1258/ijsa.2011.011099. [DOI] [PubMed] [Google Scholar]
- 14.Mondy KE. Gottdiener J. Overton ET, et al. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 15.Cade WT. Left ventricular dysfunction in human immunodeficiency virus infection. J Cardiometab Syndr. 2008;3:83–87. doi: 10.1111/j.1559-4572.2008.07581.x. [DOI] [PubMed] [Google Scholar]
- 16.Kelly KM. Tarwater PM. Karper JM, et al. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- 17.Fiala M. Popik W. Qiao JH, et al. HIV-1 induces cardiomyopathy by cardiomyocyte invasion and gp120, Tat, and cytokine apoptotic signaling. Cardiovasc Toxicol. 2004;4:97–107. doi: 10.1385/ct:4:2:097. [DOI] [PubMed] [Google Scholar]
- 18.Friis-Moller N. Sabin CA. Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 19.Yarasheski KE. Tebas P. Sigmund C, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr. 1999;21:209–216. doi: 10.1097/00126334-199907010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadigan C. Miller K. Corcoran C. Anderson E. Basgoz N. Grinspoon S. Fasting hyperinsulinemia and changes in regional body composition in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1999;84:1932–1937. doi: 10.1210/jcem.84.6.5738. [DOI] [PubMed] [Google Scholar]
- 21.Grunfeld C. Kotler DP. Hamadeh R. Tierney A. Wang J. Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- 22.Calza L. Manfredi R. Chiodo F. Dyslipidaemia associated with antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53:10–14. doi: 10.1093/jac/dkh013. [DOI] [PubMed] [Google Scholar]
- 23.Safrin S. Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13:2493–2505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]
- 24.Carr A. Samaras K. Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Lakka HM. Laaksonen DE. Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 26.Ninomiya JK. L'Italien G. Criqui MH. Whyte JL. Gamst A. Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 27.Azevedo A. Bettencourt P. Almeida PB, et al. Increasing number of components of the metabolic syndrome and cardiac structural and functional abnormalities—cross-sectional study of the general population. BMC Cardiovasc Disord. 2007;7:17. doi: 10.1186/1471-2261-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de las Fuentes L. Brown AL. Mathews SJ, et al. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–559. doi: 10.1093/eurheartj/ehl526. [DOI] [PubMed] [Google Scholar]
- 29.Gong HP. Tan HW. Fang NN, et al. Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pract. 2009;83:300–307. doi: 10.1016/j.diabres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Mogelvang R. Sogaard P. Pedersen SA, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 31.Grundy SM. Cleeman JI. Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 32.Mondy K. Overton ET. Grubb J, et al. Metabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient population. Clin Infect Dis. 2007;44:726–734. doi: 10.1086/511679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samaras K. Wand H. Law M. Emery S. Cooper D. Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: Associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30:113–119. doi: 10.2337/dc06-1075. [DOI] [PubMed] [Google Scholar]
- 34.Adeyemi O. Rezai K. Bahk M. Badri S. Thomas-Gossain N. Metabolic syndrome in older HIV-infected patients: Data from the CORE50 cohort. AIDS Patient Care STDS. 2008;22:941–945. doi: 10.1089/apc.2008.0119. [DOI] [PubMed] [Google Scholar]
- 35.Jerico C. Knobel H. Montero M, et al. Metabolic syndrome among HIV-infected patients: Prevalence, characteristics, and related factors. Diabetes Care. 2005;28:132–137. doi: 10.2337/diacare.28.1.132. [DOI] [PubMed] [Google Scholar]
- 36.Alencastro PR. Wolff FH. Oliveira RR, et al. Metabolic syndrome and population attributable risk among HIV/AIDS patients: Comparison between NCEP-ATPIII, IDF and AHA/NHLBI definitions. AIDS Res Ther. 2012;9:29. doi: 10.1186/1742-6405-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonfanti P. Giannattasio C. Ricci E, et al. HIV and metabolic syndrome: A comparison with the general population. J Acquir Immune Defic Syndr. 2007;45:426–431. doi: 10.1097/QAI.0b013e318074ef83. [DOI] [PubMed] [Google Scholar]
- 38.Bajraktari G. Koltai MS. Ademaj F, et al. Relationship between insulin resistance and left ventricular diastolic dysfunction in patients with impaired glucose tolerance and type 2 diabetes. Int J Cardiol. 2006;110:206–211. doi: 10.1016/j.ijcard.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Ryan MC. Fenster Farin HM. Abbasi F. Reaven GM. Comparison of waist circumference versus body mass index in diagnosing metabolic syndrome and identifying apparently healthy subjects at increased risk of cardiovascular disease. Am J Cardiol. 2008;102:40–46. doi: 10.1016/j.amjcard.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 40.Alberti KG. Eckel RH. Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 41.Reeds DN. Yarasheski KE. Fontana L, et al. Alterations in liver, muscle, and adipose tissue insulin sensitivity in men with HIV infection and dyslipidemia. Am J Physiol Endocrinol Metab. 2006;290:E47–E53. doi: 10.1152/ajpendo.00236.2005. [DOI] [PubMed] [Google Scholar]
- 42.Schiller NB. Shah PM. Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 43.Lang RM. Bierig M. Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Quinones MA. Otto CM. Stoddard M. Waggoner A. Zoghbi WA. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 45.de las Fuentes L. Waggoner AD. Brown AL. Davila-Roman VG. Plasma triglyceride level is an independent predictor of altered left ventricular relaxation. J Am Soc Echocardiogr. 2005;18:1285–1291. doi: 10.1016/j.echo.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Peterson LR. Waggoner AD. Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: Assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 47.Nagueh SF. Middleton KJ. Kopelen HA. Zoghbi WA. Quinones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 48.Oki T. Tabata T. Yamada H, et al. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol. 1997;79:921–928. doi: 10.1016/s0002-9149(97)00015-5. [DOI] [PubMed] [Google Scholar]
- 49.Brucks S. Little WC. Chao T, et al. Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am J Cardiol. 2005;95:603–606. doi: 10.1016/j.amjcard.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Munagala VK. Jacobsen SJ. Mahoney DW. Rodeheffer RJ. Bailey KR. Redfield MM. Association of newer diastolic function parameters with age in healthy subjects: A population-based study. J Am Soc Echocardiogr. 2003;16:1049–1056. doi: 10.1016/S0894-7317(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 51.Peterson LR. Saeed IM. McGill JB, et al. Sex and type 2 diabetes: Obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring) 2012;20:802–810. doi: 10.1038/oby.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chai Y. Zhang DM. Lin YF. Activation of cGMP-dependent protein kinase stimulates cardiac ATP-sensitive potassium channels via a ROS/calmodulin/CaMKII signaling cascade. PLoS One. 2011;6:e18191. doi: 10.1371/journal.pone.0018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou YT. Grayburn P. Karim A, et al. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cade WT. Reeds DN. Overton ET, et al. Effects of human immunodeficiency virus and metabolic complications on myocardial nutrient metabolism, blood flow, and oxygen consumption: A cross-sectional analysis. Cardiovasc Diabetol. 2011;10:111. doi: 10.1186/1475-2840-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kan H. Xie Z. Finkel MS. HIV gp120 enhances NO production by cardiac myocytes through p38 MAP kinase-mediated NF-kappaB activation. Am J Physiol Heart Circ Physiol. 2000;279:H3138–3143. doi: 10.1152/ajpheart.2000.279.6.H3138. [DOI] [PubMed] [Google Scholar]
- 56.Kan H. Xie Z. Finkel MS. p38 MAP kinase-mediated negative inotropic effect of HIV gp120 on cardiac myocytes. Am J Physiol Cell Physiol. 2004;286:C1–7. doi: 10.1152/ajpcell.00059.2003. [DOI] [PubMed] [Google Scholar]
- 57.Kan H. Xie Z. Finkel MS. iPLA2 inhibitor blocks negative inotropic effect of HIV gp120 on cardiac myocytes. J Mol Cell Cardiol. 2006;40:131–137. doi: 10.1016/j.yjmcc.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Berzingi C. Chen F. Finkel MS. p38 MAP kinase inhibitor prevents diastolic dysfunction in rats following HIV gp120 injection in vivo. Cardiovasc Toxicol. 2009;9:142–150. doi: 10.1007/s12012-009-9047-1. [DOI] [PubMed] [Google Scholar]
- 59.Espinola-Klein C. Gori T. Blankenberg S. Munzel T. Inflammatory markers and cardiovascular risk in the metabolic syndrome. Front Biosci. 2011;16:1663–1674. doi: 10.2741/3812. [DOI] [PubMed] [Google Scholar]
- 60.Kan H. Finkel MS. Inflammatory mediators and reversible myocardial dysfunction. J Cell Physiol. 2003;195:1–11. doi: 10.1002/jcp.10213. [DOI] [PubMed] [Google Scholar]
- 61.Lugnier C. PDE inhibitors: A new approach to treat metabolic syndrome? Curr Opin Pharmacol. 2011;11:698–706. doi: 10.1016/j.coph.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Raidel SM. Haase C. Jansen NR, et al. Targeted myocardial transgenic expression of HIV Tat causes cardiomyopathy and mitochondrial damage. Am J Physiol Heart Circ Physiol. 2002;282:H1672–1678. doi: 10.1152/ajpheart.00955.2001. [DOI] [PubMed] [Google Scholar]
- 63.Fang Q. Kan H. Lewis W. Chen F. Sharma P. Finkel MS. Dilated cardiomyopathy in transgenic mice expressing HIV Tat. Cardiovasc Toxicol. 2009;9:39–45. doi: 10.1007/s12012-009-9035-5. [DOI] [PubMed] [Google Scholar]
- 64.Lewis W. Miller YK. Haase CP, et al. HIV viral protein R causes atrial cardiomyocyte mitosis, mesenchymal tumor, dysrhythmia, and heart failure. Lab Invest. 2005;85:182–192. doi: 10.1038/labinvest.3700222. [DOI] [PubMed] [Google Scholar]
- 65.Lubega S. Zirembuzi GW. Lwabi P. Heart disease among children with HIV/AIDS attending the paediatric infectious disease clinic at Mulago Hospital. Afr Health Sci. 2005;5:219–226. doi: 10.5555/afhs.2005.5.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okoromah CA. Ojo OO. Ogunkunle OO. Cardiovascular dysfunction in HIV-infected children in a sub-Saharan African country: Comparative cross-sectional observational study. J Trop Pediatr. 2012;58:3–11. doi: 10.1093/tropej/fmr009. [DOI] [PubMed] [Google Scholar]
- 67.Fisher SD. Easley KA. Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: The prospective P2C2 HIV Multicenter Study. Am Heart J. 2005;150:439–447. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Simone G. Palmieri V. Bella JN, et al. Association of left ventricular hypertrophy with metabolic risk factors: The HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 69.de las Fuentes L. Waggoner AD. Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376–2381. doi: 10.1016/j.jacc.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levy D. Garrison RJ. Savage DD. Kannel WB. Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 71.Norton GR. Majane OH. Libhaber E, et al. The relationship between blood pressure and left ventricular mass index depends on an excess adiposity. J Hypertens. 2009;27:1873–1883. doi: 10.1097/HJH.0b013e32832dca53. [DOI] [PubMed] [Google Scholar]
- 72.Owan TE. Hodge DO. Herges RM. Jacobsen SJ. Roger VL. Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto K. Redfield MM. Nishimura RA. Analysis of left ventricular diastolic function. Heart. 1996;75:27–35. doi: 10.1136/hrt.75.6_suppl_2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zile MR. Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: Diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 75.Lam CS. Donal E. Kraigher-Krainer E. Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gottdiener JS. McClelland RL. Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 77.MacDonald MR. Petrie MC. Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]