Abstract
Stroke is the second leading cause of death and the third leading cause of disability worldwide. Approximately 16 million first-ever strokes occur each year, leading to nearly 6 million deaths. Nevertheless, currently, very few therapeutic options are available. Cell therapies have been applied successfully in different hematological diseases, and are currently being investigated for treating ischemic heart disease, with promising results. Recent preclinical studies have indicated that cell therapies may provide structural and functional benefits after stroke. However, the effects of these treatments are not yet fully understood and are the subject of continuing investigation. Meanwhile, different clinical trials for stroke, the majority of them small, nonrandomized, and uncontrolled, have been reported, and their results indicate that cell therapy seems safe and feasible in these conditions. In the last 2 years, the number of published and registered trials has dramatically increased. Here, we review the main findings available in the field, with emphasis on the clinical results. Moreover, we address some of the questions that have been raised to date, to improve future studies.
Introduction
Stroke is responsible for ∼11.1% of all deaths, and is the second leading cause of death worldwide after ischemic heart disease [1]. After a stroke, roughly a quarter of patients die within a month, and half within 1 year [2]. There were an estimated 16 million first-ever strokes and 5.7 million deaths in 2005 [3]. These numbers are expected to increase to 23 million first-ever strokes and 7.8 million deaths in 2030 [3]. Stroke was responsible for 102 million disability-adjusted life years (DALYs) in 2010, an increase to the third leading cause of DALYS from the fifth leading cause in 1990 [4]. Approximately 80% of all strokes are ischemic, and currently, tissue plasminogen activator (tPA) is the only pharmacological agent approved for treatment of acute ischemic stroke. However, tPA therapy has important limitations, notably the narrow therapeutic window of 4.5 h, which limits its use to a small minority (2% to 4%) of patients [5]. Moreover, tPA prevents disability in only six patients per 1000 ischemic strokes, and does not reduce the mortality rate [6]. The administration of aspirin within 48 h of onset of ischemic stroke decreases the mortality rate or the incidence of disability in about nine patients per 1000 treated, probably due to early secondary prevention [2]. The injury produced by stroke is largely complete after 24–48 h, and neuroprotective therapies that must be administered within a time window such as 3–6 h are difficult to apply in clinical practice [7]. On the other hand, neurorestorative therapies, including cell therapies, seek to enhance regenerative mechanisms such as angiogenesis, neurogenesis, and synaptogenesis, and have been investigated extensively in the preclinical models of ischemia [7,8]. Neurorestorative cell therapies can be grossly divided into endogenous or exogenous. Endogenous therapies are those that aim to stimulate, for example, bone marrow-cell migration to the blood stream, with pharmacological agents such as granulocyte-colony stimulating factor (G-CSF). The exogenous approach involves the injection of a variety of cells to produce structural or functional benefits, and will be the focus of this article. Although excellent reviews have been recently made on different aspects of cell therapies for stroke [9–13], there has been a dramatic increase in the number of published and registered trials in the past years that has not been comprehensively assessed. In the following sections, we will review the main preclinical and clinical results to date and comment on some of the questions that have been raised.
Main Cell Types Used in Neurorestorative Cell Therapies for Stroke
Neural stem/progenitor cells
Neural stem/progenitor cells (NSPC) are cells with a self-renewing capacity and the potential to generate neurons and glial cells. NSPC can be isolated from the fetal brain or from one of the two neurogenic niches that persist in the adult brain: the subventricular zone of the lateral ventricles and the hippocampal subgranular zone [14–16]. Despite the evidence that transplanted fetal NSPC can functionally integrate into the brain of patients with Parkinson's disease [17], there are several obstacles to the use of NSPC from these two sources in clinical trials in stroke. For instance, the need for multiple fetal donors to treat a single patient could raise ethics concerns and may not be feasible in large-scale trials. Moreover, the isolation of adult NSPC for autologous transplantation would require brain biopsies and many days in culture for expansion, and may have some limitations, given that adult NSPC are regionally specified to generate a limited number of neuronal subtypes, even after cerebral ischemia [18].
NSPC can also be generated from pluripotent stem cells, including embryonic stem cells (ES, derived from the inner cell mass of blastocysts) and induced pluripotent stem cells (iPS, obtained after epigenetic reprogramming of adult cells by a combination of transcription factors). In each case, NSPC can be expanded in vitro, forming floating cell clusters called neurospheres, composed of a heterogeneous population of proliferating cells, which can be induced to differentiate into diverse phenotypes of the neuronal or glial lineage. However, the clinical use of ES-derived NSPC is still associated with the risks of neural overgrowth or teratoma formation, if undifferentiated ES persist in the transplant pool [19]. In addition, transplantation of allogeneic NSPC grafts requires immunosuppression, which is also associated with several side effects.
iPS-derived NSPC can be obtained after reprogramming of somatic cells from the patient himself, allowing an autologous transplantation. Although a recent study has cautioned that mouse iPS-derived teratomas can trigger immunogenicity in matched mice through a T-cell immune response [20], immunogenicity may not occur when ES- or iPS-derived terminally differentitated cells are transplanted [21]. Nevertheless, the creation of public banks of human leukocyte antigen-typed ES- or iPS-derived cell lines (and their differentiated cells) could be a more practical form of generating these cells using good manufacturing practices, at the appropriate time for transplantation, while reducing the immunogenicity of NSPC [22]. Other potential sources of neural cells for transplantation include induced neuronal cells and induced NSPC generated directly from fibroblasts or other somatic cells by a combination of transcription factors [23–25]. Human teratocarcinoma-derived neurons have also been used in clinical trials in stroke, as discussed below [26–30].
Non-NSPC
Mesenchymal stem cells (MSC) and hematopoietic stem/progenitor cells (HSPC) are the two non-neural cell types that are most frequently used in preclinical and clinical neurorestorative studies in stroke.
HSPC can be isolated from bone marrow or from umbilical-cord blood (UCB), or can be mobilized into the blood by the administration of pharmacological agents such as G-CSF and plerixafor. Most of the studies in animal models of stroke have transplanted the whole mononuclear cell (MNC) fraction from one of these sources, which also contains other cell types, including monocytes and lymphocytes, in addition to HSPC, MSC, and endothelial progenitor cells [31]. Alternatively, a smaller group of studies have transplanted human CD34+ MNCs, a subpopulation enriched in HSPC and endothelial progenitor cells.
MSC are multipotent cells with the capacity to give rise to cells of the osteogenic, chondrogenic, and adipogenic lineages. MSC can be isolated, and the culture expanded from several tissues, including bone marrow, adipose tissue, and UCB. Although a set of minimal criteria defined by the International Society for Cellular Therapy can be used to identify MSC, there are some functional and phenotypic differences among MSC derived from different sources [32,33].
Potential Mechanisms of Action of Cell-Based Therapies in Stroke
Neural stem/progenitor cells
Intracerebrally administered human NSPC migrate toward the sites of injury in the ischemic brain [34], where they survive for up to 2 months and differentiate into functional neurons, astrocytes, and oligodendrocytes [35]. However, the need to generate several neuronal subtypes that must extend long axons and form the appropriate synaptic connections is still one of the main challenges in regenerative medicine, and has been extensively reviewed elsewhere [13,36].
In addition to the potential of NSPC to replace the lost neurons, recent preclinical studies have observed that part of the therapeutic effects of NSPC in the ischemic brain could be attributed to a paracrine mechanism, since NSPC constitutively express mRNA and secrete several neurotrophic and growth factors in vitro [37–39]. For example, it has been shown that human NSPC transplantation increases neovascularization and enhances the integrity of the blood–brain barrier after stroke, through a human vascular endothelial growth factor (VEGF)-dependent mechanism [40]. VEGF is also one of the main factors involved in the modulatory role of an NSPC-derived conditioned medium in microglia function [41]. Accordingly, NSPC remain in close contact with microglial cells, even when injected into the brain of control animals [34], suggesting that a similar mechanism may occur in vivo [41].
Interestingly, NSPC transplantation contributes to the functional recovery in animal models of stroke, independent of the route of injection [42–44]. NSPC migrate to the sites of injury, even when intra-arterially delivered, and this recruitment is dependent on the chemokine receptor CCR2 [43,45]. In contrast, intravenous (IV) transplantation of NSPC results only in marginal migration of cells to the damaged brain, and in an animal model of intracerebral hemorrhage, the injected NSPC migrated mainly to the spleen. Nevertheless, the treatment resulted in the reduction of inflammation, edema formation, and apoptosis in the brain. Since these effects were not observed in splenectomized animals, the authors suggested that NSPC could provide neuroprotection by modulating the inflammatory response in the spleen [46].
Similarly, despite the low levels of engraftment and neuronal differentiation in the ischemic brain, intravenously transplanted adult NSPC showed neuroprotective and anti-inflammatory effects in a rodent model of stroke [42].
Taken together, these studies provide evidence that besides neuronal replacement, NSPC could contribute to functional recovery after a stroke by a combination of mechanisms, including neuroprotection and immunomodulation. NSPC could also stimulate endogenous mechanisms of brain plasticity and regeneration, enhancing hippocampal neurogenesis [47], stimulating the repair of the neurovascular unit [40], rescuing axonal transport, and inducing dendritic plasticity and axonal sprouting [38].
Non-NSPC
Although it has been proposed that HSPC and MSC could differentiate into neural cells in vitro, HSPC- or MSC-derived neuronal-like cells do not fire action potentials [48,49], and this phenomenon has not been reproduced in vivo [50,51]. An interesting study has estimated that only a small fraction (around 0.02%) of intravenously injected bone marrow-derived HSPC migrate to the ischemic brain, where most of the transplanted cells adopt a macrophage/microglial phenotype. In spite of this, HSPC transplantation decreases the infarct size and reduces inflammation in the brain and the spleen of the treated animals [51]. Moreover, it has been observed that MSC only transiently engraft the ischemic brain after an intra-arterial infusion [52], and that systemically delivered UCB-MNCs promote the behavioral recovery in an animal model of stroke, despite the low engraftment level in the host brain [53]. In summary, MSCs, bone marrow MNCs (BM-MNCs), and UCB-MNCs can improve neurological function in several models of stroke, through a combination of effects, such as neuroprotection, immunomodulation, and stimulation of neural plasticity [54–64], but these effects are not necessarily due to the presence of the cells at the injury site. In addition, MSC and HSPC transplantation can also induce angiogenesis and neurogenesis in the ischemic brain [65,66], two processes that are tightly linked by several regulatory mechanisms [67]. These mechanisms of action seem to rely on the secretion of neurotrophic factors and immunomodulatory molecules by the transplanted cells [68,69], an effect that can be further modulated by the host microenvironment. A recent study has raised the possibility that MSC could also exert their therapeutic actions by a mechanism of exosome-mediated transfer of microRNAs to neurons and astrocytes. Interestingly, the microRNA 133b levels in MSC exosomes increased when these cells were exposed to the ischemic brain extracts [70]. Thus, the transient engraftment of the transplanted cells and the dynamic changes that occur in the ischemic brain during the repair process may suggest that multiple injections may be required to optimize the release of the appropriate factors by the injected cells [71]. In addition, stroke-induced systemic inflammation can also modulate the phenotype of the BM-MNC populations, improving their potential to induce recovery after cerebral ischemia, if the cells are harvested and transplanted on the first day after the insult [72]. Hence, it is still necessary to evaluate the best timing for bone marrow harvest after stroke, in the case of autologous transplantation.
Finally, endothelial progenitor cells can be isolated and the culture expanded from the peripheral blood or from the UCB. These cells home to the ischemic brain through a stromal-derived factor 1-dependent mechanism, reducing the infarct size and improving the neurological outcome in mice [73]. The coadministration of culture-expanded UCB-derived endothelial and smooth-muscle progenitor cells has also been shown to increase angiogenesis and neurogenesis in an animal model of stroke [74]. Therefore, preclinical studies comparing the efficacy of endothelial progenitor cells, MSC, and HSPC from different sources are needed. In this regard, it has been shown that an intravenous administration of bone marrow-derived MSC promotes a similar degree of functional recovery to bone marrow-derived mononuclear cell transplantation in a rodent model of stroke, as long as the dose is optimized for each cell type [59]. Another study showed that there was no difference in the therapeutic effects of bone marrow-derived and umbilical cord tissue-derived MSCs (UC-MSCs) in a model of focal ischemia [75].
Published Clinical Trials
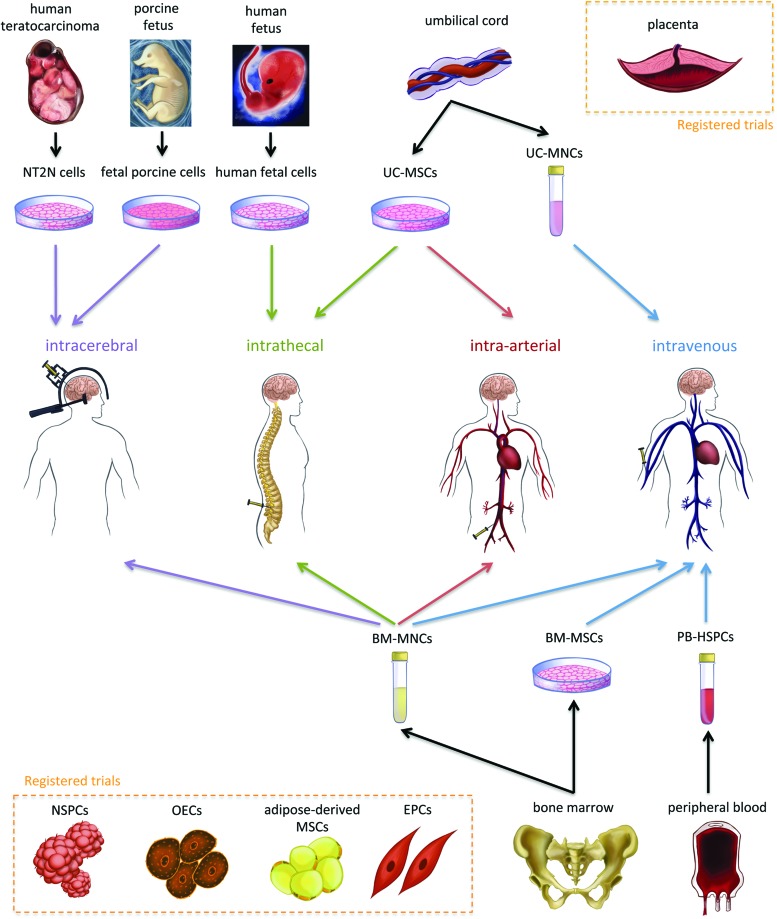
We found 31 articles in the English language involving 20 different trials of cell therapies for stroke, with a total of 243 treated patients. Sixteen of these articles and 12 of the trials were published in the last 2 years. Twelve trials were for ischemic, two for hemorrhagic, and six for ischemic or hemorrhagic strokes (Table 1 and Fig. 1). Six trials performed intravenous transplants; five injected the cells in the parenchyma; five used the intra-arterial route; three carried out intrathecal administrations; and one trial compared intra-arterial and intravenous routes (Table 1 and Fig. 1).
Table 1.
Trials with Published Clinical Results
| Study reference/Country | Study design | Route | Cell type | Type of stroke | Age range (mean) | Time range from stroke onset to transplantation | No. of treated patients (No. of controls) | No. of cells injected | Infusion volume, rate and duration | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Kondziolka et al., 2000/United States | Phase I, nonrandomized, single-blind | IC | NT2N cells | Ischemic stroke in basal ganglia (8 cases) or cortex and basal ganglia (4 cases) | 44–75 | 7 to 55 months (mean 27 months) | 12 (no controls) | 2×106 (8 patients); 6×106 (4 patients) | Not specified | 52–60 months |
| Rabinovich et al., 2005/Russia | Case series, nonrandomized, open label | IT | Human Fetal Cells | Hemorrhagic (3 cases) in MCA and ischemic (7 cases) in MCA or MCA+ACA | 35–56 | 4 to 24 months (mean 12.1 months) | 10 (10 historical controls) | 1 (5 patients) or 2 (5 patients) infusions of 2×108 | Not specified | 6 months |
| Kondziolka et al., 2005/United States | Phase II, randomized, single-blind | IC | NT2N cells | Ischemic (9 cases) or hemorrhagic (9 cases) involving basal ganglia | 40–70 | 1 to 5 years (mean 3.5 years) | 14 (4 controls without injection) | 5×106 (7 patients); 1×107 (7 patients) | Not specified | 18 to 29 months |
| Savitz et al., 2005/United States | Phase I, nonrandomized, open label | IC | Fetal Porcine cells | Ischemic involving the striatum | 25–52 (mean 39.8) | 4 to 10 years (mean 4.9 years) | 5 (no controls) | Up to 5 injections of 107 | 10 μL/min, 106 cells/μL | 4 years |
| Man et al., 2006/China | Case series, nonrandomized, open label | IV | Allogeneic UC-MNCs | Ischemic (6 cases) or hemorrhagic (4 cases) | 35–75 (mean 56) | 3 months to 7 years (mean 23.5 months) | 10 (no controls) | 6 infusions of≥1×108, 1 to 7 days apart | Not specified | 3 months |
| Mendonca et al., 2006; Correa et al., 2007/Brazil | Case reports on phase I, nonrandomized, open label | IA | Autologous BM-MNCs | MCA ischemic stroke | 54 and 37 | 5 (1 patient) and 9 days (1 patient) | 2 (no controls) | 1×108 (1 patient) and 3×107 (1 patient) | 3 mL in 10 min (first patient) | 2–4 months |
| Suarez-Monteagudo et al., 2009/Cuba | Case series, nonrandomized, open label | IC | Autologous BM-MNCs | Ischemic or hemorrhagic in thalamus, basal ganglia or cortex | 41–64 (mean 51.4) | 3 to 8 years (mean 5 years) | 5 (no controls) | 1.4×107to 5.5×107 (mean 3.4×107) | 8 seeds of 2.5 μL | 1 (4 cases) and 5 years (1 case) |
| Lee et al., 2010 (cont. of Bang et al., 2005)/South Korea | Phase I/II, randomized, single-blind | IV | Autologous BM-MSCs | MCA ischemic stroke | Mean 64 | Injections 19 to 37 days (median 32.5 days) and 2 weeks later | 16 (36 controls without injection) | 5×107 (2 doses 2 weeks apart) | Not specified | 5 years |
| Battistella et al., 2011; Rosado-de-Castro et al., 2013/Brazil | Phase I, nonrandomized, open label | IA or IV | Autologous BM-MNCs | MCA ischemic stroke | 24–68 (mean 58.5) | 19 to 89 days (mean 64.5) | 12 (no controls) | 1×108 to 5×108 (mean 3.1×108) | 10 mL in 10 min (1 mL/min) | 6 months |
| Honmou et al., 2011/Japan | Phase I, nonrandomized, open label | IV | Autologous BM-MSCs | Ischemic gray matter, white matter and mixed lesions | 41–73 (mean 59.2) | 36 to 133 days (mean 68 days) | 12 (no controls) | 0.6×108 to 1.6×108 (mean 1.1×108) | In 30 min; volume not specified | 12 months |
| Savitz et al., 2011/United States | Phase I, nonrandomized, open label | IV | Autologous BM-MNCs | MCA ischemic stroke | Mean 55 | 24 to 72 h | 10 (79 historical controls) | 7×108/kg to 1×109/kg (mean 9.6×108/kg) | In 30 min; volume not specified | 6 months |
| Han et al., 2011/South Korea | Not specified | IT | Allogeneic UC-MSCs | Ischemic in pons, midbrain and right superior cerebellum | 17 | 35 days | 1 (no controls) | 3.6×107 | Not specified | 2 months |
| Bhasin et al., 2011, 2012a, 2012b/India | Phase I, nonrandomized, single-blind (fMRI) | IV | Autologous BM-MSCs or BM-MNCs | MCA ischemic or hemorrhagic stroke | Mean 45 | mean 9.6 months | 20 (14 BM-MNC group; 6 BM-MSC group; 20 controls without injection) | 5×107 to 6×107 | 250 mL in 3 h (1.4 mL/min) | 6 months |
| Friedrich et al., 2012/Brazil | Phase I/II, nonrandomized, single-blind (CT) | IA | Autologous BM-MNCs | MCA ischemic stroke | 30–78 (mean 63) | 3 to 10 days (mean 6 days) | 20 (no controls) | 5.1×107 to 6×108 (mean 2.2×108) | 15 mL in 30 min (0.5 mL/min) | 6 months |
| England et al., 2012, United Kingdom | Subgroup of phase IIb, randomized, controlled trial with | IV | Autologous CD34+ PB-HPSCs | Ischemic stroke | Not specified for sub-group | 3 to 30 days | 8 (6 G-CSF group; 2 placebo group; no controls) | 2×107 to 4.3×108 | Not specified | 3 months |
| Sharma et al., 2012/India | Not specified | IT | Autologous BM-MNCs | Left thalamic hemorrhagic stroke | 69 | 1 year | 1 (no controls) | 5×107 | Not specified | Not specified |
| Moniche et al., 2012/Spain | Phase I/II, nonrandomized, single-blind | IA | Autologous BM-MNCs | MCA ischemic stroke | Mean 66.9 | 5 to 9 days (mean 6.4 days) | 10 (10 controls without injection) | mean 1.6×108 | 0.5 to 1 mL/min; duration not specified | 6 months |
| Prasad et al., 2012/India | Phase I, nonrandomized, open label | IV | Autologous BM-MNCs | MCA or MCA+ACA ischemic stroke | 30–70 (mean 51.5) | 8 to 29 days (mean 17 days) | 11 (no controls) | 1.9×108 to 1.9×109 (mean 8×107) | In 5 min; volume not specified | 12 months |
| Li et al., 2012/China | Phase I, nonrandomized, single-blind | IC | Autologous BM-MNCs | Basal ganglia hemorrhagic stroke | 39–74 (mean 56.3) | 5 to 7 days (mean 5.9 days) | 60 (40 controls with saline) | 2.5×108 to 2.3×109 (median 1.3×109) | 3.5 mL; duration not specified | 6 months |
| Jiang et al., 2012/China | Phase I, non-randomized, open label | IA | Allogeneic UC-MSCs | MCA ischemic (3 cases) or hemorrhagic (1 case) stroke | 40–59 (mean 49) | 11 to 50 days (mean 25.5) | 4 (no controls) | 2×107 | 20 mL in 20 min (1 mL/min) | 6 months |
IC, intracerebral; IA, intra-arterial; IV, intravenous; IT, intrathecal; BM-MNCs, bone marrow mononuclear cells; BM-MSCs, bone marrow-derived mesenchymal stem cells; UCB-MNCs, umbilical cord blood mononuclear cells; UC-MSCs, umbilical cord-derived mesenchymal stem cells; MCA, middle cerebral artery; ACA, anterior cerebral artery; NT2N, human teratocarcinoma-derived neurons; PB-HSPCs, peripheral blood hematopoietic progenitor/stem cells.
FIG. 1.
Schematic illustrating the different cells and routes of administration used in published trials. The schematic also illustrates other types of cells used in registered trials (in dotted rectangles). NT2N, human teratocarcinoma-derived neurons; UC-MSCs, umbilical cord-derived mesenchymal stem cells; UCB-MNCs, umbilical cord blood-mononuclear cells; BM-MNCs, bone marrow-mononuclear cells; BM-MSCs, bone marrow-mesenchymal stem cells; PB-HSPC, peripheral blood-hematopoietic stem/progenitor cell; NSPCs, neural stem/progenitor cells; OECs, olfactory-ensheathing cells; MSCs, mesenchymal stem cells; EPCs, endothelial progenitor cells. Color images available online at www.liebertpub.com/scd
Trials with Intracerebral Administration
Human teratocarcinoma-derived neurons
Kondziolka et al. [26] conducted the first clinical trial of cell therapy for stroke. It involved the transplantation of LBS-Neurons (Layton BioScience, Inc., Sunnyvale, CA), derived from a human teratocarcinoma cell line (NT2N) that was induced to differentiate into neurons by the addition of retinoic acid. This phase I, nonrandomized, observer-blind study included 12 patients with basal ganglia stroke and fixed motor deficits that occurred 6 months to 6 years before the transplantation. Eight of these patients received a total of 2 million cells, divided into three injections, into the area of the infarction, and the other four patients received 6 million cells divided into nine implants. Immunosuppression was accomplished with cyclosporine A started 1 week before surgery and continued for 8 weeks. One patient had a single generalized seizure 6 months after surgery, and another patient had a new brainstem stroke distant from the area of neuronal cell transplantation. However, these complications were thought not to be connected to the procedure, and no cell-related adverse effects were observed in the 5-year follow-up. Seven of 11 positron-emission tomography (PET) scans carried out at 6 months indicated an increase in fluorodeoxyglucose uptake at the implant site, while at 12 months, this number decreased to three [30]. The authors suggested that this could be related to cell viability in the area of the stroke, or alternatively to increased metabolic activity due to an inflammatory process, although no modifications indicative of inflammation were seen on magnetic resonance imaging (MRI). The procedure was evaluated as safe and feasible, and autopsy on one patient who died of myocardial infarction 27 months after cell transplantation showed that NT2N cells survived in the brain [28].
This trial was followed by a phase II, randomized, single-blind trial that included nine patients with ischemic and nine with hemorrhagic strokes from 1 to 6 years previously and with a fixed motor deficit that was stable for at least 2 months [29]. Seven patients received 5 million cells and seven patients 10 million cells, distributed in 25 sites, while 4 patients served as a nonsurgical control group; all subjects participated in a stroke rehabilitation program. One patient suffered a single seizure the day after the surgery, and another presented a burr-hole drainage of an asymptomatic chronic subdural hematoma 1 month after surgery. There was no significant improvement in the primary endpoint outcome, that is, European Stroke Scale motor score or the Fugl-Meyer (FM) Stroke Assessment, but there was improvement in the Action Research Arm Test gross hand-movement scores compared with the control and baseline values.
Fetal porcine cells
Savitz et al. [76] carried out stereotactic implantation of fetal porcine cells in five patients with basal ganglia infarcts, after pretreatment of the cells with an anti-MHC1 antibody. No immunosuppressants were administered. One patient presented transitory deterioration of motor deficits 3 weeks after cell implantation, and another patient had seizures 1 week after therapy. The study was initially designed to enroll 12 patients, but the FDA (U.S. Food and Drug Administration) ended it due to safety concerns.
Autologous BM-MNCs
Suarez-Monteagudo et al. [77] performed a trial with intracerebral transplantation of BM-MNCs, which included three patients with ischemic strokes in the thalamus, striatum, or cortex, and two patients with hemorrhagic strokes in the thalamus or striatum, from 3 to 8 years after the lesion. A total of 1.4×107 to 5.5×107 BM-MNCs were stereotactically implanted along several tracts around the lesion. There were no important adverse effects during the 1-year follow-up. The authors also reported significant neurological improvements at 12 months in comparison to baseline, with a reduction in motor defect evaluated by the Medical Research Council Scale and Ashworth's Scale for Spasticity; increased functional capacity evaluated by the Barthel index (BI); improved neurological condition evaluated by the National Institutes of Health Stroke Scale (NIHSS) and the Scandinavian Stroke Scale; and better equilibrium and locomotion, evaluated by the Tinneti scale. The same group [78] later reported the 5-year neuropsychological follow-up of one of the patients of the previous study and reported that positive cognitive changes in verbal and executive functions were maintained and seemed to be related to increased blood flow to the prefrontal areas. However, the unblind evaluation, the lack of a control group, and the small sample size did not allow definitive conclusions regarding efficacy.
In the largest clinical trial up to now, Li et al. [79] described a phase I, nonrandomized, single-blind study in which 60 patients received intraparenchymal BM-MNC transplantation 5 to 7 days after basal ganglion hemorrhagic stroke, and 40 patients formed the control group. Administered doses ranged from 2.5×108 to 2.3×109 cells. At 6 months after transplantation, the NIHSS score in the treated patients was significantly lower than in the control group, while the BI scores were higher. Moreover, there was significant neurological and functional improvement in BM-MNC-treated patients (86.7% versus 42.5% in the control group, P=0.001).
Trials with Intrathecal Administration
Human fetal cells
Rabinovich et al. [80] reported on a case-series, nonrandomized, open-label study that included three patients with hemorrhagic strokes in the middle cerebral artery (MCA) territory and seven patients with ischemic strokes in the MCA territory, with or without additional involvement of the anterior cerebral artery (ACA) territory. Subarachnoidal injections of 2×108 human fetal cells were made between 4 and 24 months after the disease onset. The cells were obtained from human fetuses after spontaneous or prostaglandin-induced abortions, and were described as a 10:1 ratio of nerve cells to hemopoetic hepatic cells. The authors reported that some patients had fever and meningism during 48 h after transplantation. Although a retrospective control group of 11 patients was described, the measures of outcome were not adequately explained, thus not permitting comparisons between the two groups. Moreover, the study lacks a detailed characterization of the phenotype of the transplanted cells.
Autologous BM-MNCs
Sharma and collaborators [81] described a case report in which 5×107 BM-MNCs were injected intrathecally in a patient 1 year after an hemorrhagic stroke. Even though there was no control group and only one patient was included, the authors attributed improvements in cognition, motor function, and activities of daily living to the cell transplantation. The follow-up period was not specified.
Allogeneic umbilical cord-MSCs
Han et al. [82] intrathecally injected 3.6×107 UC-MSCs in a patient 35 days after a basilar artery dissection that caused an infarction in the pons, midbrain, and right superior cerebellum. Two other injections were performed 15 and 41 days after the first treatment. Although the neurological and imaging were followed-up for only 2 months and in only one patient, the authors concluded that the improvement of clinical symptoms and a recanalization of the basilar artery were helped by the cell transplantation.
Trials with Intra-Arterial Administration
Autologous BM-MNCs
The first reports of studies using BM-MNC therapy for stroke were published from 2005 to 2007 [83–85] and were part of a nonrandomized, open-label phase I study. In the first case report [83,84], a 54-year-old patient was treated with intra-arterial injection of 3×107 BM-MNCs 5 days after an MCA ischemic stroke. A PET carried out 7 days after BM-MNC transplantation demonstrated augmented metabolism in the left parietal cortex, which could occur in the presence of transplanted cells or due to local inflammatory processes. In the second case report [85], a 37-year-old patient received 3×107 BM-MNCs 9 days after an MCA ischemic stroke. Approximately 1% of the cells were labeled with Technetium-99m (99mTc) by incubation with hexamethylpropylene amine oxime (HMPAO) and delivered together with the rest of the cells. Whole-body images demonstrated high uptake in the left hemisphere, liver, and spleen. Single-photon-emission computed tomography (SPECT) images 8 h after cell transplantation showed that the homing of 99mTc HMPAO-labeled cells occurred mainly in the territory of the anterior division of the MCA, while the stroke was in the territory of the posterior branch of the left MCA, probably because of the occlusion of the posterior branch. It is important to note that these patients were transplanted in the first 10 days after stroke.
Barbosa da Fonseca et al. [86,87] and Battistella et al. [88], respectively, reported the imaging and clinical results of a trial that included six patients 59 to 82 days after an MCA ischemic stroke. Afterward, another case where cells were injected 19 days after the stroke was also reported [89]. The cell dose ranged from 1×108 to 5×108 BM-MNCs, and ∼2×107 of the cells were labeled with 99mTc and delivered intra-arterially together with the unlabeled cells to the MCA. There were no cell-related adverse effects, and the cell uptake was greatest in the liver and lungs. Although cell homing was greater in the ischemic hemisphere, total uptake in the brain was low, <2% of the total activity for five of seven patients. Two patients had generalized seizures ∼200 days after cell injection, which were controlled pharmacologically, but due to the small sample, it was not possible to determine if the seizures occurred by chance or due to the cell transplantation.
In a study by Friedrich et al. [90], 20 patients with a moderate-to-severe MCA ischemic stroke received BM-MNCs infused intra-arterially between 3 and 7 days after stroke. The injected dose ranged from 5×107 to 6×108 cells. There were no procedure-related adverse events, and eight patients (40%) exhibited good clinical outcome, defined as a modified Rankin score (mRS) ≤2 at 90 days. Although the mortality level was below the expected level for similar populations, there was no control group, and the authors could not exclude the possibility that the good results could be explained by chance.
Moniche et al. [91] performed a nonrandomized single-blind phase I/II trial in which 10 patients received an intra-arterial injection of BM-MNCs 5 to 9 days after an MCA ischemic stroke, with an untreated control group of 10 patients. The mean infused dose was 1.6×108 cells. Two subjects who received BM-MNCs had an isolated partial seizure 3 months after the transplantation, which was considered a serious adverse event. In both patients, an antiepileptic medication was initiated, with no recurrent seizures. No other serious adverse events occurred during the 6-month follow-up. There was no significant improvement in neurological evaluation in comparison with the control patients. Even though there was no association involving the neurological condition and the number of injected BM-MNCs, the authors reported a trend toward a better outcome when a larger amount of CD34+ cells was injected, mainly in the BI at 1 month after cell therapy. Also, higher concentrations of ß-nerve growth factor were observed in the serum of BM-MNC-treated patients 8 days after cell transplantation.
Allogeneic umbilical cord-MSCs
Jiang et al. [92] included three patients with ischemic and one with hemorrhagic MCA strokes. One dose of 2×107 allogeneic umbilical cord-MSCs was transplanted into the MCA 11 to 50 days after the disease onset. No immunosuppression was used, and the neurological follow-up was not clearly defined; the mRS score was the only neurological scale analyzed. No adverse events such as fever, stroke, or death were observed during the 6-month follow-up. The authors reported that two of the ischemic patients demonstrated improved mRS scores, while no improvement was seen in the other two patients, which the authors interpreted as an indication that stem cells improved the neurological function after an ischemic, but not after hemorrhagic, stroke. However, the small number of patients and the absence of a control group do not permit such a conclusion regarding the efficacy of the approach.
Trials with Intravenous Administration
Allogeneic UCB-MNCs
Man et al. [93] included six patients with ischemic and four with hemorrhagic strokes that occurred 3 to 7 years before transplantation, in a trial for intravenous transplantation of allogeneic human UCB-MNCs. Each patient received six infusions of ≥1×108 cells, 1 to 7 days apart. Immunosuppressive drugs were not used, and there were no cell-related adverse events during the 3-month follow-up. Patients had a significant improvement in the neurological function deficiency, FM assessment, and BI, but there was no control group for comparison.
Autologous bone marrow-MSCs
Bang et al. [94] described the first trial with autologous bone marrow-MSCs (BM-MSCs) for stroke. In the first report of this phase I/II randomized controlled trial, 30 patients were prospectively and randomly allocated at the seventh day of admission after stroke. Five patients received two intravenous injections of 5×107 cells after culture expansion in fetal calf serum at 4 to 5 and 7 to 9 weeks after an MCA ischemic stroke, 25 patients served as controls, and all patients underwent rehabilitation therapy. At 1-year follow-up, there were no adverse cell-related, serological, or imaging-defined effects, and there was a nonsignificant trend toward improved BI and mRS. Afterward, the same group [95] included a larger number of patients in the same treatment protocol, and received a 5-year follow-up. Sixteen patients were treated, and 36 patients served as controls. No significant side effects were seen during the follow-up, and comorbidities such as seizures and recurrent strokes were similar between the groups. In comparison to the control group, there was a decrease in the mRS score of cell-treated patients. Interestingly, neurological recovery in the BM-MSC patients was related to the extent of involvement of the subventricular zone of the lateral ventricle and to the plasmatic levels of stromal cell-derived factor-1.
In another trial, Honmou et al. [96] included 12 patients with ischemic gray-matter, white-matter, and mixed lesions in a nonrandomized, open-label trial to analyze the effects of autologous BM-MSCs expanded in human serum, without a control group. They found that cell expansion was faster than in fetal bovine serum, which reduced cell preparation time. Also, they stressed that the use of human serum reduced the hazard of transmitting diseases such as bovine spongiform encephalomyelitis. BM-MSCs were infused intravenously 36 to 133 days after the cerebral infarct. There were no cell-related side effects. The authors found that the mean lesion volume as evaluated by MRI decreased by 20% or more at 1 week after cell therapy. Moreover, the median daily rate of change in the NIHSS increased in the first week after cell transplantation, and tended to be correlated with the decrease in the lesion volume.
Similarly, Bhasin et al. [97] conducted a phase I, nonrandomized, single-blind (for functional imaging interpretation) trial where six patients were included with ischemic or hemorrhagic MCA strokes ranging from 7 to 12 months previously, while 6 patients served as controls. After cell culture for 3 weeks in an animal serum-free medium (Stem Pro SFM), an intravenous injection of autologous BM-MSCs was administered. There were no cell-related adverse events during the 6-month follow-up. Although there was an improvement in the FM and modified BI at the 2- and 6-month evaluations, there was no statistical difference between the control and BM-MSC-treated groups. Moreover, there were no statistically significant differences in the functional MRI (fMRI) analysis between the BM-MSC and control groups.
Autologous BM-MNCs
Savitz et al. [98] reported the results of a trial in which 10 patients received an intravenous infusion of 7×106/kg to 1×107/kg BM-MNCs 24 to 72 h after MCA ischemic strokes. Two patients had to undergo hemicraniectomy after cell transplantation, due to infarct expansion between enrollment and bone marrow harvest. One patient died from a pulmonary embolism at 40 days after cell therapy, which was judged to be unrelated to the procedure. There were no study-related severe adverse events.
Prasad et al. [99] carried out a phase I, nonrandomized, open-label trial where 11 patients received an intravenous infusion of BM-MNCs between 8 and 29 days after MCA with or without ACA stroke, with no control group. The injected dose ranged from 1.9×108 to 1.9×109 cells. No serious adverse event was observed during the study. Seven patients had a favorable clinical outcome, defined as mRS ≤2 or a BI score of 75 to 100 at 6 months after cell transplantation.
After the first study reporting on the transplantation of BM-MSC for six patients with ischemic or hemorrhagic MCA strokes [97], Bhasin et al. reported on the intravenous transplantation of BM-MNCs for 12 patients, between 3 and 14 months after an MCA ischemic stroke [100]. Twelve patients served as controls. Statistically significant improvement was seen in the modified BI at 6 months and in the Laterality index in ipsilateral Broadmann areas 4 and 6 in fMRI at 2 months, but not at 6 months. The same group also published a comparison of the results of the six treated patients and six controls of the BM-MSC group with 14 treated patients and 14 controls of the BM-MNC group [101]. In this study, they also found statistical improvement in modified BI when comparing BM-MNC-treated patients with controls at 6 months, but no longer found improvement in fMRI. No statistical difference was found between the BM-MNC and BM-MSC groups. No adverse reactions were observed in the study in any of the groups during the follow-up.
Our group recently reported a continuation of the first trial, with intra-arterial administration of BM-MNCs in patients with a subacute stroke. In this study, five patients received an intravenous injection of BM-MNCs labeled with 99mTc. Analysis of the distribution of cells showed that intravenous administration led to higher uptake in the lungs and lower uptake in the liver and spleen at 2 and 24 h, in comparison with the intra-arterial route. Although SPECT images at 2 h indicated that intravenous injection led to a lower relative uptake in the lesioned hemisphere in comparison with the intra-arterial route, the total uptake in the brain in comparison to the whole body was low, but similar, between the two groups. All of the intravenous patients suffered seizures during the follow-up period, which were controlled pharmacologically. Although it was not possible to rule out that these seizures occurred by chance and/or because of greater stroke severity than the intra-arterial group, the incidence of seizures warrants caution, and these patients are under extended follow-up. It is possible that the infused cells could modify the excitability in the perilesional regions, generating seizures, and this possibility must be examined further in the forthcoming trials.
Autologous CD34+ HSPCs
England et al. [102] published a trial where 40 patients were included 3 to 30 days after an ischemic or hemorrhagic stroke, to receive subcutaneous injections of G-CSF once per day for 5 days, and 20 patients were treated with a placebo. Eight patients (6 from the G-CSF group and 2 from the placebo group) with ischemic strokes agreed to participate in a substudy, and on day 6 underwent peripheral blood collection with subsequent immunomagnetic separation of CD34+ cells with antibodies containing a dextran-coated iron oxide nanobead. These peripheral blood HSPCs (PB-HSPCs) were injected intravenously and could be followed by MRI due to the iron oxide labeling. Patients in the G-CSF group received 5.0×105 to 4.3×106 PB-HSPCs, while the placebo group received 2 to 7×104 cells. A hypodensity consistent with iron deposition within the stroke was seen in one G-CSF-treated patient after 10 and 90 days.
Registered Trials
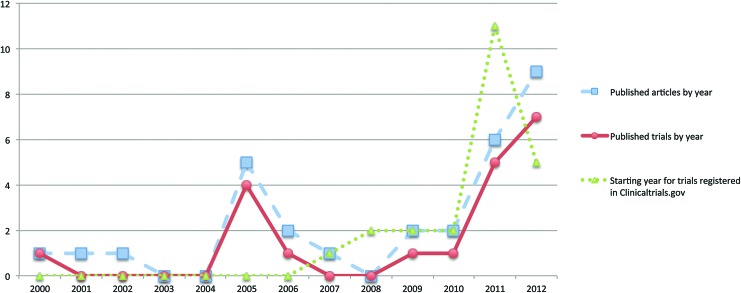
A search in the National Institutes of Health clinical trial registry (www.clinicaltrials.gov) indicated 25 completed (but unpublished) or ongoing registered studies, which are projected to enroll 1046 patients (Table 2). Of these, an exclusive intravenous, intracerebral, or intra-arterial administration was chosen by 13, 7, and 3 studies, respectively, while one study opted for intravenous and intrathecal, and another for intravenous or intra-arterial routes. The majority of the trials are being conducted in the United States and China, and a total of 16 studies were started in 2011 and 2012 (Fig. 2).
Table 2.
Completed (But Unreported) or Ongoing Trials Registered in Clinicaltrials.gov
| Trial identifier/Country | Route | Study design | Cell type | Type of stroke | Age | Time from onset to transplantation | No. of treated (controls) | No. of injected cells | Started | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT00950521/Taiwan | IC | Phase II, randomized, open-label | Autologous CD34+ PBSC | MCA ischemic | 35–70 | 6–60 months | 15 (15 controls) | 2×106 to 8×106 | 06/2009 | Completed |
| NCT01151124/United Kingdom | IC | Phase I, non-randomized, open-label | Allogeneic NSPCs (CTX0E03) | Ischemic (subcortical white matter or basal ganglia) | 60–85 | 6 months–5 years | 12 (no controls) | 4 groups: 2, 5, 10 or 20×106 | 06/2010 | Recruiting |
| NCT01287936/United States | IC | Phase I/IIA, non-randomized, open-label | Allogeneic SB623 (modified BM-MSCs) | MCA or lenticulostriate ischemic stroke | 18–75 | 6–36 months | 18 (no controls) | 3 groups: 2.5, 5 or 10×106 | 01/2011 | Recruiting |
| NCT01327768/Taiwan | IC | Phase I, randomized, single-blind (subject) | Autologous OECs | MCA ischemic stroke | 35–70 | 6–60 months | 6 (no controls) | 2×106 to 8×106 | 01/2011 | Recruiting |
| NCT01438593/China | IC | Phase I, nonrandomized, open-label | Allogeneic CD34+ UCB-MNCs | MCA ischemic stroke | 35–75 | 6–60 months | 6 (no controls) | 5×106 | 01/2013 | Not yet recruiting |
| NCT01673932/China | IC | Phase I, randomized, open-label | Allogeneic UCB-MNCs | MCA ischemic stroke | 35–65 | 6–60 months | 12 (controls not specified) | 1×107± to 4×107 | 10/2012 | Not yet recruiting |
| NCT01714167/China | IC | Phase I, non-randomized, open-label | Autologous BM-MSCs | Ischemic or hemorrhagic stroke | 40–70 | 3–60 months | 30 (controls not specified) | 2×106 to 4×106 | 06/2012 | Recruiting |
| NCT00535197/United Kingdom | IA | Phase I/II, nonrandomized, open-label | Autologous CD34+ BM-MNCs | MCA ischemic stroke | 30–80 | 7 days | 10 (no controls) | Not specified | 09/2007 | Recruiting |
| NCT01273337/United States | IA | Phase II, randomized, double-blind | ALD-401 (sorted from autologous BM) | MCA ischemic stroke | 30–83 | 13–19 days | 60 (40 controls) | Not specified | 03/2011 | Recruiting |
| NCT01518231/China | IA | Phase I, randomized, open-label | Autologous CD34+ peripheral blood HPSCs | Ischemic stroke | 40–70 | < 1 year | 20 (20 controls) | 4×106 | 01/2012 | Recruiting |
| NCT00859014/United States | IV | Phase I/IIa, nonrandomized, open-label | Autologous BM-MNCs | Ischemic stroke | 18–83 | 24–72 h | 30 (controls not specified) | Not specified | 01/2009 | Ongoing, not recruiting |
| NCT00875654/France | IV | Phase I/IIa, randomized, open-label | Autologous BM-MSCs | Ischemic stroke | 18–65 | <6 weeks | 30 (controls not specified) | Not specified | 08/2010 | Recruiting |
| NCT00908856/United States | IV | Phase I, randomized, double-blind | Autologous BM-MNCs or BM-MSCs | Supratentorial ischemic stroke | 18–85 | 4 days (BM-MNC) 23 days (MSCs) | 33 (controls not specified) | Not specified | 01/2014 | Not yet recruiting |
| NCT01028794/Japan | IV | Phase I/II, nonrandomized, open-label | Autologous BM-MNCs | Ischemic stroke | 20–75 | 7–10 days | 12 (controls not specified) | Not specified | 05/2008 | Recruiting |
| NCT01091701/Malaysia | IV | Phase I/II, randomized, double-blind | Allogeneic MSCs | Ischemic stroke | 20–80 | <10 days | 78 (controls not specified) | 4×106/kg | 12/2011 | Not yet recruiting |
| NCT01297413/United States | IV | Phase I/II, non-randomized, open-label | Allogeneic BM-MSCs | Ischemic stroke | >18 | >6 months | 35 (controls not specified) | 0.5×106/kg to 1.5×106/kg | 02/2011 | Recruiting |
| NCT01310114/United States | IV | Phase IIa, randomized, double-blind | PDA001 (human placenta-derived) | MCA or PCA ischemic stroke | 18–80 | Acute (not specified) | 34 (10 controls) | 2×108 to 8×108; 1–2 injections | 03/2011 | Ongoing, not recruiting |
| NCT01389453/China | IV and IT | Phase II/nonrandomized, open-label | Allogeneic UC-MSCs | Ischemic or hemorrhagic stroke | 40–65 | IV, 7–21 days; IT, 1 week after IV | 100 (20 controls) | Not specified | 04/2011 | Recruiting |
| NCT01436487/United States | IV | Phase II, randomized, double-blind | MultiStem® (Not specified) | Cortical ischemic stroke | 18–79 | 24–48 h | 140 (controls not specified) | Not specified | 10/2011 | Recruiting |
| NCT01453829/Mexico | IV or IA | Phase I/II, nonrandomized, open-label | Autologous adipose-derived MSCs | Ischemic or hemorrhagic stroke | 18–80 | Not specified | 10 (no controls) | Not specified | 05/2011 | Recruiting |
| NCT01461720/Malaysia | IV | Phase II, randomized, open-label | Autologous BM-MSCs | Ischemic stroke | 30–70 | 1–8 weeks | 50 (controls not specified) | Not specified | 03/2011 | Recruiting |
| NCT01468064/China | IV | Phase I/II, randomized, double-blind | Autologous BM-MSCs, EPCs | MCA ischemic stroke | 18–80 | 5 weeks | 90 (controls not specified) | 2.5×106/kg | 11/2011 | Recruiting |
| NCT01501773/India | IV | Phase II, randomized, open-label | Autologous BM-MSCs | MCA or ACA ischemic stroke | 18–70 | Not specified | 60 (60 controls) | 3×107 to 5×108 | 10/2008 | Completed |
| NCT01678534/Bolivia | IV | Phase IIa, randomized, double-blind | Allogeneic adipose-derived MSCs | MCA ischemic stroke | 60–80 | < 14 days | 20 (controls not specified) | 1×106/kg | 10/2012 | Recruiting |
| NCT01716481/South Korea | IV | Phase III, randomized, open-label | Autologous BM-MSCs | MCA ischemic stroke | 30–75 | < 90 days | 60 (controls not specified) | Not specified | 11/2012 | Recruiting |
OECs, olfactory ensheathing cells; EPCs, endothelial progenitor cells.
FIG. 2.
Graph illustrating the increase in published articles by year, published trials by year, and starting the year for trials registered in www.clinicaltrials.gov from 2000 to 2012. Color images available online at www.liebertpub.com/scd
The results available as of yet from the different above-mentioned studies suggest that cell therapies with different cell types in stroke seem to be safe and feasible, independently of the route of administration, dose, or time window after the onset of the disease. However, the many differences among them preclude further comparisons.
Discussion
Several preclinical studies have indicated that there is a structural and/or functional recovery after intracerebral, intra-arterial, and intravenous therapy with different cell types [8,103]. In clinical studies, most of the available data come from bone marrow cell therapies for malignant and nonmalignant diseases [104,105]. A meta-analysis of 50 clinical trials using cell therapies for acute and chronic ischemic heart disease with a total of 2625 patients has found that bone marrow cell treatment improves left ventricle (LV) ejection fraction, infarct size, LV end-diastolic volume, and LV end-systolic volume [106]. A recent trial investigating the transendocardial injection of autologous or allogeneic BM-MSCs in 30 patients with ischemic cardiomyopathy improved ventricular remodeling, functional capacity, and quality of life, with a 13-month follow-up [107]. For peripheral artery disease, a meta-analysis of 37 trials involving injection of bone marrow cells, peripheral blood cells, or G-CSF indicated that cell therapies, but not G-CSF, significantly improved the indices of ischemia such as the ankle–brachial index, transcutaneous oxygen tension, pain-free walking distance, and also hard endpoints such as ulcer healing and amputation [108].
Although clinical results with other ischemic diseases and preclinical studies for stroke are encouraging, there are still many questions regarding the possible mechanisms of action of the cells and the optimal treatment protocol. One of the main questions to be answered is related to the best cell type to be used in these patients. A recent meta-analysis of 117 preclinical stroke studies indicated that for structural effects, autologous stem cells were more effective than allogeneic cells, while for functional effects, allogeneic cells were more effective [109]. Interestingly, the authors found no difference between the embryonic and adult allogeneic cells for either structural or functional outcomes. This would support the use of adult cells rather than embryonic or fetal-derived cells; the former are preferred because of the ethics concerns associated with the latter. Moreover, bone marrow cells can be harvested from the patient for autologous therapy, avoiding the necessity for immunosuppressants [7,103].
To optimize future cell therapies for stroke, it is also necessary to elucidate the molecular mechanisms controlling the interaction of the grafted cells with the ischemic brain. Cerebral ischemia is immediately followed by microvascular dysfunction, oxidative stress, blood–brain barrier disruption, and excitotoxicity. These events are accompanied by the release of endogenous danger signals to the extracellular environment, the activation of the innate immune system, and the infiltration of blood leukocytes into the brain [110]. In this scenario, the interaction of transplanted cells with the ischemic tissue is mediated by a wide range of receptors, such as Toll-like receptors (TLRs), adenosine receptors, and chemokine receptors, which are activated upon the exposure to danger-associated molecular patterns and other inflammatory mediators released during the acute/subacute phases of stroke. It has been demonstrated that several chemokine receptors are involved in the recruitment of BM-MSCs and NSPC to the ischemic brain [45,111,112], and that TLR-2 mediates VEGF production and the recovery of myocardial function by transplanted BM-MSCs [113]. Thus, the postischemic environment can affect the function of transplanted stem/progenitor cells, which in turn can modulate the inflammatory response and the local microenvironment, as discussed above. Although it has been shown that human NSPC and iPS-derived long-term expandable neuroepithelial-like stem cells can give rise to functional neurons, when transplanted 48 h after stroke in T-cell-deficient rats [114,115], it is still poorly understood how the postischemic environment affects the survival, the proliferation, and the differentiation of transplanted NSPC. In one interesting study, for example, IL-6 preconditioning increased the survival of murine NSPC transplanted in the ischemic penumbra 6 h after the injury [116], suggesting that pharmacological or genetic manipulations could be used to improve the effectiveness of cell therapies for stroke.
Regarding the timing of transplantation, preclinical studies have shown that cell therapy increases functional recovery after acute, subacute, and chronic stroke [103], but few studies have compared different time windows, with differing results according to the model and cell type studied. In an animal model of focal ischemia, de Vasconcelos dos Santos et al. [59] found significant improvement in the cylinder test after intravenous injection of BM-MNCs at 1 and 7 days or BM-MSCs at 1 day after ischemia, but not in animals treated 30 days after the lesion. In a model of MCA occlusion (MCAO), Yang et al. [117] described improvement in the cylinder and corner tests if BM-MNC injection was performed at 1 or 3 days, but not at 28 days after the lesion. Also in a model of MCAO, Komatsu and colleagues [118] found a reduction of the ischemic lesion volume if BM-MSC therapy was performed at 7 days, but not at 14 or 28 days, while improvement in angiogenesis and the treadmill stess test occurred if cell transplantation was carried out up to 28 days after MCAO. In their meta-analysis of different preclinical studies, Lees and collaborators [109] found an absolute reduction in the efficacy of 1.5% for each day of delay of treatment for structural outcome, while improvement of functional outcome occurred in both early and late time windows [109].
The appropriate dose to use in clinical trials also remains unclear. The Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS) guidelines recommended a weight-based translation of cell dose from animal studies. In clinical studies of acute myocardial infarction, a metaregression indicated a dose–response correlation between the amount of CD34+ cells injected and the improvement in LVEF. A dose–response has also been reported by different preclinical studies for stroke [109,117,119], but has not been reported in the small clinical trials.
Cell tracking and imaging is also an important aspect to consider, since these techniques may improve understanding of several components of the therapy such as, cell homing, biodistribution, survival, and cell fate. One of the most often used approaches is labeling with radiopharmaceuticals for PET or SPECT imaging or exogenous contrasts such as iron oxide for MRI.
A small number of preclinical studies have compared different routes of injection, with discordant results depending on the experimental model and the moment of transplantation. Even though intracerebral transplantation may allow greater cell homing than intravascular injection, it is an invasive method and leads to poor cell distribution in the lesion [120,121]. IA administration can lead to a significant decrease in the cerebral blood flow, as assessed by laser Doppler flow, and increase in the mortality rate [120,121]. Kamiya and collaborators [122] found that IA injection of BM-MNCs resulted in greater brain cell retention and better functional outcomes compared to IV injection in a model of transient ischemia. Vasconcelos-dos-Santos et al. [123] reported that IV and IA infusions of these cells led to an equivalent functional recovery with low brain homing, in a model of permanent ischemia. Zhang et al. [124] found that IA, IV, IC, intra-cisterna magna, and lumbar intrathecal injection of human umbilical tissue-derived cells in a model of stroke led to similar structural improvements. The only meta-analysis of preclinical trials for stroke found no important impact of the delivery route on the efficacy of cell therapy [109]. In clinical trials, significant stenosis or occlusion of intracranial circulation is often an exclusion criterion, but it is possible that collateral supply may allow cells to reach the lesioned region [125].
Another aspect that must be clarified is the appropriate injection rate of the cells, and the potential effects of heparin or iodine contrast. A preclinical study by El-Khoury et al. [126] found that the IA flow rates of 5 mL/min reduced BM-MNC viability by 19%, while the rates of 2 mL/min did not affect viability or cytokine production. Although iodine and low-dose heparin exposure did not reduce cell viability, high doses of heparin were cytotoxic. With respect to IC and IV administration, information is lacking on the effects of injection rate from preclinical studies. In clinical trials published to date, the majority of studies did not report either the volume or the duration of injection (Table 1).
In addition to the different aspects previously mentioned, it is extremely important to strictly assess the safety of cell therapies. Although the currently published clinical studies indicate that cell therapies for stroke seem to be safe and feasible, there is a lack of robust scientific data, and many questions remain unanswered. For instance, the risk of teratoma formation with pluripotent stem cells must be addressed. In a recent report, Ben-David and collaborators carried out a high-throughput screen of 52,000 small molecules in cultures of different human ploripotent stem cells and identified 15 pluripotent cell-specific inhibitors, one of which prevented teratoma formation [127]. It is also important to evaluate the influence of clinical variables such as the presence of comorbidities. A preclinical study by Chen et al. indicated that BM-MSC injection 24 h after MCAO did not improve the functional outcome in Type 1 diabetic rats and increased arteriosclerosis, cerebral artery neointimal formation, and blood–brain barrier leakage [128], but this remains to be evaluated in a clinical study. Another facet that deserves attention is the influence of administering factors such as G-CSF. Clinical studies with the injection of G-CSF in patients with stroke indicate that the procedure seems to be safe [102,129–134], but only the study by England and collaborators [102] evaluated the effects of CD34+ cell transplantation after G-CSF injection. Other safety attributes such as the genetic stability and immunogenicity of cells must also be observed and have been thoroughly reviewed in an excellent article by Goldring et al. [135]. Observing such aspects will not mean a delay for the field and at the same time will allow a responsible and adequate development of cell therapies for stroke.
Conclusion
The results from preclinical studies have indicated that cell therapies can lead to the structural and functional benefits after a stroke. However, there is still a need to examine the ideal subset of stem cells to be used. Further, aspects such as the mechanisms for such improvements and the optimal treament protocol are not yet fully understood and require further evaluation. Nevertheless, different clinical studies, the majority of them small, nonrandomized and uncontrolled, have now been reported and indicate that cell therapy seems safe, feasible, and potentially efficacious. The increasing number of ongoing studies, including large randomized double-blind studies, have the potential to determine the efficacy of cell therapy for stroke and to translate the preclinical findings into clinical practice.
Acknowledgments
Dr. Rosalia Mendez-Otero was supported by a grant (PP SUS-2009 110.776/2010) from the Ministry of Health and the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). Paulo Henrique Rosado-de-Castro received a PhD Scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
The authors wish to thank Janet W. Reid for revising and editing the language in the text and Fernando Brandi for the drawings in Figure 1.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lozano R. Naghavi M. Foreman K. Lim S. Shibuya K. Aboyans V. Abraham J. Adair T. Aggarwal R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnan GA. Fisher M. Macleod M. Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 3.Strong K. Mathers C. Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ. Vos T. Lozano R. Naghavi M. Flaxman AD. Michaud C. Ezzati M. Shibuya K. Salomon JA, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 5.Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42:S16–S19. doi: 10.1161/STROKEAHA.110.598763. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W. Donnan G. Fieschi C. Kaste M. von Kummer R. Broderick JP. Brott T. Frankel M. Grotta JC, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 7.Hess DC. Hill WD. Cell therapy for ischaemic stroke. Cell Prolif. 2011;44(Suppl 1):1–8. doi: 10.1111/j.1365-2184.2010.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendez-Otero R. de Freitas GR. Andre C. de Mendonca ML. Friedrich M. Oliveira-Filho J. Potential roles of bone marrow stem cells in stroke therapy. Regen Med. 2007;2:417–423. doi: 10.2217/17460751.2.4.417. [DOI] [PubMed] [Google Scholar]
- 9.Misra V. Lal A. El Khoury R. Chen PR. Savitz SI. Intra-arterial delivery of cell therapies for stroke. Stem Cells Dev. 2012;21:1007–1015. doi: 10.1089/scd.2011.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahidy FS. Alderman S. Savitz SI. Challenges enrolling patients with acute ischemic stroke into cell therapy trials. Stem Cells Dev. 2013;22:27–30. doi: 10.1089/scd.2012.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thwaites JW. Reebye V. Mintz P. Levicar N. Habib N. Cellular replacement and regenerative medicine therapies in ischemic stroke. Regen Med. 2012;7:387–395. doi: 10.2217/rme.12.2. [DOI] [PubMed] [Google Scholar]
- 12.Glover LE. Tajiri N. Weinbren NL. Ishikawa H. Shinozuka K. Kaneko Y. Watterson DM. Borlongan CV. A step-up approach for cell therapy in stroke: translational hurdles of bone marrow-derived stem cells. Transl Stroke Res. 2012;3:90–98. doi: 10.1007/s12975-011-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindvall O. Kokaia Z. Stem cell research in stroke: how far from the clinic? Stroke. 2011;42:2369–2375. doi: 10.1161/STROKEAHA.110.599654. [DOI] [PubMed] [Google Scholar]
- 14.Kukekov VG. Laywell ED. Suslov O. Davies K. Scheffler B. Thomas LB. O'Brien TF. Kusakabe M. Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 15.Sanai N. Tramontin AD. Quinones-Hinojosa A. Barbaro NM. Gupta N. Kunwar S. Lawton MT. McDermott MW. Parsa AT, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 16.Azevedo-Pereira RL. Medei E. Mendez-Otero R. Souza JP. Alves-Leon SV. Isolation of neurosphere-like bodies from an adult patient with refractory temporal lobe epilepsy. Arq Neuropsiquiatr. 2010;68:956–958. doi: 10.1590/s0004-282x2010000600023. [DOI] [PubMed] [Google Scholar]
- 17.Lindvall O. Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol Sci. 2009;30:260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Liu F. You Y. Li X. Ma T. Nie Y. Wei B. Li T. Lin H. Yang Z. Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci. 2009;29:5075–5087. doi: 10.1523/JNEUROSCI.0201-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seminatore C. Polentes J. Ellman D. Kozubenko N. Itier V. Tine S. Tritschler L. Brenot M. Guidou E, et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010;41:153–159. doi: 10.1161/STROKEAHA.109.563015. [DOI] [PubMed] [Google Scholar]
- 20.Zhao T. Zhang ZN. Rong Z. Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 21.Araki R. Uda M. Hoki Y. Sunayama M. Nakamura M. Ando S. Sugiura M. Ideno H. Shimada A. Nifuji A. Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 22.Fairchild PJ. The challenge of immunogenicity in the quest for induced pluripotency. Nat Rev Immunol. 2010;10:868–875. doi: 10.1038/nri2878. [DOI] [PubMed] [Google Scholar]
- 23.Vierbuchen T. Ostermeier A. Pang ZP. Kokubu Y. Sudhof TC. Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han DW. Tapia N. Hermann A. Hemmer K. Hoing S. Arauzo-Bravo MJ. Zaehres H. Wu G. Frank S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Lujan E. Chanda S. Ahlenius H. Sudhof TC. Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci (USA) 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondziolka D. Wechsler L. Goldstein S. Meltzer C. Thulborn KR. Gebel J. Jannetta P. DeCesare S. Elder EM, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 27.Newman MB. Misiuta I. Willing AE. Zigova T. Karl RC. Borlongan CV. Sanberg PR. Tumorigenicity issues of embryonic carcinoma-derived stem cells: relevance to surgical trials using NT2 and hNT neural cells. Stem Cells Dev. 2005;14:29–43. doi: 10.1089/scd.2005.14.29. [DOI] [PubMed] [Google Scholar]
- 28.Nelson PT. Kondziolka D. Wechsler L. Goldstein S. Gebel J. DeCesare S. Elder EM. Zhang PJ. Jacobs A, et al. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondziolka D. Steinberg GK. Wechsler L. Meltzer CC. Elder E. Gebel J. Decesare S. Jovin T. Zafonte R, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 30.Meltzer CC. Kondziolka D. Villemagne VL. Wechsler L. Goldstein S. Thulborn KR. Gebel J. Elder EM. DeCesare S. Jacobs A. Serial [18F] fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery. 2001;49:586–591. doi: 10.1097/00006123-200109000-00011. discussion 591–582. [DOI] [PubMed] [Google Scholar]
- 31.Pimentel-Coelho PM. Rosado-de-Castro PH. da Fonseca LM. Mendez-Otero R. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2012;71:464–473. doi: 10.1038/pr.2011.59. [DOI] [PubMed] [Google Scholar]
- 32.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop D. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X. Hirai M. Cantero S. Ciubotariu R. Dobrila L. Hirsh A. Igura K. Satoh H. Yokomi I, et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- 34.Guzman R. Uchida N. Bliss TM. He D. Christopherson KK. Stellwagen D. Capela A. Greve J. Malenka RC, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci (USA) 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daadi MM. Li Z. Arac A. Grueter BA. Sofilos M. Malenka RC. Wu JC. Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Bendito G. Arlotta P. Cell replacement therapies for nervous system regeneration. Dev Neurobiol. 2012;72:145–152. doi: 10.1002/dneu.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu P. Jones LL. Snyder EY. Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 38.Andres RH. Horie N. Slikker W. Keren-Gill H. Zhan K. Sun G. Manley NC. Pereira MP. Sheikh LA, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawryluk GW. Mothe AJ. Chamankhah M. Wang J. Tator C. Fehlings MG. In vitro characterization of trophic factor expression in neural precursor cells. Stem Cells Dev. 2012;21:432–447. doi: 10.1089/scd.2011.0242. [DOI] [PubMed] [Google Scholar]
- 40.Horie N. Pereira MP. Niizuma K. Sun G. Keren-Gill H. Encarnacion A. Shamloo M. Hamilton SA. Jiang K, et al. Transplanted stem cell-secreted VEGF effects post-stroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosher KI. Andres RH. Fukuhara T. Bieri G. Hasegawa-Moriyama M. He Y. Guzman R. Wyss-Coray T. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15:1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bacigaluppi M. Pluchino S. Peruzzotti-Jametti L. Kilic E. Kilic U. Salani G. Brambilla E. West MJ. Comi G. Martino G. Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 43.Guzman R. De Los Angeles A. Cheshier S. Choi R. Hoang S. Liauw J. Schaar B. Steinberg G. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- 44.Smith EJ. Stroemer RP. Gorenkova N. Nakajima M. Crum WR. Tang E. Stevanato L. Sinden JD. Modo M. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30:785–796. doi: 10.1002/stem.1024. [DOI] [PubMed] [Google Scholar]
- 45.Andres RH. Choi R. Pendharkar AV. Gaeta X. Wang N. Nathan JK. Chua JY. Lee SW. Palmer TD. Steinberg GK. Guzman R. The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011;42:2923–2931. doi: 10.1161/STROKEAHA.110.606368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee ST. Chu K. Jung KH. Kim SJ. Kim DH. Kang KM. Hong NH. Kim JH. Ban JJ, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 47.Park DH. Eve DJ. Sanberg PR. Musso J., 3rd Bachstetter AD. Wolfson A. Schlunk A. Baradez MO. Sinden JD. Gemma C. Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev. 2010;19:175–180. doi: 10.1089/scd.2009.0172. [DOI] [PubMed] [Google Scholar]
- 48.Roybon L. Ma Z. Asztely F. Fosum A. Jacobsen SE. Brundin P. Li JY. Failure of transdifferentiation of adult hematopoietic stem cells into neurons. Stem Cells. 2006;24:1594–1604. doi: 10.1634/stemcells.2005-0548. [DOI] [PubMed] [Google Scholar]
- 49.Barnabe GF. Schwindt TT. Calcagnotto ME. Motta FL. Martinez G., Jr. de Oliveira AC. Keim LM. D'Almeida V. Mendez-Otero R. Mello LE. Chemically-induced RAT mesenchymal stem cells adopt molecular properties of neuronal-like cells but do not have basic neuronal functional properties. PLoS One. 2009;4:e5222. doi: 10.1371/journal.pone.0005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallieres L. Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarting S. Litwak S. Hao W. Bahr M. Weise J. Neumann H. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke. 2008;39:2867–2875. doi: 10.1161/STROKEAHA.108.513978. [DOI] [PubMed] [Google Scholar]
- 52.Mitkari B. Kerkela E. Nystedt J. Korhonen M. Mikkonen V. Huhtala T. Jolkkonen J. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol. 2012;239C:158–162. doi: 10.1016/j.expneurol.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 53.Borlongan CV. Hadman M. Sanberg CD. Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 54.Vendrame M. Gemma C. de Mesquita D. Collier L. Bickford PC. Sanberg CD. Sanberg PR. Pennypacker KR. Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 55.Vendrame M. Gemma C. Pennypacker KR. Bickford PC. Davis Sanberg C. Sanberg PR. Willing AE. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Ohtaki H. Ylostalo JH. Foraker JE. Robinson AP. Reger RL. Shioda S. Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci (USA) 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giraldi-Guimaraes A. Rezende-Lima M. Bruno FP. Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009;1266:108–120. doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 58.Chopp M. Li Y. Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40:S143–S145. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Vasconcelos Dos Santos A. da Costa Reis J. Diaz Paredes B. Moraes L. Jasmin Giraldi-Guimaraes A. Mendez-Otero R. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res. 2010;1306:149–158. doi: 10.1016/j.brainres.2009.09.094. [DOI] [PubMed] [Google Scholar]
- 60.Brenneman M. Sharma S. Harting M. Strong R. Cox CS., Jr. Aronowski J. Grotta JC. Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasuhara T. Matsukawa N. Hara K. Maki M. Ali MM. Yu SJ. Bae E. Yu G. Xu L, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. 2009;18:1501–1514. doi: 10.1089/scd.2009.0011. [DOI] [PubMed] [Google Scholar]
- 62.Xiao J. Nan Z. Motooka Y. Low WC. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005;14:722–733. doi: 10.1089/scd.2005.14.722. [DOI] [PubMed] [Google Scholar]
- 63.Newman MB. Willing AE. Manresa JJ. Davis-Sanberg C. Sanberg PR. Stroke-induced migration of human umbilical cord blood cells: time course and cytokines. Stem Cells Dev. 2005;14:576–586. doi: 10.1089/scd.2005.14.576. [DOI] [PubMed] [Google Scholar]
- 64.Bakondi B. Shimada IS. Peterson BM. Spees JL. SDF-1alpha secreted by human CD133-derived multipotent stromal cells promotes neural progenitor cell survival through CXCR7. Stem Cells Dev. 2011;20:1021–1029. doi: 10.1089/scd.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taguchi A. Soma T. Tanaka H. Kanda T. Nishimura H. Yoshikawa H. Tsukamoto Y. Iso H. Fujimori Y, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bao X. Feng M. Wei J. Han Q. Zhao H. Li G. Zhu Z. Xing H. An Y, et al. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes angiogenesis and neurogenesis after cerebral ischemia in rats. Eur J Neurosci. 2011;34:87–98. doi: 10.1111/j.1460-9568.2011.07733.x. [DOI] [PubMed] [Google Scholar]
- 67.Goldman SA. Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin YC. Ko TL. Shih YH. Lin MY. Fu TW. Hsiao HS. Hsu JY. Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42:2045–2053. doi: 10.1161/STROKEAHA.110.603621. [DOI] [PubMed] [Google Scholar]
- 69.Ranganath SH. Levy O. Inamdar MS. Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin H. Li Y. Buller B. Katakowski M. Zhang Y. Wang X. Shang X. Zhang ZG. Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Velthoven CT. Kavelaars A. van Bel F. Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci. 2010;30:9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang B. Xi X. Aronowski J. Savitz SI. Ischemic stroke may activate bone marrow mononuclear cells to enhance recovery after stroke. Stem Cells Dev. 2012;21:3332–3340. doi: 10.1089/scd.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan Y. Shen F. Frenzel T. Zhu W. Ye J. Liu J. Chen Y. Su H. Young WL. Yang GY. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nih LR. Deroide N. Lere-Dean C. Lerouet D. Soustrat M. Levy BI. Silvestre JS. Merkulova-Rainon T. Pocard M. Margaill I. Kubis N. Neuroblast survival depends on mature vascular network formation after mouse stroke: role of endothelial and smooth muscle progenitor cell co-administration. Eur J Neurosci. 2012;35:1208–1217. doi: 10.1111/j.1460-9568.2012.08041.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L. Li Y. Zhang C. Chopp M. Gosiewska A. Hong K. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke. 2011;42:1437–1444. doi: 10.1161/STROKEAHA.110.593129. [DOI] [PubMed] [Google Scholar]
- 76.Savitz SI. Dinsmore J. Wu J. Henderson GV. Stieg P. Caplan LR. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20:101–107. doi: 10.1159/000086518. [DOI] [PubMed] [Google Scholar]
- 77.Suarez-Monteagudo C. Hernandez-Ramirez P. Alvarez-Gonzalez L. Garcia-Maeso I. de la Cuetara-Bernal K. Castillo-Diaz L. Bringas-Vega ML. Martinez-Aching G. Morales-Chacon LM, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- 78.Bringas ML. Suarez C. Sanchez C. Alvarez LM. Valdes P. Salazar S. Chongo D. Jahanshahi M. Cognitive changes after stem cell transplantation in a patient with subcortical stroke. BMJ Case Rep. 2011. http://dx.doi.org/10.1136/bcr.03.2011.3944. http://dx.doi.org/10.1136/bcr.03.2011.3944 [DOI] [PMC free article] [PubMed]
- 79.Li ZM. Zhang ZT. Guo CJ. Geng FY. Qiang F. Wang LX. Autologous bone marrow mononuclear cell implantation for intracerebral hemorrhage-A prospective clinical observation. Clin Neurol Neurosurg. 2013;115:72–76. doi: 10.1016/j.clineuro.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 80.Rabinovich SS. Seledtsov VI. Banul NV. Poveshchenko OV. Senyukov VV. Astrakov SV. Samarin DM. Taraban VY. Cell therapy of brain stroke. Bull Exp Biol Med. 2005;139:126–128. doi: 10.1007/s10517-005-0229-y. [DOI] [PubMed] [Google Scholar]
- 81.Sharma A. Sane H. Badhe P. Kulkarni P. Chopra G. Lohia M. Gokulchandran N. Autologous bone marrow stem cell therapy shows functional improvement in hemorrhagic stroke. Indian J Clin Pract. 2012;23:100–105. [Google Scholar]
- 82.Han H. Chang SK. Chang JJ. Hwang SH. Han SH. Chun BH. Intrathecal injection of human umbilical cord blood-derived mesenchymal stem cells for the treatment of basilar artery dissection: a case report. J Med Case Rep. 2011;5:562. doi: 10.1186/1752-1947-5-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Correa P. Felix R. Mendonca ML. Freitas G. Azevedo J. Dohmann H. Alves S. Mesquita C. Dual-head coincidence gamma camera FDG-PET before and after autologous bone marrow mononuclear cell implantation in ischaemic stroke. Eur J Nucl Med Mol Imaging. 2005;32:999. doi: 10.1007/s00259-005-1808-x. [DOI] [PubMed] [Google Scholar]
- 84.Mendonca ML. Freitas GR. Silva SA. Manfrim A. Falcao CH. Gonzales C. Andre C. Dohmann HF. Borojevic R. Otero RM. [Safety of intra-arterial autologous bone marrow mononuclear cell transplantation for acute ischemic stroke] Arq Bras Cardiol. 2006;86:52–55. doi: 10.1590/s0066-782x2006000100008. [DOI] [PubMed] [Google Scholar]
- 85.Correa PL. Mesquita CT. Felix RM. Azevedo JC. Barbirato GB. Falcao CH. Gonzalez C. Mendonca ML. Manfrim A, et al. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin Nucl Med. 2007;32:839–841. doi: 10.1097/RLU.0b013e318156b980. [DOI] [PubMed] [Google Scholar]
- 86.Barbosa da Fonseca LM. Battistella V. de Freitas GR. Gutfilen B. Dos Santos Goldenberg RC. Maiolino A. Wajnberg E. Rosado de Castro PH. Mendez-Otero R. Andre C. Early tissue distribution of bone marrow mononuclear cells after intra-arterial delivery in a patient with chronic stroke. Circulation. 2009;120:539–541. doi: 10.1161/CIRCULATIONAHA.109.863084. [DOI] [PubMed] [Google Scholar]
- 87.Barbosa da Fonseca LM. Gutfilen B. Rosado de Castro PH. Battistella V. Goldenberg RC. Kasai-Brunswick T. Chagas CL. Wajnberg E. Maiolino A, et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp Neurol. 2010;221:122–128. doi: 10.1016/j.expneurol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Battistella V. de Freitas GR. da Fonseca LM. Mercante D. Gutfilen B. Goldenberg RC. Dias JV. Kasai-Brunswick TH. Wajnberg E, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6:45–52. doi: 10.2217/rme.10.97. [DOI] [PubMed] [Google Scholar]
- 89.Rosado de Castro PH. Schmidt FR. Battistella V. Lopes de Souza SA. Gutfilen B. Goldenberg RC. Kasai-Brunswick TH. Vairo L. Silva RM, et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen Med. 2013;8:145–155. doi: 10.2217/rme.13.2. [DOI] [PubMed] [Google Scholar]
- 90.Friedrich MA. Martins MP. Araujo MD. Klamt C. Vedolin L. Garicochea B. Raupp EF. Ammar JS. Machado DC, et al. Intra-arterial infusion of autologous bone-marrow mononuclear cells in patients with moderate to severe middle-cerebral-artery acute ischemic stroke. Cell Transplant. 2012;21:S13–S21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- 91.Moniche F. Gonzalez A. Gonzalez-Marcos JR. Carmona M. Pinero P. Espigado I. Garcia-Solis D. Cayuela A. Montaner J, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43:2242–2244. doi: 10.1161/STROKEAHA.112.659409. [DOI] [PubMed] [Google Scholar]
- 92.Jiang Y. Zhu W. Zhu J. Wu L. Xu G. Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of lesion artery in patients with stroke in the territory of middle cerebral artery. Cell Transplant. 2012 doi: 10.3727/096368912X658818. [Epub ahead of print]; [DOI] [PubMed] [Google Scholar]
- 93.Man Y. Li J. Yang B. Ma J. Vein transplantation using human umbilical cord blood stem cells in the treatment of stroke sequela. Neural Regen Res. 2006;1:618–621. [Google Scholar]
- 94.Bang OY. Lee JS. Lee PH. Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 95.Lee JS. Hong JM. Moon GJ. Lee PH. Ahn YH. Bang OY. collaborators S. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 96.Honmou O. Houkin K. Matsunaga T. Niitsu Y. Ishiai S. Onodera R. Waxman SG. Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhasin A. Srivastava MV. Kumaran SS. Mohanty S. Bhatia R. Bose S. Gaikwad S. Garg A. Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Savitz SI. Misra V. Kasam M. Juneja H. Cox CS., Jr. Alderman S. Aisiku I. Kar S. Gee A. Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 99.Prasad K. Mohanty S. Bhatia R. Srivastava MV. Garg A. Srivastava A. Goyal V. Tripathi M. Kumar A, et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Indian J Med Res. 2012;136:221–228. [PMC free article] [PubMed] [Google Scholar]
- 100.Bhasin A. Srivastava MV. Bhatia R. Mohanty S. Kumaran SS. Bose S. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J Stem Cells Regen Med. 2012a;8:181–189. doi: 10.46582/jsrm.0803011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhasin A. Padma Srivastava MV. Mohanty S. Bhatia R. Kumaran SS. Bose S. Stem cell therapy: A clinical trial of stroke. Clin Neurol Neurosurg. 2012b doi: 10.1016/j.clineuro.2012.10.015. In Press. [DOI] [PubMed] [Google Scholar]
- 102.England TJ. Abaei M. Auer DP. Lowe J. Jones DR. Sare G. Walker M. Bath PM. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43:405–411. doi: 10.1161/STROKEAHA.111.636449. [DOI] [PubMed] [Google Scholar]
- 103.Bliss TM. Andres RH. Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burt RK. Loh Y. Pearce W. Beohar N. Barr WG. Craig R. Wen Y. Rapp JA. Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 105.Resnick IB. Shapira MY. Slavin S. Nonmyeloablative stem cell transplantation and cell therapy for malignant and non-malignant diseases. Transpl Immunol. 2005;14:207–219. doi: 10.1016/j.trim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 106.Jeevanantham V. Butler M. Saad A. Abdel-Latif A. Zuba-Surma EK. Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hare JM. Fishman JE. Gerstenblith G. Difede Velazquez DL. Zambrano JP. Suncion VY. Tracy M. Ghersin E. Johnston PV, et al. Comparison of Allogeneic vs Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients With Ischemic Cardiomyopathy: The POSEIDON Randomized Trial. JAMA. 2012:1–11. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fadini GP. Agostini C. Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 109.Lees JS. Sena ES. Egan KJ. Antonic A. Koblar SA. Howells DW. Macleod MR. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- 110.Iadecola C. Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Imitola J. Raddassi K. Park KI. Mueller FJ. Nieto M. Teng YD. Frenkel D. Li J. Sidman RL, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci (USA) 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y. Deng Y. Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 113.Abarbanell AM. Wang Y. Herrmann JL. Weil BR. Poynter JA. Manukyan MC. Meldrum DR. Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H1529–1536. doi: 10.1152/ajpheart.01087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mine Y. Tatarishvili J. Oki K. Monni E. Kokaia Z. Lindvall O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol Dis. 2013;52:191–203. doi: 10.1016/j.nbd.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Oki K. Tatarishvili J. Wood J. Koch P. Wattananit S. Mine Y. Monni E. Tornero D. Ahlenius H, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 116.Sakata H. Narasimhan P. Niizuma K. Maier CM. Wakai T. Chan PH. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012;135:3298–3310. doi: 10.1093/brain/aws259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang B. Strong R. Sharma S. Brenneman M. Mallikarjunarao K. Xi X. Grotta JC. Aronowski J. Savitz SI. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Komatsu K. Honmou O. Suzuki J. Houkin K. Hamada H. Kocsis JD. Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res. 2010;1334:84–92. doi: 10.1016/j.brainres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 119.Saporta S. Borlongan CV. Sanberg PR. Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative dose-response analysis of cell survival and behavioral recovery. Neuroscience. 1999;91:519–525. doi: 10.1016/s0306-4522(98)00610-1. [DOI] [PubMed] [Google Scholar]
- 120.Li L. Jiang Q. Ding G. Zhang L. Zhang ZG. Li Q. Panda S. Lu M. Ewing JR. Chopp M. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab. 2010;30:653–662. doi: 10.1038/jcbfm.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walczak P. Zhang J. Gilad AA. Kedziorek DA. Ruiz-Cabello J. Young RG. Pittenger MF. van Zijl PC. Huang J. Bulte JW. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamiya N. Ueda M. Igarashi H. Nishiyama Y. Suda S. Inaba T. Katayama Y. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83:433–437. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 123.Vasconcelos-dos-Santos A. Rosado-de-Castro PH. Lopes de Souza SA. da Costa Silva J. Ramos AB. Rodriguez de Freitas G. Barbosa da Fonseca LM. Gutfilen B. Mendez-Otero R. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: is there a difference in biodistribution and efficacy? Stem Cell Res. 2012;9:1–8. doi: 10.1016/j.scr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Zhang L. Li Y. Romanko M. Kramer BC. Gosiewska A. Chopp M. Hong K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012;1489:104–112. doi: 10.1016/j.brainres.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 125.de Freitas GR. Mendez-Otero R. Intra-arterial Cell Therapy in Stroke Patients. In: Jolkkonen J, editor; Walczak P, editor. Cell-Based Therapies in Stroke. Springer; New York: 2013. pp. 181–190. [Google Scholar]
- 126.El Khoury R. Misra V. Sharma S. Cox CS. Walker P. Grotta JC. Gee A. Suzuki S. Savitz SI. The effect of transcatheter injections on cell viability and cytokine release of mononuclear cells. AJNR Am J Neuroradiol. 2010;31:1488–1492. doi: 10.3174/ajnr.A2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ben-David U. Gan QF. Golan-Lev T. Arora P. Yanuka O. Oren YS. Leikin-Frenkel A. Graf M. Garippa R, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 128.Chen J. Ye X. Yan T. Zhang C. Yang XP. Cui X. Cui Y. Zacharek A. Roberts C, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sprigg N. Bath PM. Zhao L. Willmot MR. Gray LJ. Walker MF. Dennis MS. Russell N. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37:2979–2983. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- 130.Shyu WC. Lin SZ. Lee CC. Liu DD. Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174:927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schabitz WR. Laage R. Vogt G. Koch W. Kollmar R. Schwab S. Schneider D. Hamann GF. Rosenkranz M, et al. AXIS: a trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke. 2010;41:2545–2551. doi: 10.1161/STROKEAHA.110.579508. [DOI] [PubMed] [Google Scholar]
- 132.Boy S. Sauerbruch S. Kraemer M. Schormann T. Schlachetzki F. Schuierer G. Luerding R. Hennemann B. Orso E, et al. Mobilisation of hematopoietic CD34+ precursor cells in patients with acute stroke is safe—results of an open-labeled non randomized phase I/II trial. PLoS One. 2011;6:e23099. doi: 10.1371/journal.pone.0023099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Floel A. Warnecke T. Duning T. Lating Y. Uhlenbrock J. Schneider A. Vogt G. Laage R. Koch W. Knecht S. Schabitz WR. Granulocyte-colony stimulating factor (G-CSF) in stroke patients with concomitant vascular disease—a randomized controlled trial. PLoS One. 2011;6:e19767. doi: 10.1371/journal.pone.0019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moriya Y. Mizuma A. Uesugi T. Ohnuki Y. Nagata E. Takahashi W. Kobayashi H. Kawada H. Ando K. Takagi S. Takizawa S. Phase I Study of intravenous low-dose granulocyte colony-stimulating factor in acute and subacute ischemic stroke. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.08.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 135.Goldring CE. Duffy PA. Benvenisty N. Andrews PW. Ben-David U. Eakins R. French N. Hanley NA. Kelly L, et al. Assessing the safety of stem cell therapeutics. Cell Stem Cell. 2011;8:618–628. doi: 10.1016/j.stem.2011.05.012. [DOI] [PubMed] [Google Scholar]