Abstract
Non-invasive and quantitative imaging of vascular endothelial growth factor receptor-2 (VEGFR-2) expression levels is highly important in cancer diagnosis, prognosis, and patient management. Although various literature reports have investigated the tumor expression levels of VEGFR-2 using imaging techniques such as positron emission tomography, single-photon emission computed tomography, targeted ultrasound, etc., accurate evaluation of the dynamic microdistribution of VEGFR-2 in vivo with good spatial and temporal resolution remains a major challenge. In this issue of the American Journal of Nuclear Medicine and Molecular Imaging, He at al. reported the use of a VEGFR-2 targeted probe for magnetic resonance imaging (MRI) of VEGFR-2 in two glioma models in rats (i.e. C6 and RG2). The heterogeneity of VEGFR-2 expression was non-invasively imaged with MRI and validated with various in vitro, in vivo, and ex vivo experiments. Not only was heterogeneous expression of VEGFR-2 found in different glioma tumors, it was also observed in different regions within the same tumor (e.g. tumor periphery, peri-necrotic area, and tumor interior). This report highlights the complex nature of gliomas, which may offer invaluable insights into tumor heterogeneity and potential clinical management of glioma patients. These patients have dismal clinical outcomes and are in urgent need of better tools to improve brain tumor treatment.
Keywords: Molecular MRI (mMRI), glioma, tumor angiogenesis, VEGFR-2, molecular imaging
Angiogenesis, the formation of new blood vessels from preexisting vasculature, is a hallmark of cancer which plays pivotal roles in tumor development and metastasis [1-3]. Non-invasive and quantitative imaging of tumor angiogenesis is critical for lesion detection, patient stratification, and monitoring the therapeutic response of cancer patients, including brain tumors [4,5]. Over the last decade, the field of molecular imaging has advanced tremendously, which is becoming indispensable for future personalized medicine in the clinic [6-10].
Anti-angiogenic therapy has attracted tremendous attention over the last several decades and several biomarkers related to angiogenesis have been extensively studied, such as integrin αvβ3 [11,12], vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) [12,13], CD105 (also called endoglin) [14], among many others. The VEGF/VEGFR signaling pathway plays a critical role in both normal vasculature development and many disease processes [15]. The angiogenic actions of VEGF are mainly mediated via two endothelium-specific receptor tyrosine kinases, VEGFR-1 (Flt-1/FLT-1) and VEGFR-2 (Flk-1/KDR). As the major mediator of the mitogenic, angiogenic, and permeability-enhancing effects of VEGF, VEGFR-2 is generally considered to be more functionally important than VEGFR-1 in cancer progression [16]. A number of studies have shown that overexpression of VEGF and/or VEGFRs correlated with poor prognosis in multiple cancer types, including gliomas [17]. In addition, it has been reported that intra-tumoral levels of VEGF and VEGFRs correlate with the histological grade of gliomas [18].
Knowing the dynamic distribution of VEGFRs, especially VEGFR-2, will lead to better understanding of the mechanisms underlying tumor angiogenesis, as well as provide a better tool for cancer diagnosis and treatment [12,13]. The ability to accurately evaluate VEGFR-2 expression with non-invasive molecular imaging techniques such as positron emission tomography (PET) [19-21], single-photon emission computed tomography [22], or targeted ultrasound [23] can help with decisions about whether and when to start anti-angiogenic treatment, which may significantly improve cancer patient management. Although PET is highly attractive for imaging of VEGF/VEGFRs because of its high sensitivity and quantitative capability, it is difficult or even impossible for PET to provide high resolution images of VEGFR expression in different tumor regions due to its limited special resolution (a few mm). Magnetic resonance imaging (MRI) can offer sufficient spatial resolution on the order of micrometers. However, direct measurement of VEGF/VEGFR expression using molecular MRI (mMRI) is quite challenging and remains understudied to date.
In this issue of the American Journal of Nuclear Medicine and Molecular Imaging (AJNMMI, http://www.ajnmmi.us), He et al. reported mMRI investigation of VEGFR-2 in two different glioma models (i.e. C6 and RG2) in rats using a high magnetic field (7.0 Tesla) small animal MRI scanner and a VEGFR-2 specific contrast agent [24]. The mMRI contrast agent used in this study is composed of 4 distinct components: an anti-VEGFR-2 antibody which provides target specificity, biotin which can be used for ex vivo validation of the in vivo data using fluorescently labeled streptavidin, Gd-DTPA which confers MRI sensitivity, and bovine serum albumin (BSA) which serves as the platform for covalently linking the 3 abovementioned components together (Figure 1). In addition to the VEGFR-2 specific contrast agent for mMRI, normal rat IgG conjugated biotin-BSA-Gd-DTPA was also synthesized and used as a control.
Figure 1.
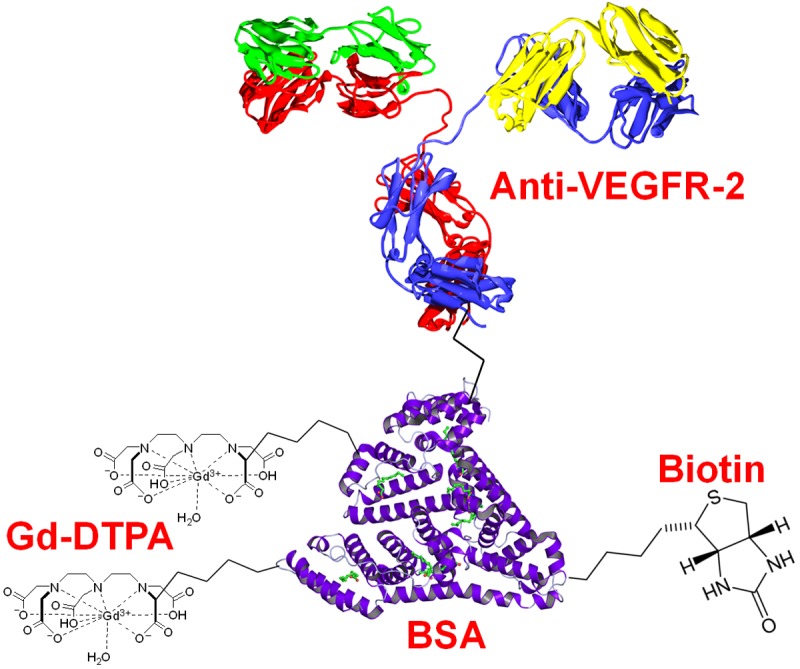
The probe used for molecular MRI of VEGFR-2.
This report is an extension of their previous work on mMRI of VEGFR-2 in the C6 glioma model using the same contrast agent [25]. In this work, the heterogeneous expression of VEGFR-2 in C6 and RG2 glioma tumors and different regions within the tumor tissue was successfully imaged non-invasively with MRI and comprehensively investigated [24]. It was demonstrated that when compared to the RG2 glioma, the C6 tumor exhibited a more heterogeneous pattern with more VEGFR-2 expression in the tumor periphery and peri-necrotic areas, but less in the tumor interior. These findings indicated that the C6 glioma likely have more active angiogenesis occurring in the relatively large vessels within the tumor periphery, whereas the RG2 glioma may have increased angiogenesis in the microcapillaries within the tumor interior [24].
A number of experiments were carried out to validate the in vivo findings with mMRI. Interestingly, the C6 and RG2 glioma cells were also found to express VEGFR-2 at a significant level, as evidenced by both in vitro MRI studies (i.e. decreased T1 values were observed when either C6 or RG2 cells were incubated with the VEGFR-2 specific contrast agent) and ex vivo immunohistological examination of the tumor tissue (i.e. prominent fluorescence signal were found on the tumor cells with VEGFR-2 staining). However, it is unclear whether the expression level of VEGFR-2 on the C6 and RG2 cells is comparable to that on the tumor vasculature, which deserves further investigation in the future. VEGFR-2 specificity of the MRI signal observed in the glioma models was evidenced by the significantly lower signal intensity in the contralateral normal brain after intravenous injection of the VEGFR-2 specific agent, as well as the lower MRI signal in the tumor when non-specific rat IgG-conjugated contrast agent were injected. These findings were corroborated by ex vivo staining of the tumor tissue using fluorescently labeled streptavidin (which binds with high affinity to biotin within the MRI contrast agent), where stronger fluorescence signal in the C6 tumor tissue (both the tumor cells and the vasculature) was observed when compared to the RG2 tumor.
Aside from measurement of VEGFR-2 on the glioma cells and tumor tissue, the investigators also assessed another marker which plays important roles in tumor angiogenesis: HIF-1α. Surprisingly, histological examination revealed significantly higher HIF-1α expression in the RG2 glioma tissue than the C6 tumor tissue, which is not in agreement with the VEGFR-2 expression level in the two tumor models. Although the exact mechanism for this finding remains to be elucidated, the histologic data for laminin (an endothelial cell marker) appears to correlate with the HIF-1α result: larger blood vessels were present in the C6 glioma tissue whereas more abundant, smaller and diffuse blood vessels were found in the RG2 tumors.
The molecular weight of the MRI contrast agent was estimated to be 232 kDa, which is quite high and may not have efficient extravasation into the tumor tissue. Therefore, distribution of the contrast agent was more diffused in the C6 tumor tissue, which has many large vessels and hence is more “leaky”. In the RG2 tumor tissue, the vessels are much smaller and therefore less “leaky”. Staining of the RG2 tumor tissue with fluorescently labeled streptavidin showed that the intravenously injected contrast agent was mostly located within the vasculature or close to the vasculature, with significantly less extravasation than in the C6 tumors. The blood-brain barrier and blood-tumor barrier also play important roles in the tumor uptake of such a macromolecular contrast agent, since both the tumor vasculature and tumor cells express certain level of VEGFR-2, which warrants more detailed examination in follow-up studies.
Using 64Cu-labeled VEGF121 as a tracer, we have previously examined the dynamic nature of VEGFR-2 expression in U87MG human glioblastoma tumors of different sizes by non-invasive PET imaging [26]. Based on both the in vivo data and ex vivo histology, smaller sized tumors were found to have significantly higher VEGFR-2 expression than similar tumors that were either too small (e.g. < 2 mm in diameter) or larger in sizes (e.g. > 8 mm in diameter). Clearly, these studies highlight the complex and dynamic nature of brain tumors even in small animal models, where dynamic and heterogeneous expression of a given protein is observed not only during different stages of tumor development but also within the same tumor tissue [24,26]. With further optimization of the already existing probes or future generations of molecular imaging probes, the dynamic and high resolution distribution of VEGFR-2 in the tumor microenvironment will no longer be a mystery, which may provide researchers/clinicians new tools for non-invasive imaging of tumor angiogenesis with sufficient spatial and temporal resolution.
Much more future investigations are warranted and expected. For example, it will be desirable to apply similar techniques/probes to assess VEGFR-2 expression levels of the same tumor at different sizes during growth or at different stages of development, which may potentially become an efficient non-invasive tool for accurate molecular profiling of brain tumors in the clinic. Multimodality imaging of VEGFR-2 expression, in which the same probe can be simultaneously detected by two or more imaging techniques (e.g. PET and MRI), can offer many advantages over the currently used probe. To further reduce the possible false-positive signal caused by the detachment of Gd-DTPA from the probe, conjugation of anti-VEGFR-2 antibody to suitable nanoplatforms with intrinsic properties for imaging applications (e.g. Gd3+-doped upconversion nanoparticles [27,28]) may provide more accurate information about the dynamic status of VEGFR-2 expression. Furthermore, invasion of tumor cells into the normal brain is one of the main reasons for treatment failure in gliomas [4]. With mMRI and a VEGFR-2 specific contrast agent, a better strategy for evaluating/monitoring the invasion of brain tumors may become possible in the near future. Lastly, these probes can also allow longitudinal imaging of VEGFR-2 expression in gliomas, which can be used for evaluating the therapeutic responses of anti-angiogenic therapy in preclinical studies as well as potentially providing new opportunities for improving brain tumor patient management.
Since mMRI contrast agents are molecule specific rather than disease specific, these agents can also have potential impacts in other cancer types as well (VEGFR-2 plays important roles in multiple solid tumor types [16]). For most molecular imaging agents, rapid clinical translation is a bottleneck. Future translation of optimized imaging agents into clinical investigation can shed new light on the development of novel mMRI probes, which will ultimately lead to better brain tumor patient management with improved tumor detection/staging, patient stratification, tumor invasion monitoring, prediction of therapeutic responses, and personalized therapies.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 4.Price SJ, Gillard JH. Imaging biomarkers of brain tumour margin and tumour invasion. Br J Radiol. 2011;84 Spec No 2:S159–67. doi: 10.1259/bjr/26838774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagner JP, Law M, Fischer I, Newcomb EW, Zagzag D. Angiogenesis in gliomas: imaging and experimental therapeutics. Brain Pathol. 2005;15:342–363. doi: 10.1111/j.1750-3639.2005.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 7.Alauddin MM. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am J Nucl Med Mol Imaging. 2012;2:55–76. [PMC free article] [PubMed] [Google Scholar]
- 8.Holland JP, Cumming P, Vasdev N. PET radiopharmaceuticals for probing enzymes in the brain. Am J Nucl Med Mol Imaging. 2013;3:194–216. [PMC free article] [PubMed] [Google Scholar]
- 9.Nolting DD, Nickels ML, Guo N, Pham W. Molecular imaging probe development: a chemistry perspective. Am J Nucl Med Mol Imaging. 2012;2:273–306. [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Zhang W, Li J, Zhang Y. Optical imaging of tumor microenvironment. Am J Nucl Med Mol Imaging. 2013;3:1–15. [PMC free article] [PubMed] [Google Scholar]
- 11.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 13.Cai W, Chen X. Multimodality imaging of vascular endothelial growth factor and vascular endothelial growth factor receptor expression. Front Biosci. 2007;12:4267–4279. doi: 10.2741/2386. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Yang Y, Hong H, Cai W. Multimodality molecular imaging of CD105 (Endoglin) expression. Int J Clin Exp Med. 2011;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 18.Samoto K, Ikezaki K, Ono M, Shono T, Kohno K, Kuwano M, Fukui M. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res. 1995;55:1189–1193. [PubMed] [Google Scholar]
- 19.Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007;13:504–509. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 20.Hao G, Hajibeigi A, Leon-Rodriguez LM, Oz OK, Sun X. Peptoid-based PET imaging of vascular endothelial growth factor receptor (VEGFR) expression. Am J Nucl Med Mol Imaging. 2011;1:65–75. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Cai W, Chen K, Li ZB, Kashefi A, He L, Chen X. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging. 2007;34:2001–2010. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- 22.Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007;13:504–509. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 23.Deshpande N, Ren Y, Foygel K, Rosenberg J, Willmann JK. Tumor angiogenic marker expression levels during tumor growth: longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology. 2011;258:804–811. doi: 10.1148/radiol.10101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He T, Smith N, Saunders D, Pittman BP, Lerner M, Lightfoot S, Mansat RS, Lupu F, Towner RA. Molecular MRI Differentiation of VEGF Receptor-2 Levels in C6 and RG2 Glioma Models. Am J Nucl Med Mol Imaging. 2013;3:300–311. [PMC free article] [PubMed] [Google Scholar]
- 25.He T, Smith N, Saunders D, Doblas S, Watanabe Y, Hoyle J, Silasi-Mansat R, Lupu F, Lerner M, Brackett DJ, Towner RA. Molecular MRI assessment of vascular endothelial growth factor receptor-2 in rat C6 gliomas. J Cell Mol Med. 2011;15:837–849. doi: 10.1111/j.1582-4934.2010.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen K, Cai W, Li ZB, Wang H, Chen X. Quantitative PET imaging of VEGF receptor expression. Mol Imaging Biol. 2009;11:15–22. doi: 10.1007/s11307-008-0172-1. [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Bu WB, Zhang SJ, Liu XH, Liu JN, Xing HY, Xiao QF, Zhou LP, Peng WJ, Wang LZ, Shi JL. Positive and Negative Lattice Shielding Effects Co-existing in Gd (III) Ion Doped Bifunctional Upconversion Nanoprobes. Advanced Functional Materials. 2011;21:4285–4294. [Google Scholar]
- 28.Zhou J, Liu Z, Li F. Upconversion nanophosphors for small-animal imaging. Chem Soc Rev. 2012;41:1323–1349. doi: 10.1039/c1cs15187h. [DOI] [PubMed] [Google Scholar]


