Abstract
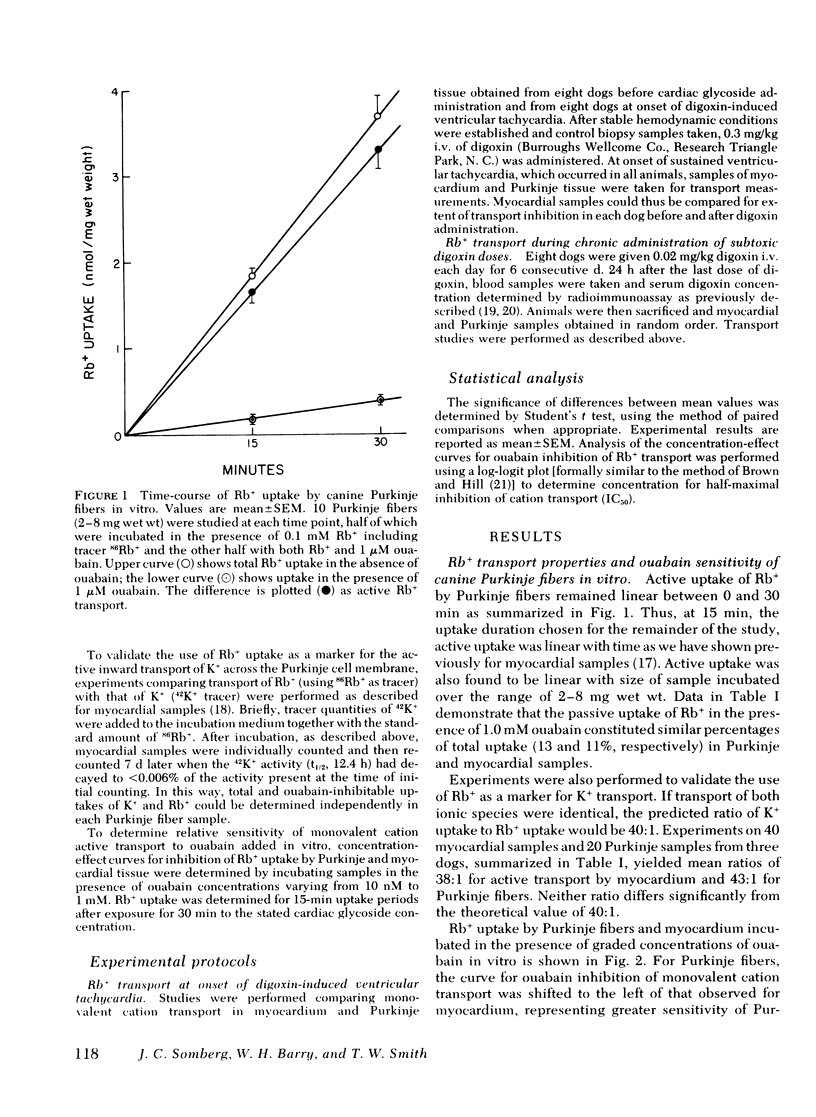
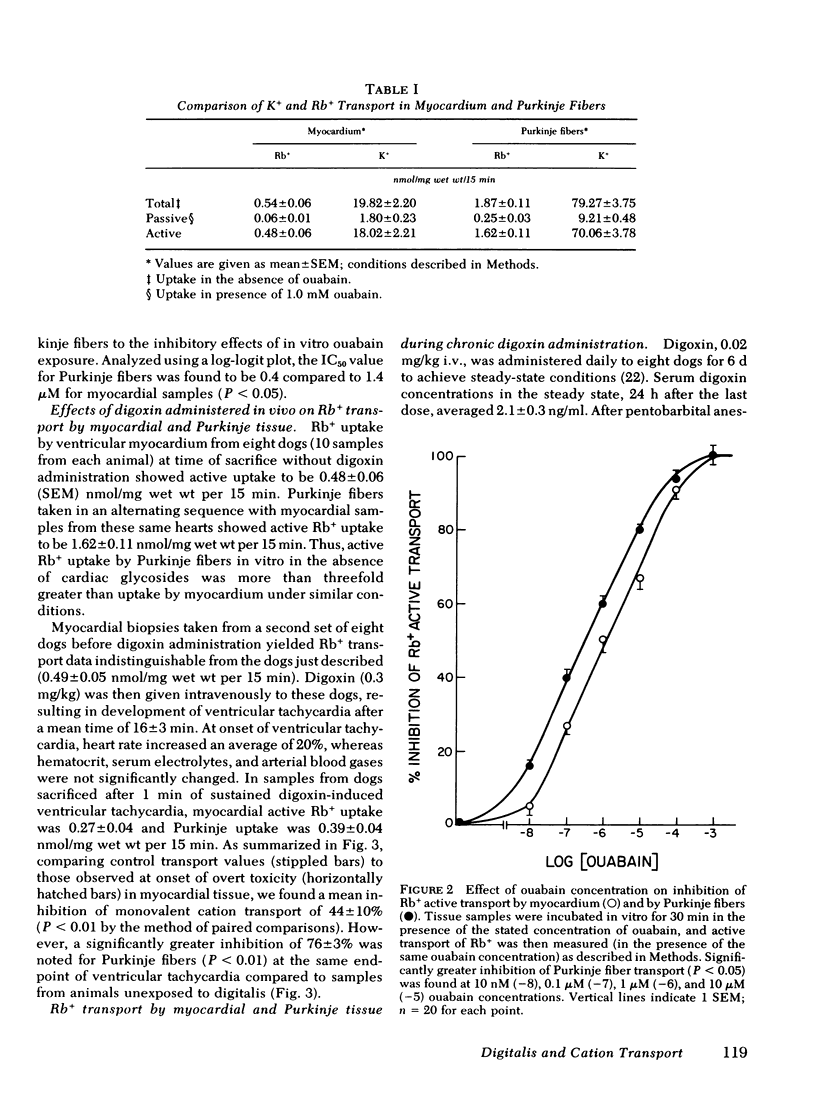
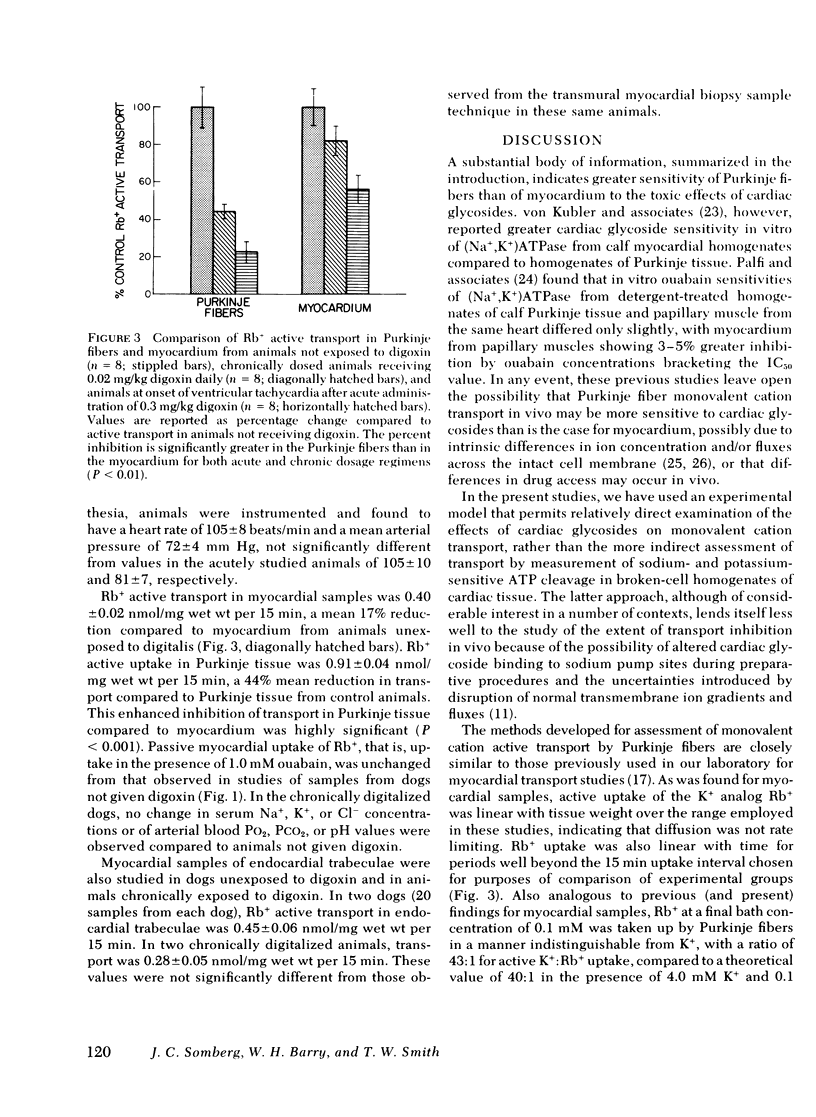
The extent of inhibition of monovalent cation active transport in Purkinje fibers and myocardium in response to toxic and inotropic doses of digitalis were studied in the dog to elucidate the factors underlying the different relative sensitivities of these tissues to the toxic arrhythmogenic effects of digitalis. Monovalent cation transport inhibition was assessed by measuring uptake of the K+ analog Rb+ in samples of myocardium and Purkinje fibers after in vitro ouabain exposure and after acute or chronic administration of digoxin in vivo. The active uptake of Rb+ was determined as the difference between total uptake and uptake in the presence of 1.0 mM ouabain. Mean active uptake of Rb+ by Purkinje fibers from control hearts was 1.62±0.11 (SEM) nmol/mg wet wt per 15 min, significantly greater than the value of 0.49±0.05 for myocardium (P < 0.001). Concentration-effect curves for inhibition of monovalent cation active transport by in vitro exposure to graded concentrations of ouabain showed that the concentration for half-maximal inhibition of monovalent cation transport, IC50, for Purkinje fiber transport was 0.4 μM, significantly less than the value of 1.4 μM for myocardial samples. Dogs were given toxic doses of digoxin (0.3 mg/kg i.v.). At onset of sustained ventricular tachycardia, they were killed and monovalent cation transport measured in myocardial and Purkinje fiber samples. Active Rb+ uptake was inhibited by 44% in myocardial samples, whereas a significantly greater inhibition of 76% was noted in Purkinje fibers (P < 0.01). Similar data were obtained for both transmural myocardial biopsy samples and subendocardial trabecular samples obtained from regions adjacent to Purkinje fibers. Another group of dogs received a nontoxic dose of 0.02 mg/kg i.v. daily for 6 d. Myocardium showed a 17% reduction in Rb+ active uptake compared to control animals receiving no drug, whereas Purkinje fiber transport was reduced in these dogs to a significantly greater extent averaging 44% (P < 0.001). Thus, both toxic and inotropic (non-toxic) doses of digoxin inhibited monovalent cation transport in Purkinje fibers to a greater extent than in myocardium. This difference in apparent sensitivity of monovalent cation transport to digoxin may contribute to the previously reported tendency of digitalis toxic arrhythmias to arise in Purkinje fibers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera D. K., Brody T. M., Ku D., Pew C. L. Cardiac glucosides: correlations among Na+ K+-ATPase, sodium pump and contractility in the guinea pig heart. Naunyn Schmiedebergs Arch Pharmacol. 1974;285(2):185–200. doi: 10.1007/BF00501153. [DOI] [PubMed] [Google Scholar]
- Akera T., Larsen F. S., Brody T. M. Correlation of cardiac sodium- and potassium-activated adenosine triphosphatase activity with ouabain-induced inotropic stimulation. J Pharmacol Exp Ther. 1970 May;173(1):145–151. [PubMed] [Google Scholar]
- Bernstein J. C., Israel Y. Active transport of Rb86 in human red cells and rat brain slices. J Pharmacol Exp Ther. 1970 Aug;174(2):323–329. [PubMed] [Google Scholar]
- Besch H. R., Jr, Allen J. C., Glick G., Schwartz A. Correlation between the inotropic action of ouabain and its effects on subcellular enzyme systems from canine myocardium. J Pharmacol Exp Ther. 1970 Jan;171(1):1–12. [PubMed] [Google Scholar]
- Biedert S., Barry W. H., Smith T. W. Inotropic effects and changes in sodium and calcium contents associated with inhibition of monovalent cation active transport by ouabain in cultured myocardial cells. J Gen Physiol. 1979 Oct;74(4):479–494. doi: 10.1085/jgp.74.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORABOEUF E., DE LOZE C., BOISTEL J. Action de la digitale sur les potentiels de membrane et d'action du tissu conducteur du coeur de chien étudiée à l'aide de microélectrodes intracellulaires. C R Seances Soc Biol Fil. 1953 Jul;147(13-14):1169–1172. [PubMed] [Google Scholar]
- Curfman G. D., Crowley T. J., Smith T. W. Thyroid-induced alterations in myocardial sodium-potassium-activated adenosine triphosphatase, monovalent cation active transport, and cardiac glycoside binding. J Clin Invest. 1977 Mar;59(3):586–590. doi: 10.1172/JCI108675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. D. Effect of changes in cycle length on diastolic depolarization produced by ouabain in canine Purkinje fibers. Circ Res. 1973 Feb;32(2):206–214. doi: 10.1161/01.res.32.2.206. [DOI] [PubMed] [Google Scholar]
- Dutta S., Rhee H. M., Marks B. H. 3H-ouabain accumulation and Na+-K+-ATPase activity in relation to therapeutic, toxic, and lethal doses of ouabain in dogs. Recent Adv Stud Cardiac Struct Metab. 1974;4:119–130. [PubMed] [Google Scholar]
- Ettinger P. O., Calabro J., Regan T. J., Oldewurtel H. A. Origin of acetyl strophanthidin-induced ventricular arrhythmias. J Clin Invest. 1977 Feb;59(2):193–202. doi: 10.1172/JCI108629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier G. R. Digitalis arrhythmias: role of oscillatory afterpotentials. Prog Cardiovasc Dis. 1977 May-Jun;19(6):459–474. doi: 10.1016/0033-0620(77)90010-x. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R., Saunders J. H., Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973 May;32(5):600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Hougen T. J., Lloyd B. L., Smith T. W. Effects of inotropic and arrhythmogenic digoxin doses and of digoxin-specific antibody on myocardial monovalent cation transport in the dog. Circ Res. 1979 Jan;44(1):23–31. doi: 10.1161/01.res.44.1.23. [DOI] [PubMed] [Google Scholar]
- Hougen T. J., Smith T. W. Inhibition of myocardial monovalent cation active transport by subtoxic doses of ouabain in the dog. Circ Res. 1978 Jun;42(6):856–863. doi: 10.1161/01.res.42.6.856. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler W., Endres G., Smekal P. V. Vergleichende Untersuchungen über die Aktivität und Digitalisempfindlicheit der Transport-ATPase im Arbeitsmyokard und im Reizleitungssystem. Verh Dtsch Ges Kreislaufforsch. 1970;36:305–309. [PubMed] [Google Scholar]
- MOE G. K., MENDEZ R. The action of several cardiac glycosides on conduction velocity and ventricular excitability in the dog heart. Circulation. 1951 Nov;4(5):729–734. doi: 10.1161/01.cir.4.5.729. [DOI] [PubMed] [Google Scholar]
- Nowak G., Haustein K. O. Different toxic effects of ouabain and 16-epi-gitoxin on Purkinje fibres and ventricular muscle fibres. Pharmacology. 1976;14(3):256–264. doi: 10.1159/000136603. [DOI] [PubMed] [Google Scholar]
- Ochs H. R., Vatner S. F., Smith T. W. Reversal of inotropic effects of digoxin by specific antibodies and their Fab fragments in the conscious dog. J Pharmacol Exp Ther. 1978 Oct;207(1):64–71. [PubMed] [Google Scholar]
- Palfi F. J., Besch H. R., Jr, Watanabe A. M. Ouabain sensitivity of the Na+, K+-ATPase activity from single bovine cardiac Purkinje fiber and adjacent papillary muscle. J Mol Cell Cardiol. 1978 Dec;10(12):1149–1155. doi: 10.1016/0022-2828(78)90359-0. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I., Vassalle M. On the mechanism of ouabain toxicity in Purkinje and ventricular muscle fibers at rest and during activity. Am J Cardiol. 1971 Jun;27(6):622–629. doi: 10.1016/0002-9149(71)90226-8. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H. Effects of ouabain on canine Purkinje fibers in situ or perfused with blood. J Pharmacol Exp Ther. 1973 Aug;186(2):366–372. [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Merker C., Hoffman B. F. Mechanisms of digitalis toxicity. Effects of ouabain on phase four of canine Purkinje fiber transmembrane potentials. Circulation. 1973 Apr;47(4):681–689. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Merker C., Hoffman B. F. Mechanisms of digitalis toxicity. Effects of ouabain on phase four of canine Purkinje fiber transmembrane potentials. Circulation. 1973 Apr;47(4):681–689. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- SWAIN H. H., WEIDNER C. L. A study of substances which alter intraventricular conduction in the isolated dog heart. J Pharmacol Exp Ther. 1957 Jun;120(2):137–146. [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Van Winkle W. B., Munson R. Further studies on the correlation between the inotropic action of ouabain and its interaction with the Na+,K+-adenosine triphosphatase: isolated perfused rabbit and cat hearts. J Pharmacol Exp Ther. 1974 Oct;191(1):119–127. [PubMed] [Google Scholar]
- Smith T. W., Haber E. Digitalis (third of four parts). N Engl J Med. 1973 Nov 15;289(20):1063–1072. doi: 10.1056/NEJM197311152892005. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Haber E. Digoxin intoxication: the relationship of clinical presentation to serum digoxin concentration. J Clin Invest. 1970 Dec;49(12):2377–2386. doi: 10.1172/JCI106457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. W., Wagner H., Jr Effects of (Na+ + K+)-ATPase-specific antibodies of enzymatic activity and monovalent cation transport. J Membr Biol. 1975;25(3-4):341–360. doi: 10.1007/BF01868583. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Wagner H., Jr, Markis J. E., Young M. Studies on the localization of the cardiac glycoside receptor. J Clin Invest. 1972 Jul;51(7):1777–1789. doi: 10.1172/JCI106979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somberg J. C., Risler T., Smith T. W. Neural factors in digitalis toxicity: protective effect of C-1 spinal cord transection. Am J Physiol. 1978 Nov;235(5):H531–H536. doi: 10.1152/ajpheart.1978.235.5.H531. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Carpenter D. O. Ionic mechanisms of pacemaker activity in cardiac Purkinje fibers. Fed Proc. 1978 Jun;37(8):2127–2131. [PubMed] [Google Scholar]
- VASSALLE M., KARIS J., HOFFMAN B. F. Toxic effects of ouabain on Purkinje fibers and ventricular muscle fibers. Am J Physiol. 1962 Sep;203:433–439. doi: 10.1152/ajplegacy.1962.203.3.433. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Musso E. On the mechanisms underlying digitalis toxicity in cardiac Purkinje fibers. Recent Adv Stud Cardiac Struct Metab. 1976;9:355–376. [PubMed] [Google Scholar]
- Vick R. L., Hazlewood C. F., Nichols B. L. Distribution of potassium, sodium, and chloride in canine Purkinje and ventricular tissues. Relation to cellular potential. Circ Res. 1970 Aug;27(2):159–169. doi: 10.1161/01.res.27.2.159. [DOI] [PubMed] [Google Scholar]


