Abstract
Purpose
To evaluate the usefulness of 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography (FDG-PET/CT) in the early detection of resectable recurrences of colorectal cancer (CRC) and the impacts on the clinical disease management.
Methods
FDG-PET/CT was performed on patients with elevated serum carcinoembryonic antigen (CEA) levels >5 ng/mL (Group 1) or suspicious recurrences without rise in serum CEA levels (Group 2). The results were analyzed on the basis of histological data, disease progression, and/or clinical follow-up. Recurrence was defined as evidence of recurrent lesions within 6 months of the FDG-PET/CT scan. Resectable recurrences and changes in management were calculated based on medical records.
Results
In our study, 128 consecutive FDG-PET/CT analyses (n=49 in Group 1 and n=79 in Group 2) were performed on 96 recruited patients. Recurrences were proven in 63. The overall sensitivity, specificity, and accuracy of FDG-PET/CT were 98.4%, 89.2%, and 93.8%, respectively, and were 100%, 88.9%, and 95.9% in Group 1 and 96.9% and 89.4% and 92.4% in Group 2, respectively. Surgical resections were performed in 38.7% (12/31) of Group 1 patients and 53.1% (17/32) of Group 2 patients. FDG-PET/CT induced changes in planned management in 48.4% (62/128) of all patients, which included 63.3% (31/49) of Group 1 patients and 39.2% (31/79) of Group 2 patients (p=0.008). After a follow-up, 3.4% (1/29) of patients who underwent surgical resection of recurrent lesions and 34.3% (11/34) patients who did not undergo resection died at the end of study (p=0.004).
Conclusions
The surgical resection of limited recurrent disease, as determined by FDG-PET/CT, improves the survival of CRC patients. FDG-PET/CT should be performed not only in patients with elevated serum CEA levels, but also in those in whom recurrences are suspected to improve the early detection of resectable disease.
Introduction
Colorectal cancer (CRC) is increasingly the leading cause of cancer-related mortality in Taiwan, as well as in the Western world.1 Surgery remains the mainstay of therapy for patients with CRC, but recurrences after curative resection of CRC occur in 30%–40% of patients.2,3 Accordingly, various follow-up strategies have been applied after curative surgeries for CRC to detect recurrent disease.
Carcinoembryonic antigen (CEA) has been widely used as a tumor marker to detect asymptomatic or early recurrences that might be amenable to curative surgery. The elevation of serum CEA levels has been shown to be a sensitive marker of recurrent CRC, with a detection sensitivity range of 88%–91%.4,5 Increases in serum CEA levels precede clinically apparent recurrences by an average of 1.5–6 months.6–9 The use of this tumor marker has been shown to be cost effective, however, additional imaging examinations are needed to detect the exact locations and extensions of the recurrent lesions. Further, 30%–40% of all CRC recurrences are not associated with measurable elevations in serum CEA levels.10
Positron emission tomography with the glucose analog tracer 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) is an imaging method that is based on the increased glucose metabolism in malignant tumors. A meta-analysis of 11 clinical reports that encompassed 577 patients determined that the sensitivity and specificity of FDG-PET in the detection of recurrent CRC were 97% and 76%, respectively, which led to changes in treatment for 29% of the patients.11 A review of the literature, in which 2244 patients were evaluated for recurrences, showed that the sensitivity and specificity of FDG-PET and computed tomography (CT) are 94% and 87%, and 79% and 73%, respectively.12 In a prospective randomized trial of either conventional or FDG-PET procedures during follow-up evaluations of CRC, recurrences were detected after a shorter time (12.1 vs. 15.4 months, p=0.01) and were more frequently cured by surgery (10 vs. 2 patients) in the PET group.13 Recently, combined PET and CT systems (PET/CT) have emerged as promising imaging modalities and are more routinely applied in clinical situations. PET/CT offers the evaluation of both metabolic activity and anatomy at the same location in the body and is useful in the recognition of local recurrences of CRC. The reported sensitivity and specificity of FDG-PET/CT for the detection of recurrent CRC range from 86% to 98% and 83.3% to 98%, respectively.14–22
The early detection of recurrent disease has been reported to improve the survival of CRC patients, particularly for those with the potential to be surgically cured due to limited sites of metastatic disease. Five year survival rates have been reported to approach 40% in patients who underwent partial hepatectomies for limited hepatic metastases and 35%–45% in selected patients who underwent metastasectomies for lung metastases.23–27
FDG-PET/CT might be useful in the early diagnosis of recurrent CRC and thus might improve the survival rates of CRC patients. The aim of this study was to evaluate the ability of FDG-PET/CT to detect early resectable recurrent CRC in patients with elevated serum levels of tumor markers and/or suspected recurrence due to clinical or radiologic evaluations, and the impact of FDG-PET/CT on the clinical disease management.
Patients and Methods
We retrospectively collected patients with prior histories of CRC and complete responses to treatment (primary surgery and/or chemotherapy) who were undergoing FDG-PET/CT scans, and divided them into two groups: in Group 1 patients, the decision to obtain a FDG-PET/CT scan was based on serum CEA levels >5 ng/mL; in Group 2, FDG-PET/CT scans were performed due to suspicions of recurrence in the absence of elevated serum CEA levels. At the authors' institution, serum CEA levels were monitored every 3 months in CRC patients after complete responses to treatment. The exclusion criteria for this study included vital sign instability, severe diabetes, severe illness, the confirmed existence of 1 or more additional tumors and an inability to remain supine for 30 minutes. From June 2006 to January 2012, 128 consecutive FDG-PET/CT studies were performed on 96 recruited patients with prior CRC. Of these patients, 3 underwent 4 FDG-PET/CT studies, 5 patients underwent 3 studies, 13 patients underwent 2 studies, and the remaining patients each underwent 1 study. Overall, 49 studies in 40 patients were referred due to increased serum CEA levels (Group 1) and 79 studies in 56 patients were referred due to various reasons in the absence of elevated serum CEA levels (Group 2). Almost all studies in Group 1 were prescribed to determine the source of recurrence, while many studies in Group 2 were intended to rule out other lesions and to identify patients who might potentially be cured surgically due to limited sites of metastatic disease. According to the study application forms, the indications for suspected recurrences included suspicious or indeterminate CT scans (n=33); magnetic resonance imaging (n=3); abdominal sonography (n=2) and chest film (n=1); abdominal tenderness (n=5); palpable mass in abdomen (n=6) and supra-clavicular regions (n=3), body weight loss (n=5); elevation of other serum tumor markers such as CA 19–9, SCC, and so on (n=7); poor appetite (n=6), general malaise, and weakness (n=4); and low-level increases in the levels of serum CEA<5 ng/mL (n=4). Detailed patient characteristics are presented in Table 1. The American Joint Committee on Cancer (AJCC) classification was used in the initial staging of CRC. This study was approved by the institutional review board and the hospital ethics committee, and all the participants provided written informed consent.
Table 1.
Patient Characteristics
| Group 1 (n =40) | Group 2 (n =56) | |
|---|---|---|
| Median age, years (range) | 62.0 (37–79) | 60.8 (34–85) |
| Location of tumors | ||
| Colon | 18 | 35 |
| Rectum | 22 | 20 |
| Colon & rectum | 0 | 1 |
| Tumor, differentiation | ||
| Adenocarcinoma, good | 20 | 24 |
| Adenocarcinoma, intermediate | 16 | 18 |
| Adenocarcinoma, poor | 4 | 13 |
| Intraepithelial carcinoma | 0 | 1 |
| Stage at diagnosis | ||
| 0 | 0 | 1 |
| I | 7 | 8 |
| II | 12 | 19 |
| III | 17 | 22 |
| IV | 4 | 5 |
| Unknown | 0 | 1 |
FDG-PET/CT, 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography.
FDG-PET/CT imaging
The patients fasted for at least 6 hours prior to the FDG-PET/CT imaging. An intravenous catheter was placed for radiopharmaceutical administration, and the blood glucose levels of the patients were measured prior to the tracer injection. All of the patients demonstrated blood glucose levels below 150 mg/dL at the time of injection. Each patient received 370–555 MBq of 18F FDG according to the patient's body weight (7.03 MBq/kg). After the tracer injection, the patients rested for 1 hour on a comfortable bed in a dark room. Whole-body FDG-PET/CT imaging (Discovery ST-16; GE Healthcare, Milwaukee, WI) was performed from the head to the upper thigh while the patients were in a supine position. A delayed image with or without the use of diuretics was obtained when necessary. CT scanning was performed prior to the acquisition of the PET data using the following parameters: 0.6 seconds per rotation: 120 kV, 100 mA, and 3.75-mm thick slices. After the completion of a plain CT, PET images of the same areas were acquired in a two-dimensional mode and 4 minutes of data collected per bed position. Attenuation-corrected PET images were reconstructed with an ordered subset expectation maximization iterative reconstructed algorithm. The 3.75-mm thick transaxial CT images were reconstructed at 3.27 mm intervals for fusion with the PET images. PET, CT, and fused PET/CT images were generated on a Xeleris image display and processing platform (GE Healthcare) for review on a computer workstation.
Image analysis
The PET, CT, and fused PET/CT images were interpreted by two qualified nuclear medicine physicians who were allowed to manipulate the image contrast, image intensities, and three-dimensional images on a computer screen. The final diagnoses were made by consensus. Physicians were not blinded with regards to the reasons for the examinations or the outcomes at the times of the image analyses. Because this was a retrospective study, the reasons for the examination were described on the application form. Each study was regarded as an independent event, and prior imaging, including prior PET/CT and contrast-enhanced CT scans, were available at the time of review for the fullest analysis. Both physicians independently reviewed the data before developing a consensus. Any increases in FDG uptake were compared with the corresponding anatomical findings on the CT scan. Areas of increased FDG uptake that corresponded to normal structures such as muscle and fat tissue or the bowel were considered to indicate physiologic uptake or benign processes. For lesions that demonstrated abnormal FDG uptake, the physicians outlined the region of interest, which indicated the area with the greatest amount of uptake. A standardized uptake value (SUV) was semiautomatically determined using the SUV tools that are available in the Xeleris software (SUV=activity in the region of interest (Bq/g)/[injected dose (Bq)/body weight (g)]). The two-dimensional regions of interest were drawn around the tumor on each transaxial slice that contained tumor tissue. A single-pixel SUVmax was determined for each region, and the slice that demonstrated the highest SUV was designated as the SUVmax for the entire tumor.
Impact of FDG-PET/CT on patient management
The impact of FDG-PET/CT on patient management was assessed by comparing the management plans before and after the performance of FDG-PET/CT. Before FDG-PET/CT, patients with serum CEA levels >5 ng/mL were expected to start chemotherapy, and those without increased serum CEA levels were scheduled for observation until any changes were noted. The intended management was dichotomized as either treatment (e.g., surgery, chemotherapy, radiation, or other treatment, alone or in combination) or nontreatment (e.g., observation or supportive care). A major change in management was defined as a switch from treatment to nontreatment or vice versa. A minor change in management was defined as a modification of the intended treatment methods (e.g., from chemotherapy to a combined surgery or radiation). Resectable recurrences were calculated according to the surgical resections of limited metastasis on medical records.
Statistical analysis
The results were analyzed on a pathological basis when histological sampling was possible, on disease progression, and/or on clinical follow-up evaluations. Recurrent CRC was defined as the detection of recurrent lesions within 6 months of the FDG-PET/CT scan. The chi-square test was used to determine the significance of the differences between the two groups with respect to the detection of recurrent disease and patient outcomes. Differences were considered to be significant if p<0.05. All of the calculations were performed with SPSS version 12.0 (SPSS, Chicago, IL).
Results
The outcomes of the FDG-PET/CT studies in Group 1 and Group 2 patients are listed in Table 2. Among the 128 FDG-PET/CT studies, the mean serum CEA levels at the time of the studies were 31.7 ng/mL (range: 5–351.9 ng/mL) and 2.2 ng/mL (range: 1–4.5 ng/mL) for Group 1 and Group 2, respectively (p=0.002). Recurrences were proven in 63 patients, including 31 of 49 (63.3%) in Group 1 and 32 of 79 (40.5%) in Group 2, (p=0.012). Of the recurrences, 36 (57.1%) were proven on a histological basis, and the others were proven by disease progression or responses to therapy during follow-up. Surgical resections were performed in 38.7% (12/31) of the Group 1 patients and 53.1% (17/32) of the Group 2 patients. As shown in Table 3, most patients (25/29, 86.2%) had liver and lung lesions (Fig. 1). Lesions in other sites included one in the peritoneum (Fig. 2) and three in the bowel (two in anastomotic sites and the other in the ascending colon). Most of the studies (26/29, 89.7%) had delayed scans, and 24 of these studies (24/26, 92.3%) showed increased SUVmax on the delayed scans. Of the remaining 2 patients, the first showed a decrease from 2.5 to 1.9 in the SUVmax of a 1.2 cm pulmonary nodule, while the second retained a SUVmax of 1.3 in a 0.8 cm pulmonary nodule. After discussion, both physicians reached a consensus. The reduced or equal tracer uptake on the delayed scans was likely due to the partial volume effect, respiration movement, or both. After follow-up studies, 8 of the 31 (25.8%) recurrent patients in Group 1 and 4 of the 32 (12.5%) recurrent patients in Group 2 died at the end of study (p>0.05). The time from recurrence to death was 18.3 months in Group 1 and 25 months in Group 2 (p>0.05).
Table 2.
The Outcome of FDG-PET/CT Studies in the Group 1 and Group 2 Patients
| Group 1 (n=49) | Group 2 (n=79) | p | |
|---|---|---|---|
| Mean interval to last surgery, months (range) | 42.1 (3–226) | 33.7 (3–276) | 0.25 |
| Mean CEA level, ng/mL (range) | 31.7 (5–351.9) | 2.2 (1–4.5) | 0.002 |
| Mean follow-up time, months (range) | 27.9 (4–60) | 25.5 (6–69) | 0.41 |
| Recurrence, n (% over studies) | 31 (63.3%) | 32 (40.5%) | 0.012 |
| Time to recurrence, months (range) | 34.9 (3–226) | 24.6 (4–97) | 0.23 |
| Time from recurrence to therapy, months (range) | 1.4 (0–6) | 1.8 (0–6) | 0.44 |
| Histology, n (% over recurrences) | 15 (48.4%) | 21 (65.6%) | 0.17 |
| Surgical resection, n (% over recurrences) | 12 (38.7%) | 17 (53.1%) | 0.25 |
| Death, n (% over recurrences) | 8 (25.8%) | 4 (12.5%) | 0.21 |
| Time from recurrence to death, months (range) | 18.3 (4–31) | 25 (16–45) | 0.42 |
CEA, carcinoembryonic antigen.
Table 3.
FDG-PET/CT Findings and Disease Management in the 29 Patients Who Underwent Surgical Resection of Recurrent Lesions
| Case | Age (year) | CEA (ng/mL) | Stage | FDG-PET/CT findings | SUVmax (early) | SUVmax (delayed) | Management changed | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | 7.7 | IIIB | A 2 cm nodule over peritoneum | 3.3 | 4.4 | C/T to OP | 20 |
| 2 | 58 | 10.4 | I | Local recurrence over anastomotic site | 7.0 | 7.9 | C/T to OP | 37 |
| 3 | 69 | 31.8 | II | Ascending colon | 13.6 | 16.2 | C/T to OP | 42 |
| 4 | 52 | 24.2 | IIIB | A 1.2 cm nodule in RLL | 2.5 | 1.9 | C/T to OP | 35 |
| 5 | 74 | 351.9 | IV | Two nodules in liver | 6.4 | 7.2 | C/T to OP | 40 |
| 6 | 47 | 98.1 | IIIC | A 2.3 cm nodule in liver | 6.1 | 10 | C/T to OP | 24 |
| 7 | 49 | 45.6 | II | Two nodules in liver | 9.7 | 13.1 | C/T to OP | 11 |
| 8 | 69 | 16.8 | II | A 5.5 cm mass in LUL | 13.6 | 15.7 | C/T to OP | 11 |
| 9 | 63 | 5.4 | III | A 2.5 cm nodule in liver | 4.0 | 8.9 | C/T to OP | 10 |
| 10 | 65 | 12.7 | I | A 5.5 cm mass in liver | 10.6 | 14 | C/T to OP | 4 |
| 11 | 37 | 10.5 | IIIB | Several nodules in liver | 7.4 | C/T to OP | 7 | |
| 12 | 57 | 16.2 | IV | A 3.3 cm nodule in liver, a 0.7 cm nodule in RML, and a 1.3 cm nodule in RLL | 14.4 | C/T to OP | 24 | |
| 13 | 58 | 3.2 | IIA | A 0.8 cm nodule in LUL | 1.3 | 1.3 | None to OP | 34 |
| 14 | 77 | 2 | IIIB | A 3.0 cm nodule in liver | 7.9 | 13.3 | None to OP | 29 |
| 15 | 57 | 1.9 | IIIB | A 1.0 cm nodule in RML | 2.0 | 2.1 | None to OP | 27 |
| 16 | 57 | 2.3 | IIIB | Several lung nodules in both lungs | 5.8 | None to OP | 6 | |
| 17 | 61 | 1.5 | IIIC | A 0.7 cm nodule in RLL | 1.5 | 1.7 | None to OP | 16 |
| 18 | 60 | 3.4 | IIIB | Two isodense foci in liver | 13.3 | 17.1 | None to OP | 2 |
| 19 | 46 | <1.0 | IVB | A 1.2 cm nodule in RLL | 1.0 | 2.1 | None to OP | 13 |
| 20 | 62 | 2.8 | III | Two nodules in RLL | 2.0 | 2.3 | None to OP | 13 |
| 21 | 55 | 1.6 | IIA | A 3.7 cm nodule in LLL | 10.1 | 11 | None to OP | 12 |
| 22 | 48 | 1.9 | IIIB | Two nodules in liver | 5.0 | 6.7 | None to OP | 12 |
| 23 | 67 | 4.4 | IIIB | A 1.7 cm nodule and an isodense focus in liver | 12.7 | 18.6 | None to OP | 11 |
| 24 | 75 | 1 | IIIB | A 1.5 cm nodule in RML | 3.1 | 6.1 | None to OP | 8 |
| 25 | 67 | 3.1 | IV | A 1.5 cm nodule in liver | 4.2 | 6.9 | None to OP | 7 |
| 26 | 62 | 1.8 | IIB | An isodense focus in liver | 4.4 | 5.0 | None to OP | 17 |
| 27 | 59 | 3.9 | I | Local recurrence over anastomotic site | 4.6 | 5.6 | None to OP | 36 |
| 28 | 48 | 1.5 | IIIC | An isodense focus in liver | 2.9 | 3.4 | None to OP | 52 |
| 29 | 50 | 3.9 | IIIC | Several nodules in liver | 4.7 | 7.7 | None to OP | 19 |
SUV, standardized uptake value; LLL, left lower lobe of lung; LUL, left upper lobe of lung; RLL, right lower lobe of lung; RML, right middle lobe of lung; RUL, right upper lobe of lung; C/T, chemotherapy; OP, operation.
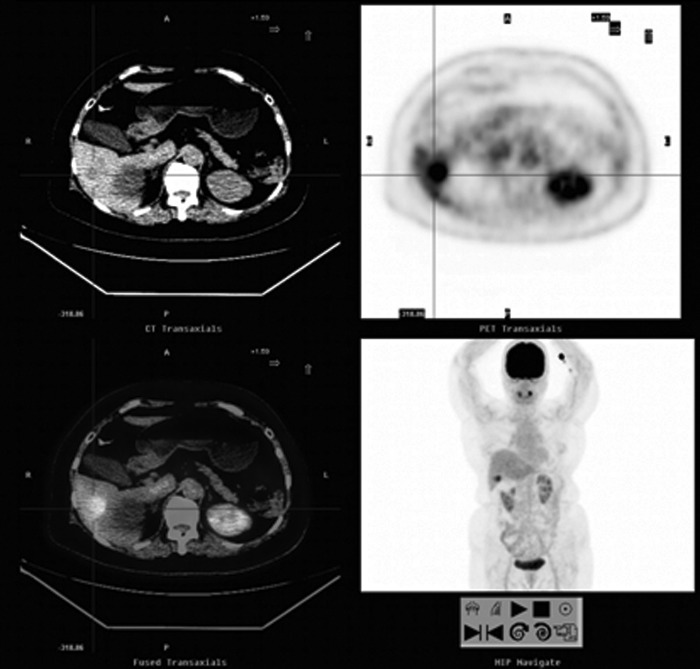
FIG. 1.
A 77-year-old woman with a history of colon cancer (moderately differentiated adenocarcinoma of the descending-sigmoid junction, stage IIIB) and a normal serum CEA level. Abdominal sonography revealed a new nodule in the liver. FDG-PET/CT showed a 3.0 cm FDG-avid hypodense nodule in the inferior portion of the right hepatic lobe (SUVmax: 7.9, cross cursor). A partial hepatectomy was performed and metastasis was demonstrated by pathological analysis. CEA, carcinoembryonic antigen; FDG-PET/CT, 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography; SUV, standardized uptake value.
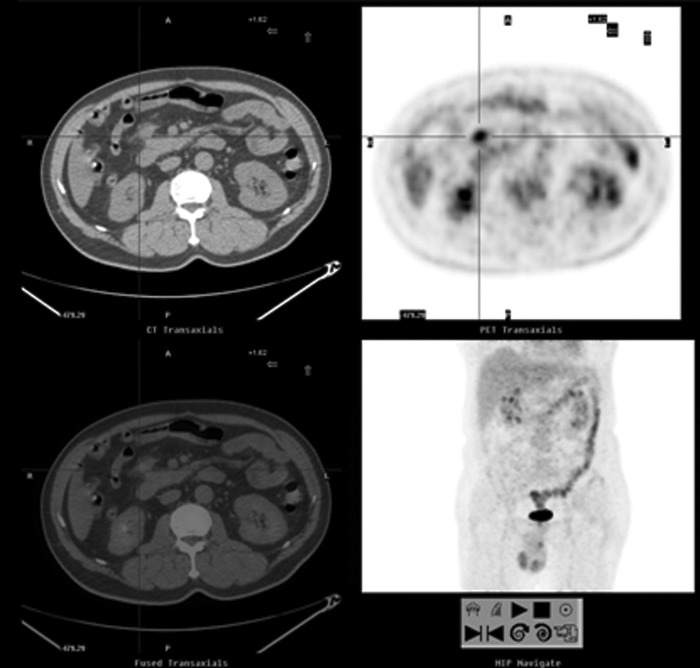
FIG. 2.
A 66-year-old man with a history of colon cancer (moderately differentiated adenocarcinoma in the hepatic flexure of transverse colon, stage IIIB) presented with an elevated serum CEA level of 7.7 ng/mL. FDG-PET/CT showed a 2 cm nodule anterior to the pancreatic head, abutted the right gastroepiploic vein (SUVmax: 4.4, cross cursor). The pathological analysis confirmed the presence of a metastatic lesion. After therapy, serum CEA levels were reduced to the normal range. The patient remained on clinical follow-up at the end of the study.
The use of FDG-PET/CT allowed for the detection of 62 recurrent cancers and 7 false positives. Of the latter, four were proven with histological analysis, including a tubulovillous adenoma, a sclerosing angiomatoid nodular transformation of the spleen, and one case each of second primary lung cancer (Fig. 3) and malignant teratoma. The remaining cases were confirmed as benign on follow-up studies and included one case of pulmonary tuberculosis and two cases with unknown causes of increased FDG uptake in an anastomotic site and a soft tissue lesion in the left paracolic region. A false negative diagnosis was noted in a Group 2 patient, who had two recurrent presacral lesions within the 6-month poststudy period. In the remaining patients, no recurrent disease was detected during a follow-up period of at least 6 months, which included close surveillance, the periodic evaluation of serum CEA levels, and other imaging modalities. In Group 1, FDG-PET/CT detected 31 recurrent cancers and 2 false positives, which were found to be due to a tubulovillous adenoma and pulmonary tuberculosis. In Group 2, FDG-PET/CT detected 31 recurrent cancers, 2 second primary cancers, and the 3 aforementioned benign lesions. Accordingly, the overall sensitivity, specificity, and accuracy of FDG-PET/CT were found to be 98.4%, 89.2%, and 93.8%, respectively, in comparison to those of serum CEA levels, which were 48.4%, 72.3%, and 60.2%, respectively. With regard to each group, the sensitivity, specificity, and accuracy of FDG-PET/CT were 100%, 88.9%, and 95.9% in Group 1 and 96.9%, 89.4%, and 92.4% in Group 2, respectively (Table 4). FDG-PET/CT induced major changes in management in 36.7% (47/128) of the overall patients; in Group 1, 32.7% (16/49) of the patients were moved from treatment to nontreatment approaches and in Group 2, 39.2% (31/79) of the patients were moved from nontreatment to treatment approaches. Together with the surgical resections in 12 of the 31 recurrent CRC patients and radiotherapy approaches in another 3 patients in Group 1, FDG-PET/CT induces changes in the planned management for 48.4% (62/128) of the patients, which included 63.3% (31/49) in Group 1 and 39.2% (31/79) in Group 2 (p=0.008).
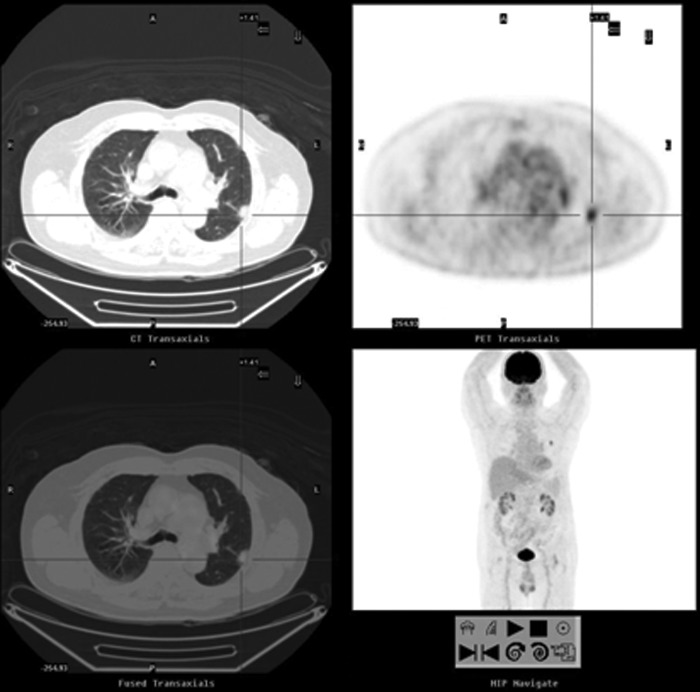
FIG. 3.
A 79-year-old man with a history of adenocarcinoma of rectal cancer (moderately differentiated, stage IIA). A nodule was noted on the chest film and the serum CEA level of 2.9 ng/mL was within the normal range. FDG-PET/CT showed a 1.9 cm pleural-based nodule over the left upper lobe (SUVmax: 5.0, cross cursor) and no FDG-avid lesions elsewhere. After a wedge resection of the left upper lobe, the pathological analysis showed a moderately differentiated adenocarcinoma of pulmonary origin.
Table 4.
Performance of Serum Carcinoembryonic Antigen Levels and FDG-PET/CT in the Detection of Recurrent Cancer
| TP | FP | TN | FN | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|---|
| Serum CEA (n=128) | 30 | 18 | 47 | 32 | 48.4%a | 72.3%b | 60.2%a |
| FDG-PET/CT (n=128) | 62 | 7 | 58 | 1 | 98.4%a | 89.2%b | 93.8%a |
| Group 1 FDG-PET/CT (n=49) | 31 | 2 | 16 | 0 | 100% | 88.9% | 95.9% |
| Group 2 FDG-PET/CT (n=79) | 31 | 5 | 42 | 1 | 96.9% | 89.4% | 92.4% |
p<0.001 FDG-PET/CT versus serum CEA.
p=0.025 FDG-PET/CT versus serum CEA.
FN, false negative; FP, false positive; TN, true negative; TP, true positive.
For the comparison of recurrent patients with surgical resection and those without, the mean serum CEA level at the time of FDG-PET/CT imaging, mean SUVmax, mean follow-up time, time to recurrence, and time from recurrence to therapy were not found to be significantly different (Table 5). All of the 29 (100%) patients with surgical resection and 7 of the 34 (20.6%) patients without resection had histologically proven recurrences (p<0.001). Of these, 1 of 29 (3.4%) patients with surgical resection and 11 of the 34 (34.3%) patients without resection died at the end of the study (p=0.004).
Table 5.
Outcomes of Recurrent Patients With and Without Surgical Resection
| Surgical resection (n=29) | No surgical resection (n=34) | p | |
|---|---|---|---|
| Mean CEA level, ng/mL (range) | 23.2 (1–351.9) | 23.4 (1–220.2) | 0.99 |
| Mean SUVmax (range) | 6.7 (1.3–17.1) | 8.2 (1.1–41.3) | 0.30 |
| Mean follow-up time, months (range) | 20.9 (2–52) | 19.3 (4–45) | 0.69 |
| Time to recurrence, months (range) | 27.4 (1–112) | 30.7 (3–226) | 0.62 |
| Time from recurrence to therapy, months (range) | 1 (0–6) | 1.7 (0–5) | 0.53 |
| Histology, n (% over recurrences) | 29 (100%) | 7 (20.6%) | <0.001 |
| Death, n (% over recurrences) | 1 (3.4%) | 11 (34.3%) | 0.004 |
| Time from recurrence to death, months (range) | 4 | 22 (6–45) |
Discussion
The serum CEA level is the most frequently used tumor marker when monitoring therapeutic responses in CRC patients and in surveillance for recurrent disease. The serial measurement of serum CEA levels has been recommended for the identification of patients who might be cured by resection.28,29 In the present study, the sensitivity of serum CEA levels in the detection of recurrences was found to be only 48.4%, which was comparable to that reported in other studies30–33; thus, more than half of the recurrent patients were missed when elevated serum CEA levels were used as the only criteria. Although the incidences of recurrence and mortality in patients with elevated serum CEA levels were higher than those without, increased incidences of surgical resections were noted in patients without elevated serum CEA levels. Therefore, simply monitoring serum CEA levels might not be sufficient for the early detection of recurrent and resectable disease.
FDG-PET/CT is highly useful for the detection of recurrent CRC. In the present study, FDG-PET/CT detected 62 of the 63 recurrent CRC cases. The sensitivity, specificity, and accuracy of FDG-PET/CT for recurrent CRC were 98.4%, 89.2%, and 93.8%, respectively; and 100%, 88.9%, and 95.9%, and 96.9%, 89.4%, and 92.4% in Group 1 and Group 2, respectively. No significant differences were observed in serum CEA levels with respect to the detection of recurrent CRC by FDG-PET/CT. Increased serum CEA levels were used as an inclusion criterion in some studies.34,35 Kyoto et al. conducted a retrospective review of 75 FDG-PET/CT scans in 57 patients for the detection of recurrent CRC, and found that sensitivity values were 88% and 95%, and specificity values were 78% and 70% for patients with serum CEA concentrations of 5–10 and >10 ng/mL, respectively. The study concluded that FDG-PET/CT could accurately detect recurrent CRC, irrespective of elevated serum CEA levels.34 In a retrospective study of patients with clinically suspected recurrent disease and normal serum CEA levels, the sensitivity, specificity, and accuracy for FDG-PET/CT were 96.3%, 86.1%, and 90.5%, respectively,36 which was comparable to those observed in the present study. Therefore, elevated serum CEA might indicate only the recurrences with higher probabilities of detection by FDG-PET/CT, as was observed in the present study (63.3% vs. 40.5%, p=0.012). Our study confirmed that FDG-PET/CT is valuable in the detection of suspected recurrent CRC, irrespective of the serum CEA levels.
Recently published data from the National Oncologic PET Registry from patients with a variety of cancers showed that the intended disease management was changed in 36.5% of patient after PET imaging analysis.37 In a multicenter prospective study of 191 patients with recurrent CRC, a change in the planned management was documented for 65.6% of the symptomatic patients with residual structural lesions and in 43.9% of the patients with pulmonary or hepatic metastases that were considered to be potentially resectable.38 In the present study, FDG-PET/CT changed the planned management for 48.4% (62/128) of the total patients, which included 63.3% (31/49) of the patients with and 39.2% (31/79) of the patients without increased serum CEA levels (p=0.008). Surgery is potentially curative for patients with limited sites of metastatic disease, particularly those involving the liver and lung.23–27 Of the patients whose recurrent diseases were detected early and who underwent surgical resections of the recurrent lesions, only 1 patient died, in comparison to those who did not undergo resections (3.4% vs. 34.3%, p=0.004). The earlier recognition of resectable lesions hopefully will provide more effective treatment options and improve patient survival rates. FDG-PET/CT is helpful in the selection of patients who might derive significant survival benefits from optimal surgical strategies.
FDG-PET/CT is advantageous in that it can merge the metabolic information from FDG-PET with the anatomical information from CT. However, there are still inherent false positive and false negative results, though patients might benefit from the false positive findings. In the present study, false positives resulted in the surgical resections of two primary cancers that were independent of CRC and the colonoscopic excision of a tubulovillous adenoma. Further, a patient with pulmonary tuberculosis was appropriately treated. The earlier recognition of synchronous cancers might allow for the provision of more effective treatment options and could improve patient survival rates. A false negative was determined by the definition of recurrent lesions as those that were found within 6 months of FDG-PET/CT. In this case, FDG-PET/CT was limited by the resolution and the inability to detect microscopic metastases. A size threshold of 0.5 cm was identified for the largest lesion that was missed by FDG-PET/CT.39
The present study had some limitations. First, pathology was available for only 36 of the 63 recurrent patients (57.1%). Recurrent tumors were confirmed during follow-ups in 20 patients on the basis of therapeutic response and in another 7 according to disease progression. Second, 128 consecutive FDG-PET/CT studies in 96 patients were retrospectively recruited which meant that some patients were studied more than once. Further, FDG-PET/CT was performed in the Group 2 patients for various reasons. Some of the patients also underwent contrast-enhanced CT at the time of the image analyses. Because the physicians collected the available information, including prior PET/CT and contrast-enhanced CT studies for the most complete analyses, bias might have been introduced. Third, the total case numbers were small. Although more of the recurrent patients without elevated serum CEA levels received surgical resections (53.1% vs. 38.7%) and fewer of these patients died (12.5% vs. 25.8%), these differences were not found to be statistically significant. Further studies that include a greater numbers of cases and prospective trials that focus on the specific issue of resectable recurrences would be of interest.
Conclusions
In the present study, FDG-PET/CT was revealed to be highly sensitive and specific in the detection of recurrent CRC, both in patients with and without elevated serum CEA levels. FDG-PET/CT could detect early resectable recurrences and thus prompted changes to the planned disease management. Surgical resection of limited recurrent disease that was detected by FDG-PET/CT improved the survival rates of CRC patients. Patients with suspected recurrences but no elevations in serum CEA levels might benefit from surgical resection as indicated by FDG-PET/CT. Therefore, we recommend that FDG-PET/CT should be performed not only in patients with elevated serum CEA levels but also in those with suspected recurrences for the earlier detection of resectable disease.
Acknowledgments
We thank Miss Yi-Ru Chen and Ching-Jie Peng for their assistance in the procedures of patient arrangement and data management. This work was supported by the grant VGHKS 98-071 from the Kaohsiung Veterans General Hospital, Taiwan, Republic of China.
Disclosure Statement
There are no existing financial conflicts.
References
- 1.Siegel R. Ward E. Brawley O, et al. Cancer statistics. CA Cancer J Clin. 2011;61:212. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Detry R. Follow-up after curative surgery for colorectal cancer. Acta Gastroenterol Belg. 2001;64:268. [PubMed] [Google Scholar]
- 3.Korner H. Soreide K. Stokkeland PJ, et al. Systematic follow-up after curative surgery for colorectal cancer in Norway: A population-based audit of effectiveness, costs, and compliance. J Gastrointest Surg. 2005;9:320. doi: 10.1016/j.gassur.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Chau I. Allen MJ. Cunningham D, et al. The value of routine serum carcino-embryonic antigen measurement and computed tomography in the surveillance of patients after adjuvant chemotherapy for colorectal cancer. J Clin Oncol. 2004;22:1420. doi: 10.1200/JCO.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Kievit J. Follow-up of patients with colorectal cancer: Numbers needed to test and treat. Eur J Cancer. 2002;38:986. doi: 10.1016/s0959-8049(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 6.Minton JP. Hoehn JL. Gerber DM, et al. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer. 1985;55:1284. doi: 10.1002/1097-0142(19850315)55:6<1284::aid-cncr2820550622>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.McCall JL. Black RB. Rich CA, et al. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum. 1994;37:875. doi: 10.1007/BF02052591. [DOI] [PubMed] [Google Scholar]
- 8.Hine KR. Dykes PW. Serum CEA testing in the post-operative surveillance of colorectal carcinoma. Br J Cancer. 1984;49:689. doi: 10.1038/bjc.1984.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin EW., Jr Cooperman M. Carey LC, et al. Sixty second-look procedures indicated primarily by rise in serial carcinoembryonic antigen. J Surg Res. 1980;28:389. doi: 10.1016/0022-4804(80)90100-6. [DOI] [PubMed] [Google Scholar]
- 10.Benson AB., 3rd Desch CE. Flynn PJ, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18:3586. doi: 10.1200/JCO.2000.18.20.3586. [DOI] [PubMed] [Google Scholar]
- 11.Huebner RH. Park KC. Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG PET detection of colorectal cancer. J Nucl Med. 2000;41:1177. [PubMed] [Google Scholar]
- 12.Gambhir SS. Czernin J. Schimmer J, et al. A tabulated review of the literature. J Nucl Med. 2001;42(Suppl):9S. [PubMed] [Google Scholar]
- 13.Sobhani J. Tiret E. Aparicio T, et al. Early detection of recurrence by F-18 FDG-PET in the follow-up of patients with colorectal cancer. Bri J Cancer. 2008;98:875. doi: 10.1038/sj.bjc.6604263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamim SA. Kumar R. Halanaik D, et al. Role of FDG-PET/CT in detection of recurrent disease in colorectal cancer. Nucl Med Commun. 2010;31:590. doi: 10.1097/MNM.0b013e328338a120. [DOI] [PubMed] [Google Scholar]
- 15.Chen LB. Tong JL. Song HZ, et al. F-18 FDG PET/CT in detection of recurrence and metastasis of colorectal cancer. World J Gastroenterol. 2007;13:5025. doi: 10.3748/wjg.v13.i37.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukunaga H. Sekimoto M. Ikeda M, et al. Fusion image of positron emission tomography and computed tomography for the diagnosis of local recurrence of rectal cancer. Ann Surg Oncol. 2005;12:561. doi: 10.1245/ASO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Soyka J. Veit-Haibach P. Strobel K, et al. Staging pathways in recurrent CRC: Is contrast-enhanced F-18 FDG PET/CT the diagnostic tool of choice? J Nucl Med. 2008;49:354. doi: 10.2967/jnumed.107.048249. [DOI] [PubMed] [Google Scholar]
- 18.Votrubova J. Belohlavek O. Jaruskova M, et al. The role of FDG-PET/CT in the detection of recurrent CRC. Eur J Nucl Med Mol Imaging. 2006;33:779. [Google Scholar]
- 19.Even-Sapir E. Parag Y. Lerman H, et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology. 2004;232:815. doi: 10.1148/radiol.2323031065. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH. Czernin J. Allen-Auerbach M, et al. Comparison between F-18 FDG PET, in-line PET/CT, and software fusion for restaging of recurrent CRC. J Nucl Med. 2005;46:587. [PubMed] [Google Scholar]
- 21.Nakamoto Y. Sakamoto S. Okada T. Clinical value of manual fusion of PET and CT images in patients with suspected recurrent CRC. Am J Roentgenol. 2007;188:257. doi: 10.2214/AJR.05.0708. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt GP. Baur-Melnyk A. Haug A, et al. Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. Eur Radiol. 2009;19:1366. doi: 10.1007/s00330-008-1289-y. [DOI] [PubMed] [Google Scholar]
- 23.McAfee MK. Allen MS. Trastek VF, et al. Colorectal lung metastases: Results of surgical excision. Ann Thorac Surg. 1992;53:780. doi: 10.1016/0003-4975(92)91435-c. [DOI] [PubMed] [Google Scholar]
- 24.Saito Y. Omiya H. Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg. 2002;124:1007. doi: 10.1067/mtc.2002.125165. [DOI] [PubMed] [Google Scholar]
- 25.Ike H. Shimada H. Ohki S, et al. Results of aggressive resection of lung metastases from colorectal carcinoma detected by intensive follow-up. Dis Colon Rectum. 2002;45:468. doi: 10.1007/s10350-004-6222-0. [DOI] [PubMed] [Google Scholar]
- 26.Rizk NP. Downey RJ. Resection of pulmonary metastases from colorectal cancer. Semin Thorac Cardiovasc Surg. 2002;14:29. doi: 10.1053/stcs.2002.31742. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg RM. Fleming TR. Tangen CM, et al. Surgery for recurrent colon cancer: Strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med. 1998;129:27. doi: 10.7326/0003-4819-129-1-199807010-00007. [DOI] [PubMed] [Google Scholar]
- 28.Desch CE. Benson AB., 3rd Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 29.Locker GY. Hamilton S. Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 30.Hara M. Kanemitsu Y. Hirai T, et al. Negative serum carcinoembryonic antigen has insufficient accuracy for excluding recurrence from patients with Dukes C colorectal cancer: Analysis with likelihood ratio and posttest probability in a follow-up study. Dis Colon Rectum. 2008;51:1675. doi: 10.1007/s10350-008-9406-1. [DOI] [PubMed] [Google Scholar]
- 31.Zeng Z. Cohen AM. Urmacher C. Usefulness of carcinoembryonic antigen monitoring despite normal preoperative values in node-positive colon cancer patients. Dis Colon Rectum. 1993;36:1063. doi: 10.1007/BF02047301. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharjya S. Aggarwal R. Davidson BR. Intensive follow-up after liver resection for colorectal liver metastases: Results of combined serial tumour marker estimations and computed tomography of the chest and abdomen—A prospective study. Br J Cancer. 2006;95:21. doi: 10.1038/sj.bjc.6603219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park IJ. Choi GS. Lim KH, et al. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: Clinical significance of the preoperative level. Ann Surg Oncol. 2009;16:3087. doi: 10.1245/s10434-009-0625-z. [DOI] [PubMed] [Google Scholar]
- 34.Kyoto Y. Momose M. Kondo C, et al. Ability of F-18 FDG PET/CT to diagnose recurrent colorectal cancer in patients with elevated CEA concentrations. Ann Nucl Med. 2010;24:395. doi: 10.1007/s12149-010-0372-z. [DOI] [PubMed] [Google Scholar]
- 35.Tutt ANJ. Plunkett TA. Barrington SF, et al. The role of positron emission tomography in the management of colorectal cancer [review] Colorectal Dis. 2004;6:2. doi: 10.1111/j.1463-1318.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH. Park SG. Jee KN, et al. Performance of FDG PET/CT in postoperative colorectal cancer patients with a suspected recurrence and a normal CEA level. Nucl Med Commun. 2010;31:576. doi: 10.1097/MNM.0b013e32833845b7. [DOI] [PubMed] [Google Scholar]
- 37.Hillner BE. Siegel BA. Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: Initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155. doi: 10.1200/JCO.2007.14.5631. [DOI] [PubMed] [Google Scholar]
- 38.Scott AM. Gunawardana DH. Kelley B, et al. PET changes management and improves prognostic stratification in patients with recurrent colorectal cancer: Results of a multicenter prospective study. J Nucl Med. 2008;49:1451. doi: 10.2967/jnumed.108.051615. [DOI] [PubMed] [Google Scholar]
- 39.Peng NJ. Liou WS. Liu RS, et al. Early detection of recurrent ovarian cancer in patients with low-level increases in serum CA-125 levels by FDG-PET/CT. Cancer Biother Radiol. 2011;26:175. doi: 10.1089/cbr.2010.0872. [DOI] [PubMed] [Google Scholar]