Abstract
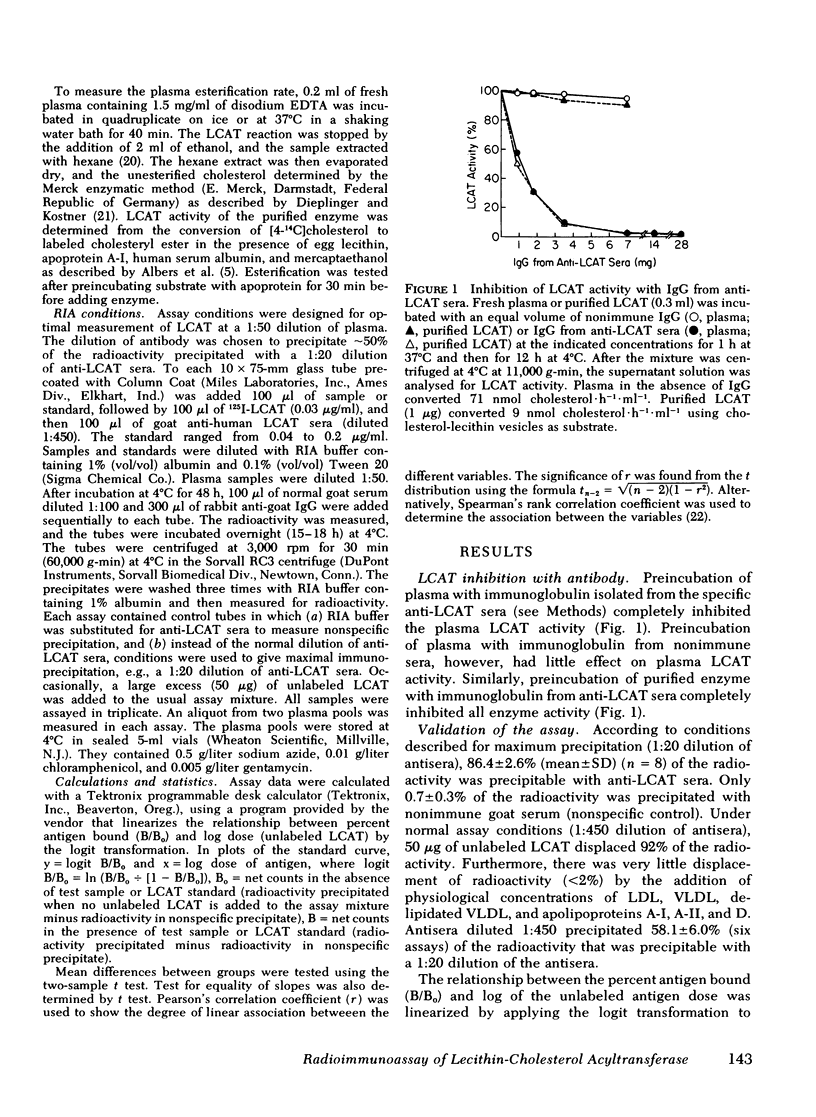
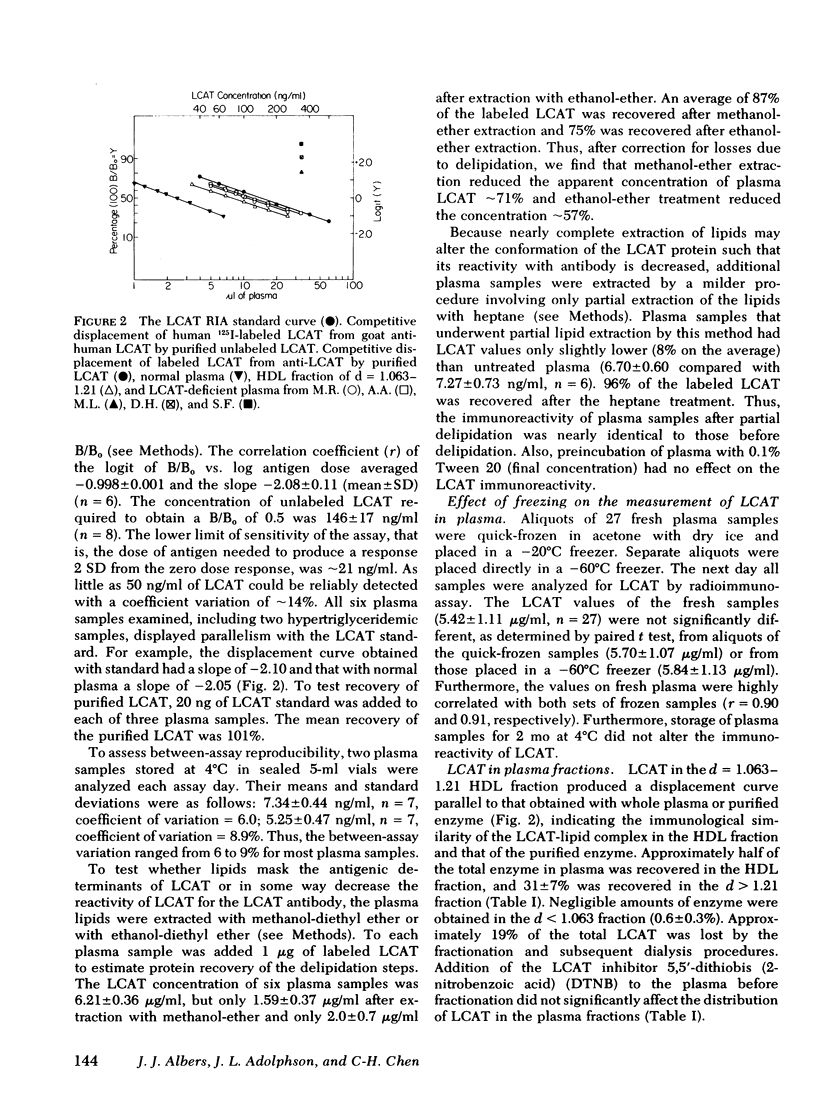
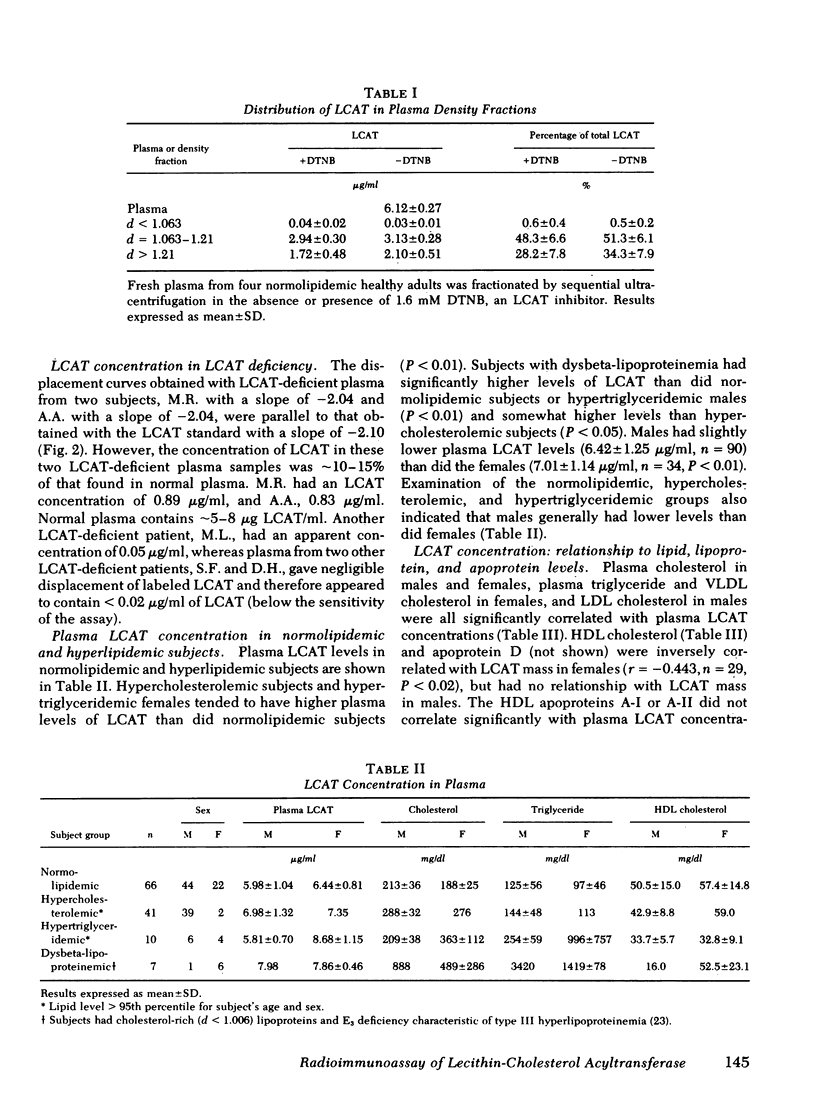
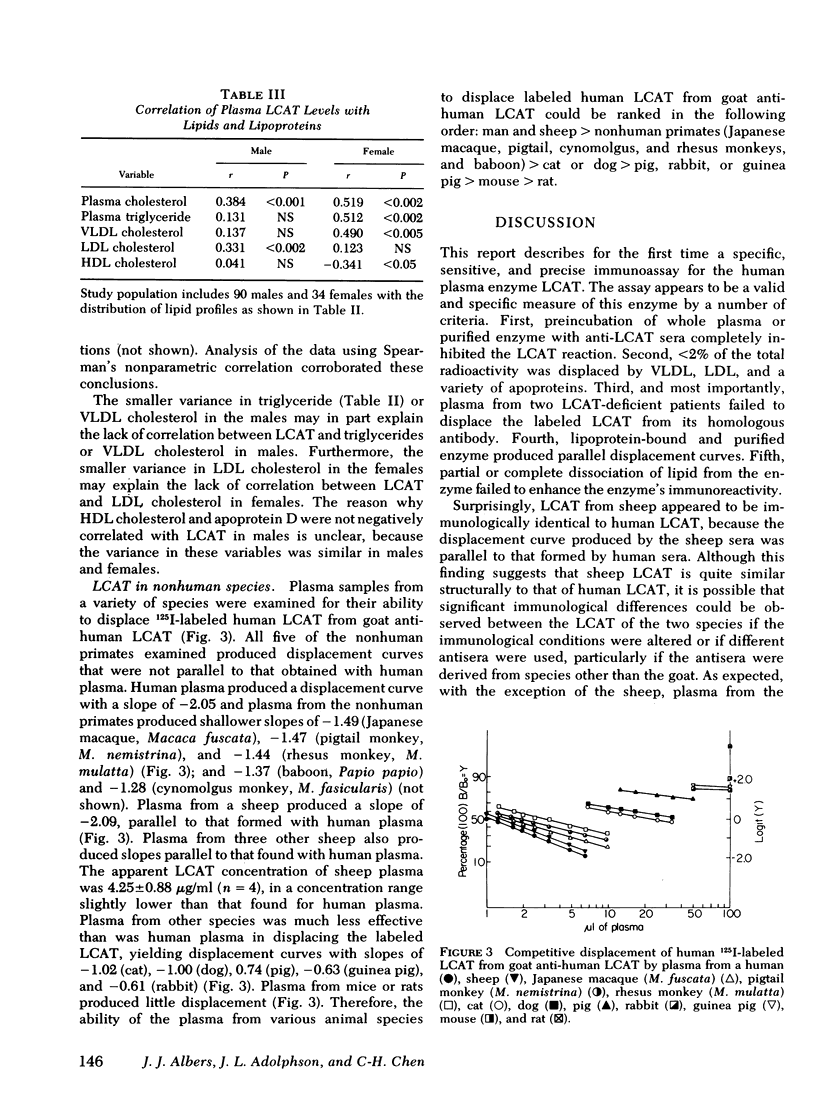
A sensitive and precise competitive-displacement double-antibody radioimmunoassay was developed for the human plasma enzyme lecithin-cholesterol acyltransferase (LCAT; Ec 2.3 1.43). The ability of plasma from various animal species to displace labeled human LCAT from goat anti-human LCAT could be ranked in the following order: man and sheep > nonhuman primates > cat or dog > pig > rabbit or guinea pig > mouse > rat. Normolipidemic subjects had levels of LCAT of 6.14 +/- 0.98 micrograms/ml (mean +/- SD, n = 66). Subjects with dysbeta-lipoproteinemia had the highest plasma LCAT levels (7.88 +/- 0.39 micrograms/ml, n = 7, P < 0.05), followed by hypercholesterolemic subjects (7.00 +/- 1.30, n = 41) and hypertriglyceridemic subjects (6.96 +/- 1.3, n = 10). LCAT-deficient subjects had the lowest enzyme levels (0.89, 0.83, and 0.05 micrograms/ml, respectively, and two subjects with no detectable enzyme). Males had lower LCAT levels (6.42 +/- 1.05 micrograms/ml, n = 90, for all subjects; 5.99 +/- 1.03, n = 44, for normolipidemics) than females (7.01 +/- 1.14, n = 34, for all subjects P < 0.01; 6.44 +/- 0.79, n = 22, for normolipidemics, P < 0.01). LCAT levels correlated significantly with total cholesterol (males, r = 0.384, P < 0.001; females, r = 0.519, P < 0.002); and total triglyceride (only in females, r = 0.512, P < 0.002). LCAT levels in females correlated inversely with HDL cholesterol (r = 0.341, P < 0.05) and apoprotein D (r = 0.443, P < 0.02), but no such relationship existed in males.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Cabana V. G., Dee Barden Stahl Y. Purification and characterization of human plasma lecithin:cholesterol acyltransferase. Biochemistry. 1976 Mar 9;15(5):1084–1087. doi: 10.1021/bi00650a020. [DOI] [PubMed] [Google Scholar]
- Albers J. J. Effect of human plasma apolipoproteins on the activity of purified lecithin:cholesterol acyltransferase. Scand J Clin Lab Invest Suppl. 1978;150:48–52. [PubMed] [Google Scholar]
- Albers J. J., Lin J., Roberts G. P. Effect of human plasma apolipoproteins on the activity of purified lecithin: cholesterol acyltransferase. Artery. 1979 Jan;5(1):61–75. [PubMed] [Google Scholar]
- Albers J. J., Scanu A. M. Isoelectric fractionation and characterization of polypeptides from human serum very low density lipoproteins. Biochim Biophys Acta. 1971 Apr 27;236(1):29–37. doi: 10.1016/0005-2795(71)90145-0. [DOI] [PubMed] [Google Scholar]
- Albers J. J., Wahl P. W., Cabana V. G., Hazzard W. R., Hoover J. J. Quantitation of apolipoprotein A-I of human plasma high density lipoprotein. Metabolism. 1976 Jun;25(6):633–644. doi: 10.1016/0026-0495(76)90060-3. [DOI] [PubMed] [Google Scholar]
- Cheung M. C., Albers J. J. The measurement of apolipoprotein A-I and A-II levels in men and women by immunoassay. J Clin Invest. 1977 Jul;60(1):43–50. doi: 10.1172/JCI108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Frohlich J., Godolphin W. J., Reeve C. E., Evelyn K. A. Familial LCAT deficiency. Report of two patients from a Canadian family of Italian and Swedish descent. Scand J Clin Lab Invest Suppl. 1978;150:156–161. [PubMed] [Google Scholar]
- GILMAN A. M., NISONOFF A., DRAY S. SYMMETRICAL DISTRIBUTION OF GENETIC MARKERS IN INDIVIDUAL RABBIT GAMMA-GLOBULIN MOLECULES. Immunochemistry. 1964 Jun;1:109–120. doi: 10.1016/0019-2791(64)90075-8. [DOI] [PubMed] [Google Scholar]
- GLOMSET J. A., WRIGHT J. L. SOME PROPERTIES OF A CHOLESTEROL ESTERIFYING ENZYME IN HUMAN PLASMA. Biochim Biophys Acta. 1964 Aug 26;89:266–276. doi: 10.1016/0926-6569(64)90215-9. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Hamnström B., Gjone E., Norum K. R. Familial plasma lecithin: cholesterol acyltransferase deficiency. Br Med J. 1969 May 3;2(5652):283–286. doi: 10.1136/bmj.2.5652.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacko A. G., Rutenberg H. L., Soloff L. A. The influence of age and sex on the esterification of human serum cholesterol. Biochem Med. 1977 Jun;17(3):275–283. doi: 10.1016/0006-2944(77)90033-3. [DOI] [PubMed] [Google Scholar]
- Marcel Y. L., Vezina C. A method for the determination of the initial rate of reaction of lecithin: cholesterol acyltransferase in human plasma. Biochim Biophys Acta. 1973 Jun 21;306(3):497–504. doi: 10.1016/0005-2760(73)90188-4. [DOI] [PubMed] [Google Scholar]
- McConathy W. J., Alaupovic P. Isolation and partial characterization of apolipoprotein D: a new protein moiety of the human plasma lipoprotein system. FEBS Lett. 1973 Dec 1;37(2):178–182. doi: 10.1016/0014-5793(73)80453-3. [DOI] [PubMed] [Google Scholar]
- Nordby G., Norum K. R. Aspects of the role of lecithin:cholesterol acyltransferase in metabolism of triglycerides. Scand J Clin Lab Invest Suppl. 1978;150:111–114. [PubMed] [Google Scholar]
- Patsch W., Lisch H. J., Sailer S., Braunsteiner H. Initial cholesterol esterification rate in hyperlipoproteinaemia: effects of triglyceride-rich lipoproteins. Eur J Clin Invest. 1978 Aug;8(4):209–213. doi: 10.1111/j.1365-2362.1978.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Schenkein I., Levy M., Uhr J. W. The use of glucose oxidase as a generator of H 2 O 2 in the enzymatic radioiodination of components of cell surfaces. Cell Immunol. 1972 Nov;5(3):490–493. doi: 10.1016/0008-8749(72)90076-7. [DOI] [PubMed] [Google Scholar]
- Subbaiah P. V., Albers J. J., Chen C. H., Bagdade J. D. Low density lipoprotein-activated lysolecithin acylation by human plasma lecithin-cholesterol acyltransferase. Identity of lysolecithin acyltransferase and lecithin-cholesterol acyltransferase. J Biol Chem. 1980 Oct 10;255(19):9275–9280. [PubMed] [Google Scholar]
- Wallentin L., Angelin B., Einarsson K., Leijd B. Lecithin:cholesterol acyl transfer rate in plasma and its relations to lipoprotein concentrations and to kinetics of bile acids and triglycerides in hyperlipoproteinemic subjects. Scand J Clin Lab Invest Suppl. 1978;150:103–110. [PubMed] [Google Scholar]
- Warnick G. R., Mayfield C., Albers J. J., Hazzard W. R. Gel isoelectric focusing method for specific diagnosis of familial hyperlipoproteinemia type 3. Clin Chem. 1979 Feb;25(2):279–284. [PubMed] [Google Scholar]


