Abstract
Background and purpose
Operative findings during revision of metal-on-metal hip arthroplasty (MOMHA) vary widely and can involve massive soft tissue and bone disruption. As a result, planning of theater time and resources is difficult, surgery is challenging, and outcomes are often poor. We describe our experience with revision of MOMHA and provide recommendations for management.
Patients and methods
We present the findings and outcomes of 39 consecutive MOMHAs (in 35 patients) revised in a tertiary unit (median follow-up time 30 (12–54) months). The patients underwent a preoperative work-up including CT, metal artifact reduction sequence (MARS) MRI, and blood metal ion levels.
Results
We determined 5 categories of failure. 8 of 39 hips had conventional failure mechanisms including infection and impingement. Of the other 31 hips, 14 showed synovitis without significant disruption of soft tissue; 6 had a cystic pseudotumor with significant soft tissue disruption; 7 had significant osteolysis; and 4 had a solid pseudotumor. Each category of failure had specific surgical hazards that could be addressed preoperatively. There were 2 reoperations and 1 patient (2 hips) died of an unrelated cause. Median Oxford hip score (OHS) was 37 (9–48); median change (ΔOHS) was 17 (–10 to 41) points. ΔOHS was similar in all groups—except those patients with solid pseudotumors and those revised to metal-on-metal bearings, who fared worse.
Interpretation
Planning in revision MOMHA is aided by knowledge of the different categories of failure to enable choice of appropriate personnel, theater time, and equipment. With this knowledge, satisfactory outcomes can be achieved in revision of metal-on-metal hip arthroplasty.
In patients who present with a painful MOMHA, there is great heterogeneity of clinical presentation, imaging, and operative findings (Browne et al. 2010). This ranges from unexplained hip pain to catastrophic failure. There is a spectrum of findings on MRI, which range from being normal or demonstrating small, cystic pseudotumors—which have a high incidence in well-functioning MOMHA (Hart et al. 2012)—to demonstrating large, solid masses with widespread destruction of muscle and bone. Intraoperative findings show similar heterogeneity.
One commonly reported mechanism of failure of metal-on-metal (MOM) hips is the result of a high rate of material loss (through wear or corrosion, or both), frequently related to suboptimal implant positioning and subsequent edge loading. This has led to the hypothesis that the soft tissue reaction is a dose-dependent reaction to excessive nanoparticle wear debris (Kwon et al. 2009), and can be avoided by accurate implantation to minimize wear. However, other patients show low bearing surface wear rates with low loss of material from all the surfaces of the implants, suggesting that there is a patient-related susceptibility factor. Conventional, stemmed designs have an additional mechanism of failure when compared to hip resurfacings, as a result of loss of material at the trunion-taper interface (Langton et al. 2011).
The variable degree of destruction of muscle and bone makes planning and performance of revision of MOMHA challenging. Series of such revisions have shown a high incidence of dislocation, recurrence, and reoperation (Grammatopoulos et al. 2009), although this appears to improve with experience (De Smet et al. 2011).
The main aim of this study was to report our experience with revision of MOMHA, in a multidisciplinary setting receiving tertiary referrals and employing sophisticated imaging techniques. A secondary aim was to provide recommendations on the basis of this experience for improvement of pre-revision planning and outcomes in patients undergoing revision of a MOM hip.
Patients and methods
We present preoperative and outcome data on all patients who were revised for a painful MOMHA at our institution between 2007 and 2010. 39 hips in 35 patients (32 females) were revised during the study period. The median age was 61 (25–74) years. For all patients, data in the following categories were collected prospectively: preoperative and postoperative functional scoring, radiological findings, blood analysis (trace metal analysis, renal function tests, inflammatory markers), histology, and microbiology.
During the period covered by this study, our approach to the painful MOM hip has evolved as a result of published research. However, from the outset we adopted a multidisciplinary approach with the assistance of musculoskeletal radiologists, a clinical chemist, a microbiologist with an interest in orthopaedic implants, and a musculoskeletal histopathologist.
We obtained ethical approval for the prospective collection and analysis of our data, which was analyzed at a median follow-up time of 30 (12–54) months. Patients were recruited either locally or as tertiary referrals from surgeons regionally and nationally, and therefore had a range of implant types and surgical approaches. All patients were referred with a diagnosis of unexplained pain attributable to a MOM articulation. Preoperative work-up included metal artifact reduction sequence (MARS) MRI and 3D CT scanning. MARS MRI was performed using a 1.5-Tesla scanner (Magnetom 1.5T; Siemens Medical, Erlangen, Germany). Preoperative CT images were reconstructed in 3 dimensions and anatomical inclination and version was defined with reference to the anterior pelvic plane (Dandachli et al. 2009). This was converted to radiographic values using accepted formulae (Murray 1993). Blood metal ions and functional hip scores were determined preoperatively.
Detailed operative findings were recorded. Patients were followed-up at 6, 12, 26, and 52 weeks with clinical assessment, blood metal ions, plain radiographs, and functional hip scores. Postoperative cross-sectional imaging was reserved for patients who became symptomatic after revision without any clear explanation.
For the purposes of this study, MRI and CT images were reviewed by a consultant radiologist who was blinded regarding the clinical and operative findings, and using a previously published protocol (Sabah et al.. 2011). Specific findings recorded included the presence and appearance of a soft tissue mass, muscle loss, bone death, and loosening/osteolysis. Muscle damage (gluteus maximus, medius, and minimus, piriformis, obturator internus, and obturator externus) was rated from 0 to 3, with 0 being no loss of muscle, 1 being loss of < 30% of the muscle surface area, 2 being 30–70% loss, and 3 being > 70% loss. Patient-reported outcome was recorded using a validated hip score (Murray et al. 2007). On the basis of preoperative and intraoperative findings, and histological examination of tissues collected at operation, failures were classified as being either bearing-related or due to conventional causes such as infection or impingement. After removal, implants underwent wear analysis as described by Matthies et al. (2011).
Statistics
Descriptive statistics had a non-normal distribution of data and non-parametric tests were used to summarize the data and compare between the categories of failure and implant types. Spearman’s rank correlation was used to determine the presence and strength of any correlation between pre-revision investigation results and clinical outcome. A contingency table was used to determine the sensitivity and specificity of the MRI findings in predicting the operative findings, and the chi-squared test was used to determine the statistical significance of any associations. Wilcoxon’s signed rank test was used to compare clinical outcomes before and after revision. The group was divided into subgroups, which were analyzed using the Mann-Whitney U test and included hips showing high and low degrees of wear, hips with normal and abnormal results of preoperative investigation, and hips with different types of revision procedure. For these tests, effect size was calculated using Hodges-Lehmann estimators, which calculate the differences between each possible pair in each group and give the median of the resultant list of differences. The 95% confidence intervals (CIs) for this statistic are shown alongside the Hodges-Lehmann medians of difference.
This was a consecutive series of hips, so the cohort had some bilateral cases. These bilateral cases have been included in the analyses presented below, to provide as accurate a record as possible of the revision practice of our institution over the time period studied. However, in the analysis of these findings, the statistical tests employed assume independence of all the cases, which may have been compromised by the inclusion of these bilateral cases (Bryant et al. 2006, Park et al. 2010). In order to test the validity of the tests used, the analysis was repeated using a smaller dataset in which the bilateral cases were removed. In the interests of clarity and brevity, all statistics that are shown in the Results section relate to the full dataset, except in instances where the findings of this second analysis diverged from the findings of the complete dataset, where both statistical results are given. Statistical analysis was performed using SPSS version 19.
Results
Most of the patients were tertiary referrals and this is reflected in the heterogeneity of implant designs revised (Table 1). Most cases were resurfacing prostheses (32/39), and most of these (21/32) were Birmingham Hip Resurfacings (Smith and Nephew Inc., Memphis, TN). Median survivorship after implantation of the primary prosthesis was 47 (11–131) months. Median preoperative OHS was 15 (2–31). Median follow-up time was 30 (12–54) months.
Table 1.
Preoperative and demographic details
| No. | Age | Sex | Prosthesis | Modular | Head | Cup | Incl. | Ver. | Survival (m) | Co | Cr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | F | BHR | Resurfacing | 50 | 56 | 71 | 42 | 31 | 47 | 33 |
| 2 | 55 | M | BHR | Resurfacing | 46 | 54 | 29 | -34 | 13 | 0.9 | 1.5 |
| 3 | 63 | F | ASR | Resurfacing | 47 | 54 | 55 | 48 | 29 | 30 | 32 |
| 4 | 65 | F | ASR | Resurfacing | 40 | 48 | 66 | 28 | 53 | 24 | 21 |
| 5 | 72 | F | BHR | Resurfacing | 50 | 56 | 44 | 29 | 59 | 3.1 | 2.3 |
| 6 | 65 | F | BHR | Resurfacing | 42 | 48 | 38 | 9 | 61 | 1.1 | 1.3 |
| 7 | 69 | F | BHR | Resurfacing | 42 | 48 | 56 | 23 | 82 | 1.7 | 2.7 |
| 8 | 63 | F | BHR | Resurfacing | 42 | 50 | 40 | 7 | 95 | 7.7 | 9.4 |
| 9 | 46 | F | Cormet | Resurfacing | 44 | 50 | 75 | 34 | 18 | 3.3 | 0.3 |
| 10 | 65 | F | BHR | Resurfacing | 42 | 50 | 57 | 26 | 56 | 21 | 11 |
| 11 | 66 | M | Cormet | Resurfacing | 48 | 54 | 60 | 29 | 68 | 12 | 6.0 |
| 12 | 74 | F | BHR | Resurfacing | 44 | 50 | 34 | 32 | 54 | 1.5 | 1.0 |
| 13 | 48 | F | BHR | Resurfacing | 46 | 54 | 41 | 27 | 45 | 1.8 | 6.7 |
| 14 | 48 | F | BHR | Resurfacing | 48 | 54 | 47 | 26 | 52 | 1.8 | 6.7 |
| 15 | 73 | F | Durom | THA | 50 | 56 | 55 | 37 | 38 | 6.5 | 8.4 |
| 16 | 35 | F | Cormet | Resurfacing | 44 | 50 | 43 | 26 | 11 | 1.6 | 8.6 |
| 17 | 57 | F | ASR | Resurfacing | 53 | 60 | 70 | 43 | 45 | 165 | 117 |
| 18 | 60 | M | BHR | Resurfacing | 50 | 56 | 51 | 12 | 43 | 1.0 | 2.7 |
| 19 | 67 | M | Mitch | THA | 44 | 50 | 60 | -6 | 13 | 1.2 | 0.5 |
| 20 | 64 | M | BHR | Resurfacing | 50 | 56 | 57 | 43 | 92 | 71 | 36 |
| 21 | 67 | F | Biomet | Resurfacing | 44 | 50 | 49 | 9 | 28 | 9.5 | 1.0 |
| 22 | 67 | F | Biomet | Resurfacing | 44 | 50 | 38 | -5 | 22 | 9.5 | 1.0 |
| 23 | 61 | F | BHR | Resurfacing | 42 | 50 | 64 | 13 | 43 | 2.1 | 3.1 |
| 24 | 47 | F | BHR | Resurfacing | 42 | 50 | 43 | 39 | 65 | 0.5 | 0.7 |
| 25 | 74 | F | BHR | THA | 50 | 56 | 37 | 19 | 49 | 8.8 | 3.0 |
| 26 | 69 | M | BHR | Resurfacing | 54 | 60 | 73 | 41 | 63 | 33 | 14 |
| 27 | 60 | F | BHR | Resurfacing | 46 | 52 | 36 | 26 | 68 | 1.5 | 3.5 |
| 28 | 25 | F | Cormet | Resurfacing | 40 | 48 | 47 | 45 | 39 | 70 | 32 |
| 29 | 61 | F | Pinnacle | THA | 36 | 52 | 68 | -3 | 56 | 102 | 37 |
| 30 | 55 | F | BHR | Resurfacing | 46 | 50 | 64 | 30 | 72 | 98 | 49 |
| 31 | 69 | F | ASR | Resurfacing | 43 | 48 | 55 | 27 | 62 | 11 | 6.6 |
| 32 | 45 | F | ASR | THA | 45 | 50 | 57 | 35 | 38 | 10 | 3.5 |
| 33 | 46 | F | ASR | THA | 45 | 50 | 47 | 10 | 3.5 | ||
| 34 | 66 | F | BHR | Resurfacing | 42 | 50 | 66 | 34 | 68 | 105 | 52 |
| 35 | 61 | M | BHR | Resurfacing | 46 | 52 | 43 | 22 | 131 | 3.8 | 2.9 |
| 36 | 31 | F | Cormet | Resurfacing | 40 | 46 | 42 | 24 | 30 | 20 | 6.8 |
| 37 | 58 | F | BHR | Resurfacing | 46 | 52 | 42 | 20 | 25 | 3.1 | 4.8 |
| 38 | 44 | F | Mitch | Resurfacing | 52 | 58 | 38 | 38 | 21 | 23 | 13 |
| 39 | 72 | F | Taperloc Magnum | THA | 44 | 50 | 28 | 9.4 | 0.5 |
Incl. and Ver.: inclination and version measured by CT (radiographic values);
Co and Cr: whole-blood cobalt and chromium (parts per billion);
BHR: Birmingham Hip Resurfacing (Smith and Nephew);
ASR: Anatomical Surface Replacement (DePuy Johnson and Johnson).
Biochemistry (Table 1)
Median preoperative cobalt level was 9.4 (0.5–166) parts per billion (ppb) and median preoperative chromium level was 6.0 (0.26–117) ppb. Levels were below 7 ppb (the level considered acceptable for a unilateral MOMHA, as defined by the UK Medicines and Healthcare Regulatory Agency (MHRA, 2010)) in 17 of the 39 hips for cobalt, and in 24 of the 39 hips for chromium. There was no correlation between preoperative cobalt or chromium levels and preoperative functional scoring or time to revision (Spearman’s correlation, p = 0.12–1.0).
MRI findings (Table 2)
Table 2.
MRI and intraoperative appearances; revision surgery performed
| Hip | MRI mass and class | MRI muscle destruction |
Intraoperative appearance | Type | Revision prosthesis and bearing | |
|---|---|---|---|---|---|---|
| No. | Abductor | SER | ||||
| 1 | N/A | N/A | N/A | Synovitis, no mass | II | COC |
| 2 | Nil | Moderate | Severe | Clear impingement, no mass | I | COC |
| 3 | Nil | Moderate | Severe | Synovitis, no mass | II | COC |
| 4 | Nil | Moderate | Severe | Synovitis, no mass | II | COC |
| 5 | Nil | Moderate | Severe | Osteolysis, acetabular # 2º to minor trauma, no mass | III | COC |
| 6 | Nil | Mild | None | Synovitis, no mass | II | MOM |
| 7 | N/A | N/A | N/A | Mass, abductors preserved | III | COC |
| 8 | 2a | None | Mild | Synovitis, no mass | II | COC |
| 9 | 3 | Moderate | Severe | Solid mass, pelvic extension, abductors preserved | V | Revision stem COC |
| 10 | 2a | Moderate | Moderate | Loose femoral component with osteolysis | IV | COC |
| 11 | N/A | N/A | N/A | Synovitis and acetabular loosening. Infection confirmed on histology/microbiology | I | COC |
| 12 | N/A | N/A | N/A | Femoral neck fracture, no mass | I | MOM |
| 13 | 1 | Mild | Moderate | Synovitis, no mass | II | COC |
| 14 | 2a | Mild | Moderate | Synovitis, no mass | II | COC |
| 15 | Nil | None | Severe | Impingement, no mass | I | COC |
| 16 | Nil | None | None | Synovitis, no mass | II | COC |
| 17 | 2a | Severe | Moderate | Mass, gluteal dehiscence, bare trochanter | III | COC |
| 18 | Nil | Mild | Moderate | Synovitis, no mass | II | COC |
| 19 | Nil | Moderate | Moderate | Synovitis, no mass | I | COC |
| 20 | 2a | Mild | Mild | Massive pelvic osteolysis | IV | Pelvic recon, COC |
| 21 | 2a | Mild | Moderate | No mass, osteolysis with loose cup | IV | COC |
| 22 | 2a | Mild | Severe | No mass, osteolysis with loose cup | IV | COC |
| 23 | Nil | Moderate | Severe | Loose – loss of fixation with BHR dysplasia cup | I | COC |
| 24 | Nil | Moderate | Moderate | Synovitis, no mass | II | COC |
| 25 | 2a | Severe | Moderate | Complete destruction of SERs, bone death at GT | III | Revision stem, MOP |
| 26 | 2b | Moderate | Mild | Pelvic osteolysis, no mass | IV | MOP |
| 27 | Nil | Mild | Severe | Synovitis, no mass | II | MOP |
| 28 | Nil | Nil | Nil | Synovitis, no mass | II | MOP |
| 29 | 2a | Severe | Moderate | Severe synovitis. No mass | II | MOP |
| 30 | 2a | Moderate | Moderate | Very large fluid-filled mass, no muscle dehiscence but atrophic | III | MOP |
| 31 | Nil | None | Moderate | Synovitis, no mass | II | MOP |
| 32 | 3 | Moderate | Moderate | Solid mass with massive muscle destruction bilaterally | V | Captive cup, MOP |
| 33 | 3 | Moderate | Moderate | Same patient as above | V | Captive cup, MOP |
| 34 | 3 | Mild | Severe | Pelvic osteolysis, no mass | IV | TMT cup, COC |
| 35 | Nil | Mild | Moderate | Pelvic osteolysis, no mass | IV | MOP |
| 36 | 2a | None | Moderate | Infected. No mass | I | COC |
| 37 | 1 | Moderate | Severe | Complete destruction of SERs with mass | III | COC |
| 38 | 2b | Moderate | Severe | Infected | I | COC |
| 39 | 3 | Severe | Severe | Solid mass with widespread destruction. Symptomatic encasement of sciatic nerve | V | COC |
COC = ceramic-on-ceramic; MOM = metal-on-metal;
MOP = metal-on-polyethylene; SER = short external rotators
MRI was performed in 35 of the 39 hips. A mass was present in 21 of 35 hips. Levels of muscle edema or loss varied between patients.
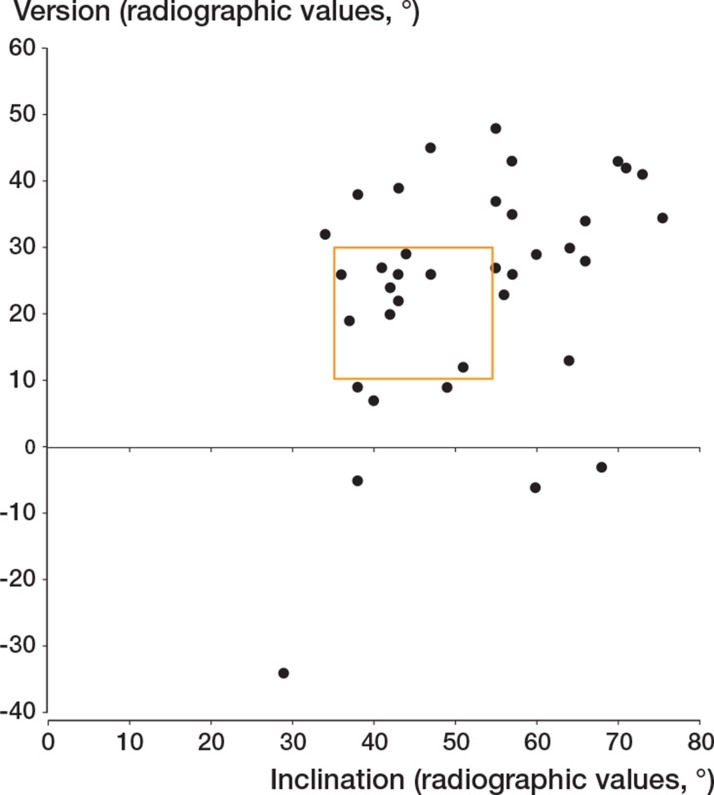
CT-measured cup position (Figure 1)
Figure 1.
Scatter plot showing inclination and version of the prostheses revised. The box represents optimal position as defined by Grammatopoulos et al. (2009).
37 of the 39 hips had preoperative CT for inclination and version. Median inclination was 51º (29–75) and mean version was 27º (–34 to 48). 10 of these 37 hips had both inclination and version within the range defined as optimal by Grammatopoulos et al. (2010) for hip resurfacing prostheses. Increasing cup inclination and version correlated positively with whole-blood cobalt (p < 0.005 and p = 0.002 for inclination and version, respectively) and chromium (p = 0.009 and p = 0.002). When analysis was restricted to unilateral cases, the correlation between metal ion levels and inclination persisted (Co: p = 0.003; Cr: p = 0.006), but there was no significant correlation with version for either (Co: p = 0.1; Cr: p = 0.07).
Intraoperative findings and mechanisms of failure (Table 3)
Table 3.
Types of failure
| Imaging | Surgical | Hazards | Surgical plan | |
|---|---|---|---|---|
| Type I: | ||||
| Conventional failure modes | Normal or small fluid-filled ‘pseudotumor’ on MRI | Variable | Misdiagnosis, Recurrent infection | |
| Type II: | ||||
| Synovitis with negative investigations | Normal or small fluid-filled ‘pseudotumor’ on MRI | Varying degrees of synovitis | Misdiagnosis | Need to exclude other causes e.g. infection, mechanical causes. Consider further imaging for psoas, frozen section during revision |
| Type III: | ||||
| Soft tissue disruption | MRI shows fluid or solid mass with variable soft tissue and muscle destruction (Figures 2 and 3) | Abductors may be atrophic or avulsed/absent | Instability after revision | Plan for possibility of muscle loss, including need for musle reconstruction (e.g. graft jacket) or captive cup. Like type V, may need pelvic surgeon for full excision of intrapelvic mass |
| Type IV: | ||||
| Bone destruction | Osteolysis on CT/plain films. MRI as type I (Figure 4) | Loose cup. Soft tissue reaction varies | Loss of bone stock and need for extensive reconstruction | May need extensive reconstruction. CT and pelvic surgeon may be helpful. Early surgery indicated to prevent fracture |
| Type V: | ||||
| Solid pseudotumor | MRI shows large mass. Mass may extend to pelvis (Figure 5) | Massive soft tissue reaction but musculature may be intact | Secondary infection if incompletely excised | Complete excision required. May need pelvic exploration |
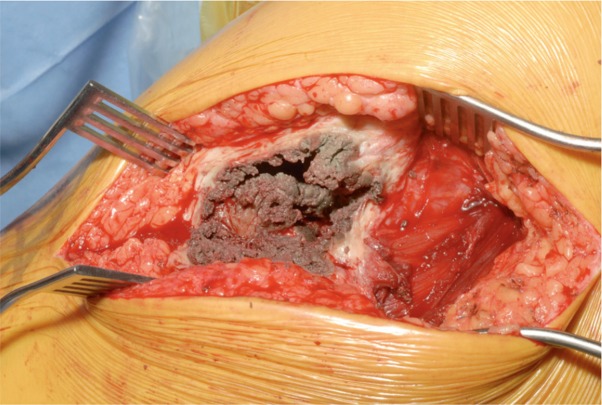
While all hips presented with unexplained pain, 8 of the 39 hips were found to have “conventional” mechanisms of failure including infection (3 patients), impingement due to malpositioning (3 patients), fracture (1 patient, who was referred with unexplained pain and sustained a fracture secondary to minor trauma after the first outpatient visit), and primary loss of acetabular fixation (1 patient). Of the remaining patients, 14 of 31 had no intraoperative features apart from synovitis. A significant soft tissue mass was determined in 10 of these 31 patients (1 further patient who was found to be infected also had a soft tissue mass); 4 of the 10 had a solid pseudotumor (Figure 5) and the remainder were fluid-filled. In 7 patients, there was significant acetabular osteolysis but no soft tissue mass (Figure 4).
Figure 5.
Case 32 with destructive solid pseudotumor.
Figure 4.
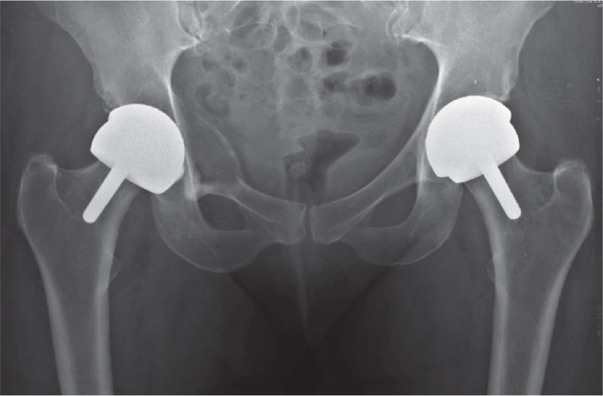
Preoperative radiograph of patient 34 demonstrating extensive osteolysis adjacent to the right acetabular component.
Figure 2.
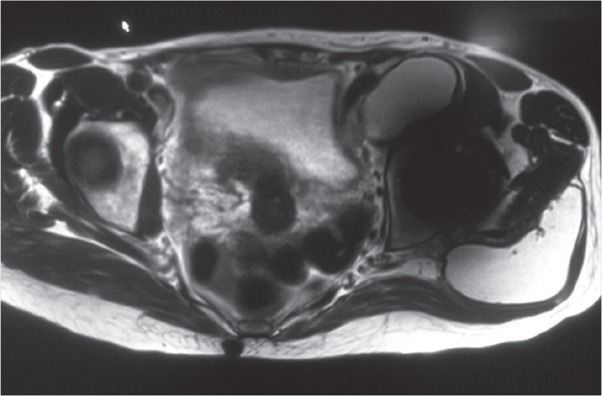
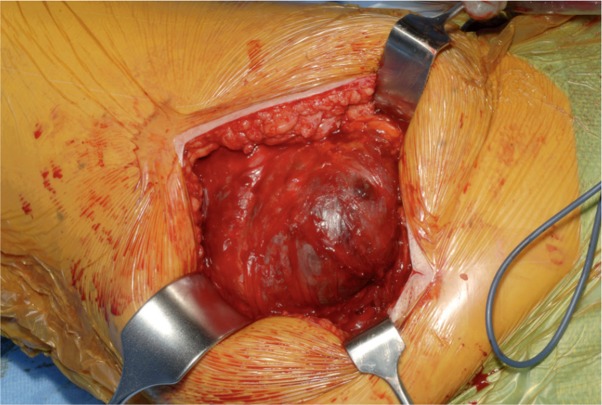
MRI of case 30, showing large fluid-filled pseudotumor.
Figure 3.
Intraoperative image of case 30.
Correlation between MRI and operative findings
The presence of a mass on MRI was predictive of a mass at operation (chi-square test, p = 0.01). MRI frequently showed a small, fluid-filled pseudotumor that was not felt to be significant intraoperatively. As a result, in this cohort MRI had a sensitivity of 85% and a specificity of 59% for the presence of a mass observed intraoperatively.
Surgery performed (Table 2)
In most cases, primary THR stems were used with metal-on-polyethylene or ceramic-on-ceramic bearings. 2 patients underwent their revision surgery early in this series to stemmed MOM arthroplasty with retention of the acetabular component, but as a result of the findings reported in this study this is no longer our practice. In cases of severe pelvic osteolysis, pelvic reconstruction was necessary. Captive cups were used in 2 hips (1 patient) with extensive muscle loss.
Analysis of retrieved components
Of the hips that failed by non-conventional means, 14 of 31 were determined to have failed with high material loss and then high levels of metal ions. The median levels of metal ions in this group were 40 (10–166) ppb for cobalt and 32 (3.5–117) ppb for chromium. Median wear rates were 20 (4.2–84) µm/year for the head and 7.0 (2.0–60) µm/year for the cup. All cases had inclination, version, or both outside the range described as acceptable by Grammatopoulos et al. (2010). The incidences of pseudotumor (9/14 vs. 9/17, p = 0.9) and of muscle or bone destruction (3/14 vs. 3/17, p = 0.6) were no different in this group than in the study population as a whole; there was no statistically significant difference in outcome between the 2 groups (Mann-Whitney U test: median ΔOHS 22 (6–35) in the wear group and 15 (4–24) in the non-wear group; p = 0.09). The remaining 19 patients with no conventional mechanism of failure showed no sign of material loss (with normal or near-normal metal ion levels and a normal wear pattern on retrieval analysis). Analysis of the patients who were found to have conventional mechanisms of failure revealed excess wear and high metal ion levels in 2 of 8 patients, both of whom were found to be infected.
Outcomes and complications (Table 4)
Table 4.
Outcomes
| Type | Description | n | Preoperative OHS median (range) | Postoperative OHS median (range) | ΔOHS (range) | p-value |
|---|---|---|---|---|---|---|
| Whole cohort | 39 | 15 (2–31) | 36.5 (9–48) | 17 (–10 to 41) | < 0.001 | |
| Subgroup | ||||||
| 1 | Conventional | 8 | 17 (2–30) | 32 (20–46) | 17 (–10 to 41) | 0.08 |
| 2 | Synovitis | 14 | 11 (4–29) | 33 (9–44) | 14 (4–33) | 0.002 |
| 3 | Soft tissue disruption | 6 | 27 (14–31) | 47 (29–48) | 16 (6–33) | 0.04 |
| 4 | Bone destruction | 7 | 20 (10–28) | 41 (39–45) | 22 (12–35) | 0.03 |
| 5 | Solid pseudotumor | 4 | 8 (2–22) | 29.5 (15–31) | 16.5 (9–23) | 0.07 |
The median OHS was 37 (9–48) at the latest follow-up, a median change of OHS (ΔOHS) of +17 points (–10 to 41) when compared to the preoperative score (Wilcoxon signed rank test, p < 0.001). Although the number of patients involved was small, the 2 patients who underwent femoral component-only revision had a statistically significantly poorer ΔOHS than those who underwent revision of both bearing surfaces (n = 37). Patients with a femoral component-only revision with retention of a MOM articulation had a median OHS of 27 at 3 years, with a median ΔOHS of –3, as compared to a median OHS of 37 with a median ΔOHS of +19 (at a median follow-up time of 19 months, p = 0.02). Patients with a higher preoperative Co level had a greater ΔOHS, although this was not the case for Cr (Spearman’s correlation coefficient was 0.40 for Co (p = 0.02) and 0.21 for Cr (p = 0.2)).
Outcome was similar in patients with failure by conventional mechanisms and in the remainder of the group (Table 4).
8 of the 36 patients who underwent MRI did not present with either a mass on MRI or with cobalt or chromium above the MHRA-recommended maximum of 7 ppb (3 other patients had normal metal ions but did not undergo MRI; they have been excluded from this analysis). Median latest OHS was 31 (19–46) in this group, as compared to 37 (9–48) in the group with at least one abnormal investigation, but this difference did not reach significance (median of differences = 7, CI: –4 to 15; p = 0.2). Similarly, ΔOHS appeared worse in the group with normal investigations (median 15 (4–24) vs. 18 (5–35) in patients with at least one abnormal investigation; median of differences = 5, CI: –2 to 14), but this was not statistically significant (p = 0.2).
There were 2 reoperations. 1 patient (who had a solid pseudotumor and massive destruction) had recurrent dislocations and underwent a revision of the acetabular component to a captive cup. 1 patient underwent a re-exploration for recurrent solid pseudotumor; at reoperation, the acetabular component was loose and it was revised. No patients developed a secondary infection but 1 patient (2 hips) died from a cause unrelated to the surgery.
Discussion
This paper outlines our experience of revision of MOMHA, gained over a 4-year period in a tertiary referral unit, with a median follow-up time of 30 months. Due to the nature of the unit’s practice, the study population shows great heterogeneity of demographics, implant type, and manufacturer, and as a result we have encountered a wide variety of failure mechanisms. This has provided a challenge, which has been addressed using a multidisciplinary approach to preoperative planning, revision surgery, and postoperative follow-up. As the revision burden of this type of implant increases, these challenges are being encountered by more surgeons and more units. It is our intention that the lessons we have learned will be of help to units with less experience.
Overall, outcomes of revision surgery were acceptable with an overall median improvement in OHS of 17 points and with only 2 re-revisions. There was no difference in postoperative functional scores between patients revised for conventional failure mechanisms and those for mechanisms directly attributable to the MOM bearing. There were 2 groups of patients who had a poorer outcome. Firstly, patients who were revised to a stemmed MOM prosthesis had poorer results than those revised to other bearing types. On this basis, we do not recommend retaining a MOM articulation, regardless of the reason for revision. Secondly, 2 of 4 hips with solid pseudotumors have since undergone reoperation. These patients are particularly challenging and the presence of a solid pseudotumor at preoperative MRI is a significant finding. Surgeons should be aware of the difficulties posed by this group of patients, and the involvement of plastic surgeons or those with pelvic expertise may be helpful.
There was great variation in the mechanism of failure. A striking proportion of patients (8 of the 39 patients, all in patients referred with unexplained pain) were found to have conventional mechanisms of failure. Of those whose failure was directly attributable to the MOM articulation, only 14 showed component malpositioning with subsequent high wear rates and high blood metal ion levels. The remaining 17 patients had normal or near-normal metal ion levels and acceptable component positioning. The proportions of patients with a pseudotumor and overall outcomes were similar in each group.
There was also great variation in the preoperative and intraoperative findings encountered. We have divided patients into 5 broad groups on the basis of preoperative and intraoperative findings, which we have found to be helpful in determining the amount of theater time required, the ordering of additional equipment for reconstruction, and the need for pelvic, vascular, or plastic surgical assistance with revision surgery (Table 3). In particular, each group has different requirements in terms of surgical technique and each presents particular challenges.
Misdiagnosis
8 of 39 hips (all in patients referred with a diagnosis of unexplained pain), turned out to have conventional mechanisms of failure such as infection or impingement. A further 14 patients presented with pain in the absence of any significant soft tissue lesion or osteolysis—either on MRI or intraoperatively. Intraoperatively, the only finding was synovitis, but histological examination revealed appearances suggestive of a reaction to metal debris. Caution must be exercised in such cases, and there should be a low threshold for 2-stage surgery if infection is suspected. Particular care should be taken with patients with low metal ion levels and a normal MRI. The outcomes for this group of patients appeared to be worse overall, although this did not reach statistical significance and some patients had a favorable outcome. We do not consider normal investigations to be an absolute contraindication to revision surgery, but we now use diagnostic hip aspiration and injection of local anesthetic in all such patients and our threshold for revision has risen.
Osteolysis
7 hips showed significant osteolysis at preoperative CT and intraoperatively. In 2 cases, significant pelvic reconstruction was performed with trabecular metal augments and posterior column plating for incipient pelvic discontinuity. The osteolysis observed in these cases may be due to the activation of osteoclasts secondary to the release of proinflammatory cytokines by osteoblasts exposed to high local levels of cobalt and chromium ions (Mabilleau et al. 2008), but over-reaming of the acetabulum and stress-shielding by rigid acetabular components may have played a role. All patients considered for surgery in our unit now undergo preoperative CT to assess acetabular orientation and the degree of osteolysis. We recommend that the equipment and expertise for pelvic reconstruction should be available for all cases, and that surveillance of asymptomatic MOMHA should include at least plain radiographs.
Incomplete resection of pseudotumor and damage to surrounding structures
1 patient (1 hip) required reoperation for incompletely resected pelvic pseudotumor. Complete resection of pseudotumors is important to avoid recurrence and prevent secondary infection. MARS MRI is essential for preoperative planning in these cases, and can reveal the presence of a solid pseudotumor, intrapelvic extension requiring pelvic exploration, or involvement of other structures such as nerves and vessels. In 1 case, the pseudotumor entirely encased the sciatic nerve and dissection of the pseudotumor was performed with the aid of intraoperative nerve monitoring.
Muscle destruction and instability
Previous series have shown a high incidence of dislocation following revision of MOMHA. Preoperative MRI is helpful to determine the presence of a large mass and muscle involvement, but these patients are challenging and require significant preoperative preparation. In 1 case, we have had to re-revise a patient with recurrent dislocation and in 2 cases, captive cups were necessary in the presence of significant soft tissue destruction.
Joint guidelines for the management of MOMHA from the European Hip Society and the European Federation of National Associations of Orthopaedics and Traumatology (EFORT) concur with earlier guidelines from the UK MHRA in suggesting close follow-up of large-diameter MOM bearings for the lifetime of the implant, with plain radiography and determination of metal ion levels (EFORT 2012, MHRA 2010). They recommend that cross-sectional imaging should be reserved for symptomatic patients or those with elevated metal ion levels, although the maximum safe level remains debatable (an issue reflected in the large number of symptomatic patients in this series presenting with one or more ion level below the 7 ppb suggested as a cutoff by the MHRA). Our findings support the existing guidelines. In addition, the following further conclusions can be drawn:
Preoperative planning with MARS MRI is useful for prediction of muscle destruction and the extent of any solid pseudotumor in a manner with which surgeons are familiar (communication between radiologist and surgeon is more straightforward with MRI images than with ultrasound snapshots). Routine involvement of a radiologist with experience of these cases can provide valuable information for preoperative planning. Patients with solid pseudotumors and/or extensive muscle or bone destruction may benefit from undergoing surgery in units with experience of this group of cases.
Surgeons should be aware of the possibility of severe pelvic osteolysis in MOM patients and plan for such an eventuality.
Raised blood metal ion levels, while predictive of high material loss, are not predictive of the degree of soft tissue or bone damage found at revision.
Caution should be exercised when revising patients with normal MRI and blood metal ion measurements. Such patients can have a good outcome, but carry a significant risk of misdiagnosis and should be investigated fully to exclude conventional mechanisms of failure.
Revision of MOMHA for any reason should include both sides of the bearing surface and result in a non-MOM bearing couple.
Acknowledgments
ADL assembled and analyzed the data, and wrote the manuscript. KS and AWMM performed and interpreted the MARS MRI and CT imaging. JH reconstructed the CTs, performing analysis of implant positioning. SAS and KVV collected clinical follow-up data and arranged collection of metal ion samples. AL, JAS, and AJH performed the surgery, recorded the intraoperative data, collected histological samples, and arranged intraoperative photography. AJH was the head of department and principal investigator. He conceived the study and co-wrote the manuscript.
We thank Gwynneth Lloyd and Charlotte Page for their help with collection of data and preparation of the manuscript. We also thank the patients who participated in the study.
The London Implant Retrieval Centre is funded by contributions from nine implant manufacturers (Zimmer, Stryker, de Puy, Finsbury, Smith and Nephew, Biomet, Corin, JRI, and Mathys) and operates a retrieval programme for the ASR hip funded separately by de Puy. AJH has a contract with de Puy for clinical advice for patients with ASR hips.
References
- Browne JA, Bechtold CD, Berry DJ, Hanssen AD, Lewallen DG. Failed metal-on-metal hip arthroplasties: A spectrum of clinical presentations and operative findings. Clin Orthop. 2010;468(9):2313–20. doi: 10.1007/s11999-010-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D, Havey TC, Roberts R, Guyatt G. How many patients? How many limbs? Analysis of patients or limbs in the orthopaedic literature: a systematic review. J Bone Joint Surg (Am) 2006;88(1):41–5. doi: 10.2106/JBJS.E.00272. [DOI] [PubMed] [Google Scholar]
- Dandachli W, Nakhla A, Iranpour F, Kannan V, Cobb JP. Can the acetabular position be derived from a pelvic frame of reference? Clin Orthop. 2009;(467)((4)):886–93. doi: 10.1007/s11999-008-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet KA, Van Der Straeten C, Van Orsouw M, Doubi R, Backers K, Grammatopoulos G. Revisions of metal-on-metal hip resurfacing: lessons learned and improved outcome. Orthop Clin North Am. 2011;42(2):259–69. ix. doi: 10.1016/j.ocl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- EFORT Consensus statement: “Current Evidence on the Management of Metal on Metal Bearings”. 2012. [DOI] [PubMed]
- Grammatopoulos G, Pandit H, Kwon Y-M, Gundle R, McLardy-Smith P, Beard DJ, Murray DW, Gill HS. Resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg (Br) 2009;91:1019–24. doi: 10.1302/0301-620X.91B8.22562. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos G, Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gill HS, Murray DW. Optimal acetabular orientation for hip resurfacing. J Bone Joint Surg (Br) 2010;92:1072–8. doi: 10.1302/0301-620X.92B8.24194. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell A. Pseudotumours in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg (Am) 2012;94(4):317–25. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- Kwon YM, Xia Z, Glyn-Jones S, Beard D, Gill HS, Murray DW. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed Mater. 2009;4(2):025018. doi: 10.1088/1748-6041/4/2/025018. [DOI] [PubMed] [Google Scholar]
- Langton DJ, Jameson SS, Joyce TJ, Gandhi JN, Sidaginamale R, Mereddy P, Lord J, Nargol AV. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg (Br) 2011;93(8):1011–6. doi: 10.1302/0301-620X.93B8.26040. [DOI] [PubMed] [Google Scholar]
- Mabilleau G, Kwon Y-M, Pandit H, Murray DW, Sabokbar A. Metal-on-metal hip resurfacing arthroplasty: A review of periprosthetic biological reactions. Acta Orthop. 2008;79(6):734–47. doi: 10.1080/17453670810016795. [DOI] [PubMed] [Google Scholar]
- Matthies A, Underwood R, Cann P, Ilo K, Nawaz Z, Skinner J, Hart AJ. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing. J Bone Joint Surg (Br) 2011;93(3):307–14. doi: 10.1302/0301-620X.93B3.25551. [DOI] [PubMed] [Google Scholar]
- MHRA Medical device alert: all metal-on-metal (MoM) hip replacements (MDA/2010/033) http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON079157. 2010
- Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg (Br) 1993;75(2):228–32. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- Murray DW, Fitzpatrick R, Rogers K, Pandit H, Beard DJ, Carr AJ, Dawson J. The use of the Oxford hip and knee scores. J Bone Joint Surg (Br) 2007;89(8):1010–4. doi: 10.1302/0301-620X.89B8.19424. [DOI] [PubMed] [Google Scholar]
- Park MS, Kim SJ, Chung CY, Choi IH, Lee SH, Lee KM. Statistical consideration for bilateral cases in orthopaedic research. J Bone Joint Surg (Am) 2010;21(92 (8)):1732–7. doi: 10.2106/JBJS.I.00724. [DOI] [PubMed] [Google Scholar]
- Sabah SA, Mitchell AW, Henckel J, Sandison A, Skinner JA, Hart AJ. Magnetic resonance imaging findings in painful metal-on-metal hips: A prospective study. J Arthroplasty. 2011;26(1):71–6. doi: 10.1016/j.arth.2009.11.008. [DOI] [PubMed] [Google Scholar]