Abstract
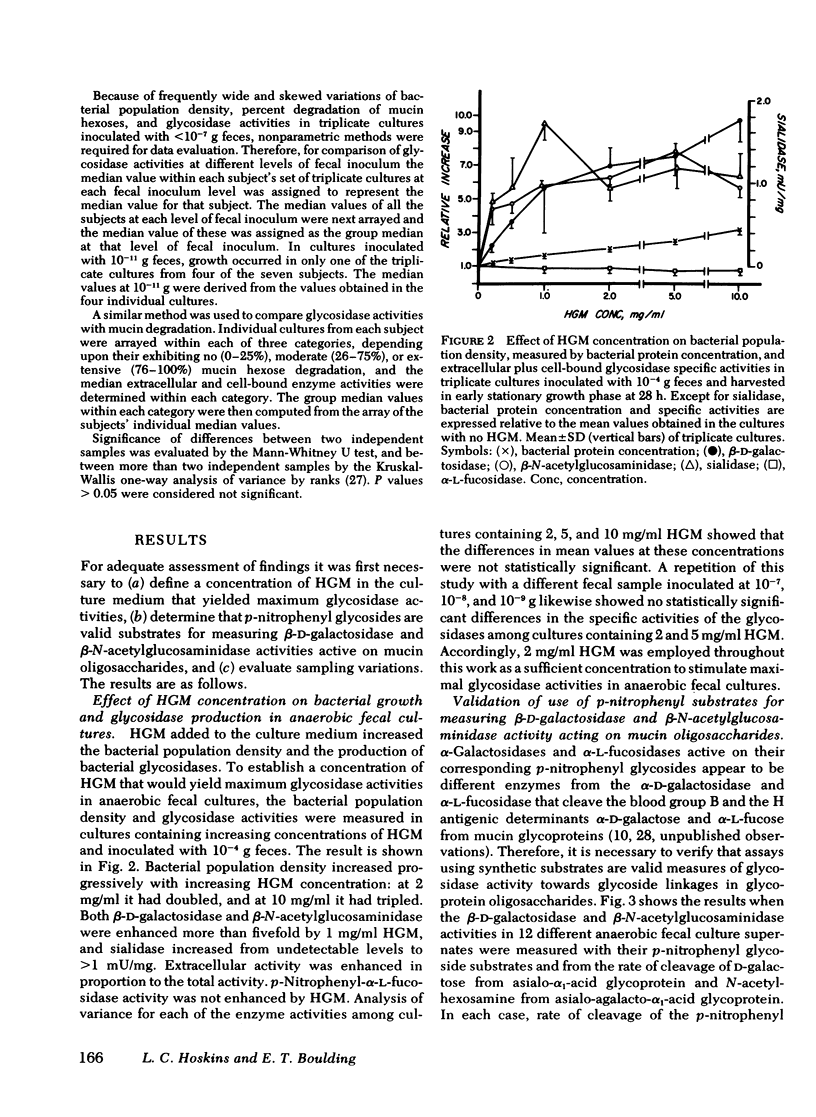
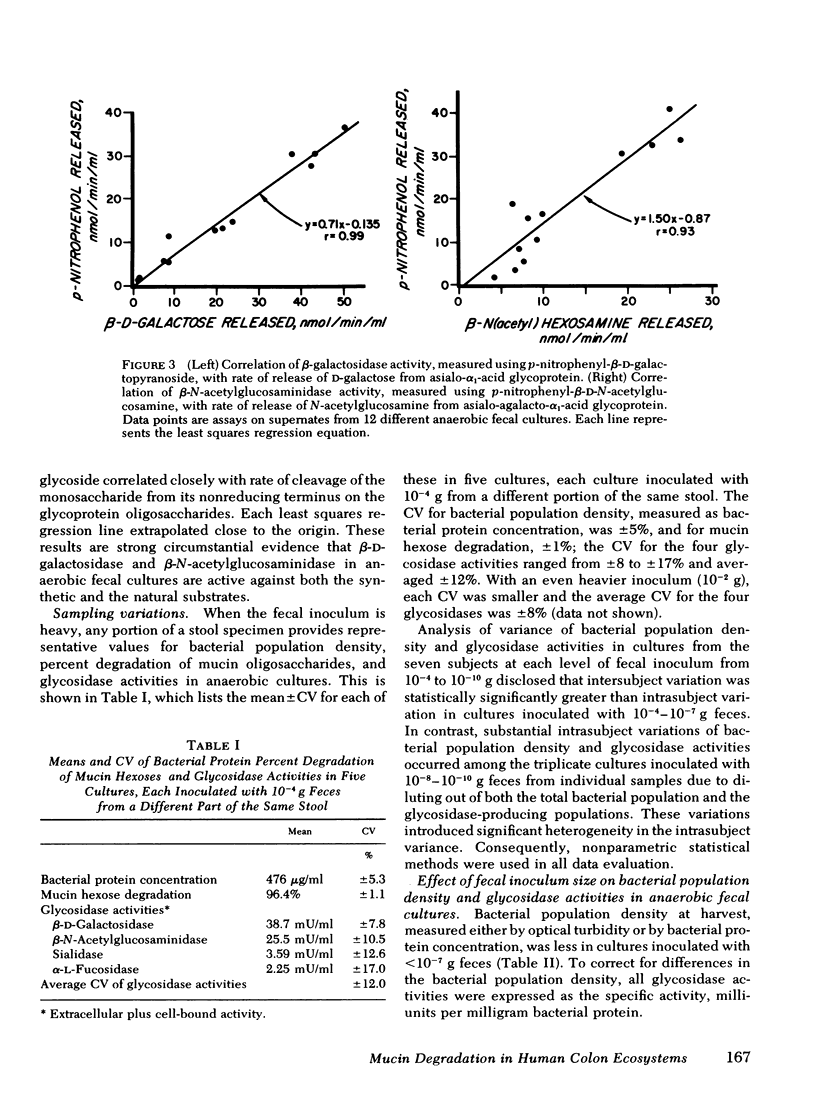
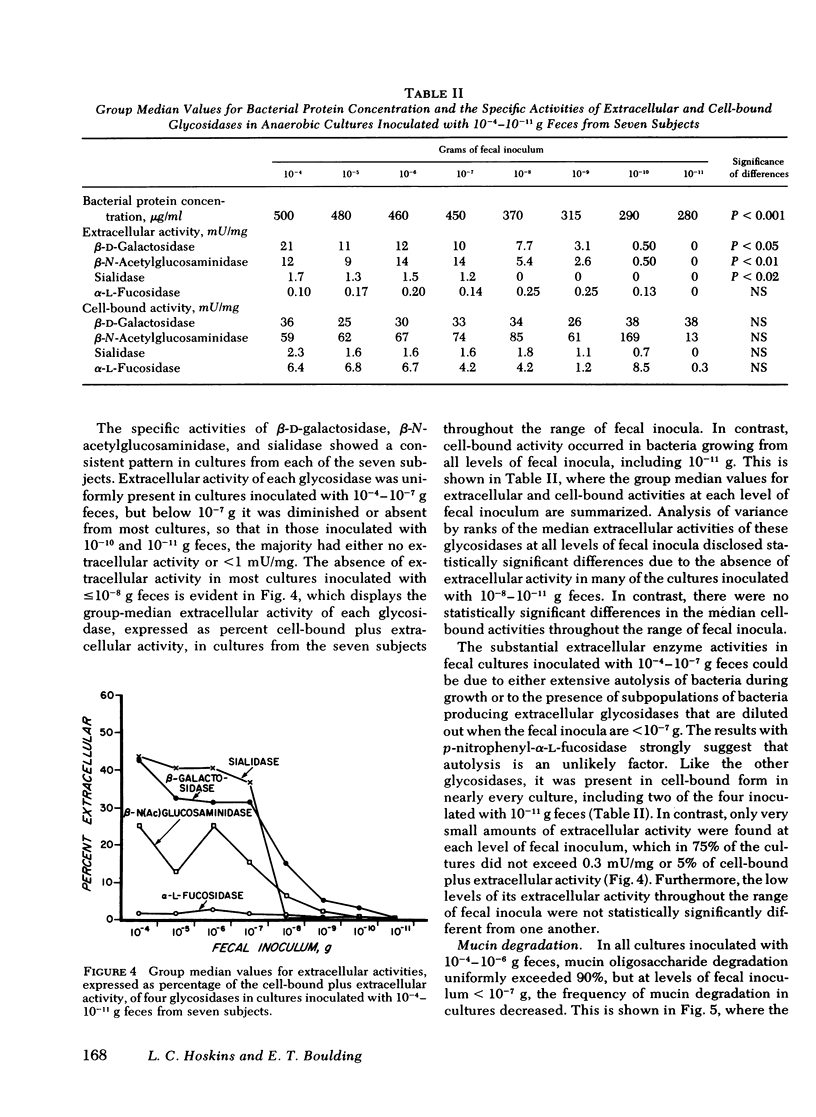
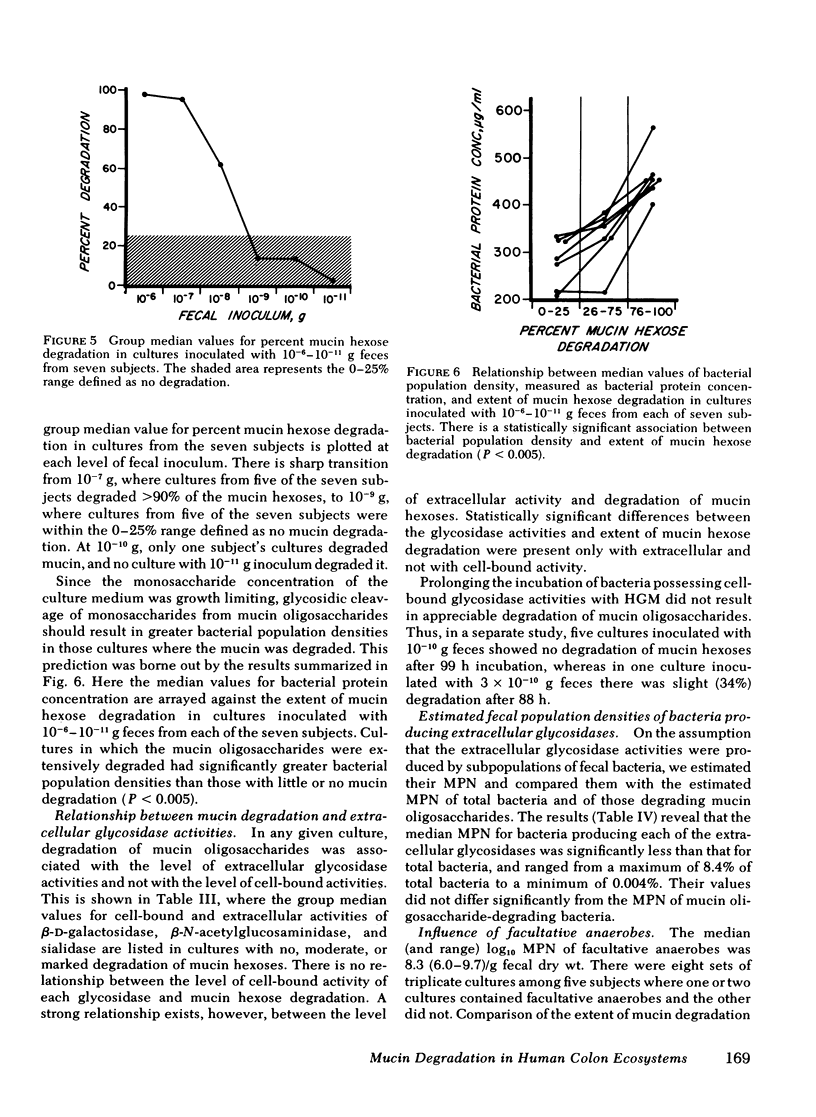
Recent work indicates that subpopulations of human fecal bacteria, averaging ∼1% of the total viable fecal flora, degrade the oligosaccharide side chains of hog gastric mucin, which structurally resembles human epithelial mucins. Here we report studies to determine whether degradation of mucin oligosaccharides is related to glycosidase production by bacteria growing in anaerobic fecal cultures. Triplicate cultures containing hog gastric mucin were inoculated with serially diluted feces from each of seven healthy subjects. When the stationary growth phase was attained, mucin oligosaccharide degradation and both cell-bound and extracellular activities of four glycosidases were measured in each culture. Cell-bound β-d-galactosidase, β-N-acetylglucosaminidase, and sialidase were present in bacteria growing at all levels of fecal inocula, including 10−11 g. In contrast, extracellular activities were present in every culture inoculated with 10−4−10−7 g feces, but were diminished or absent in cultures inoculated with 10−8−10−11 g feces. Bacterial autolysis was an unlikely cause of extracellular glycosidase activity, since p-nitrophenyl-α-l-fucosidase remained cell bound in cultures at every level of fecal inoculum. Degradation of mucin oligosaccharides was associated with extracellular, but not with cell-bound β-d-galactosidase, β-N-acetylglucosaminidase, and sialidase. Among the seven subjects, the estimated most probable numbers (MPN) of fecal bacteria producing extracellular β-d-galactosidase, β-N-acetylglucosaminidase, and sialidase ranged from 106−1010/g dry fecal wt, were comparable to the MPN of mucin-degrading bacteria, and were significantly smaller than the MPN of total fecal bacteria.
We interpret these findings as evidence for the existence of bacterial subpopulations in the normal fecal flora that produce extracellular glycosidases, and that these subpopulations have a major role in degrading the complex oligosaccharides of mucin in the gut lumen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978 Jan;34(1):28–33. [PubMed] [Google Scholar]
- Aminoff D., Furukawa K. Enzymes that destroy blood group specificity. I. Purification and properties of alpha-L-fucosidase from Clostridium perfringens. J Biol Chem. 1970 Apr 10;245(7):1659–1669. [PubMed] [Google Scholar]
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Cromwell C. L., Hoskins L. C. Antigen degradation in human colon ecosystems. Host's ABO blood type influences enteric bacterial degradation of a cell surface antigen on Escherichia coli O86. Gastroenterology. 1977 Jul;73(1):37–41. [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Toskes P. P. Jejunal brush border injury and impaired sugar and amino acid uptake in the blind loop syndrome. Gastroenterology. 1974 Nov;67(5):965–974. [PubMed] [Google Scholar]
- Glasgow L. R., Paulson J. C., Hill R. L. Systematic purification of five glycosidases from Streptococcus (Diplococcus) pneumoniae. J Biol Chem. 1977 Dec 10;252(23):8615–8623. [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hawksworth G., Drasar B. S., Hill M. J. Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol. 1971 Nov;4(4):451–459. doi: 10.1099/00222615-4-4-451. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Degradation of blood group antigens in human colon ecosystems. I. In vitro production of ABH blood group-degrading enzymes by enteric bacteria. J Clin Invest. 1976 Jan;57(1):63–73. doi: 10.1172/JCI108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Degradation of blood group antigens in human colon ecosystems. II. A gene interaction in man that affects the fecal population density of certain enteric bacteria. J Clin Invest. 1976 Jan;57(1):74–82. doi: 10.1172/JCI108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968 Feb;54(2):210–217. [PubMed] [Google Scholar]
- Jabbal I., Kells D. I., Forstner G., Forstner J. Human intestinal goblet cell mucin. Can J Biochem. 1976 Aug;54(8):707–716. doi: 10.1139/o76-102. [DOI] [PubMed] [Google Scholar]
- Kelly J. J., Alpers D. H. Blood group antigenicity of purified human intestinal disaccharidases. J Biol Chem. 1973 Dec 10;248(23):8216–8221. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOKRASCH L. C. Analysis of hexose phosphates and sugar mixtures with the anthrone reagent. J Biol Chem. 1954 May;208(1):55–59. [PubMed] [Google Scholar]
- Marshall T., Allen A. The isolation and characterization of the high-molecular-weight glycoprotein from pig colonic mucus. Biochem J. 1978 Aug 1;173(2):569–578. doi: 10.1042/bj1730569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Bahn A. N. Carbohydrate hydrolases of oral streptococci. Arch Oral Biol. 1969 Jul;14(7):735–744. doi: 10.1016/0003-9969(69)90165-4. [DOI] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Meyer K. Oligosaccharides produced by acetolysis of blood group active(A + H)sulfated glycoproteins from hog gastric mucin. J Biol Chem. 1973 Apr 10;248(7):2290–2295. [PubMed] [Google Scholar]
- Takasaki S., Yamashita K., Kobata A. The sugar chain structures of ABO blood group active glycoproteins obtained from human erythrocyte membrane. J Biol Chem. 1978 Sep 10;253(17):6086–6091. [PubMed] [Google Scholar]
- Toskes P. P., Giannella R. A., Jervis H. R., Rout W. R., Takeuchi A. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology. 1975 May;68(5 Pt 1):1193–1203. [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WEIMER H. E., WINZLER R. J. Comparative study of orosomucoid preparations from sera of six species of mammals. Proc Soc Exp Biol Med. 1955 Nov;90(2):458–460. doi: 10.3181/00379727-90-22064. [DOI] [PubMed] [Google Scholar]


