Abstract
Since the cholinergic hypothesis of memory dysfunction was first reported, extensive research efforts have focused on elucidating the mechanisms by which this intricate system contributes to the regulation of processes such as learning, memory, and higher executive function. Several cholinergic therapeutic targets for the treatment of cognitive deficits, psychotic symptoms, and the underlying pathophysiology of neurodegenerative disorders, such as Alzheimer’s disease and schizophrenia, have since emerged. Clinically approved drugs now exist for some of these targets; however, they all may be considered suboptimal therapeutics in that they produce undesirable off-target activity leading to side effects, fail to address the wide variety of symptoms and underlying pathophysiology that characterize these disorders, and/or afford little to no therapeutic effect in subsets of patient populations. A promising target for which there are presently no approved therapies is the M1 muscarinic acetylcholine receptor (M1 mAChR). Despite avid investigation, development of agents that selectively activate this receptor via the orthosteric site has been hampered by the high sequence homology of the binding site between the five muscarinic receptor subtypes and the wide distribution of this receptor family in both the central nervous system (CNS) and the periphery. Hence, a plethora of ligands targeting less structurally conserved allosteric sites of the M1 mAChR have been investigated. This Review aims to explain the rationale behind allosterically targeting the M1 mAChR, comprehensively summarize and critically evaluate the M1 mAChR allosteric ligand literature to date, highlight the challenges inherent in allosteric ligand investigation that are impeding their clinical advancement, and discuss potential methods for resolving these issues.
Keywords: M1 mAChR, allosteric ligands, Alzheimer’s disease, schizophrenia, cognitive deficits, memory
The muscarinic acetylcholine receptor (mAChR) family is a group of rhodopsin-like (Family A) G protein-coupled receptors (GPCRs) consisting of five distinct subtypes M1–M5. Activation of the M1, M3, and M5 receptor subtypes primarily results in coupling to the Gq/11 family of G proteins, activation of phospholipase C (PLC), release of inositol-1,4,5-trisphosphate (IP3), and subsequent mobilization of intracellular calcium Ca2+. Activation of the M2 and M4 receptor subtypes primarily results in coupling to the Gi/o family of G proteins, inhibition of adenylate cyclase (AC), reduction in cyclic AMP (cAMP), and a decrease in neurotransmitter release via the blockage of voltage-gated calcium channels (Figure 1). These pathways represent a generalized view of each receptor’s coupling capacity, as all five subtypes couple to a broader range of G protein- and non-G protein-mediated signaling and regulatory pathways,1 ultimately leading to the regulation of enzymes and neurotransmitters critical for intercellular chemical communication and biological function. In a broader molecular sense, this diversity is largely facilitated by the ability of GPCRs to adopt a range of conformational states that can lead to distinct functional outcomes.2
Figure 1.
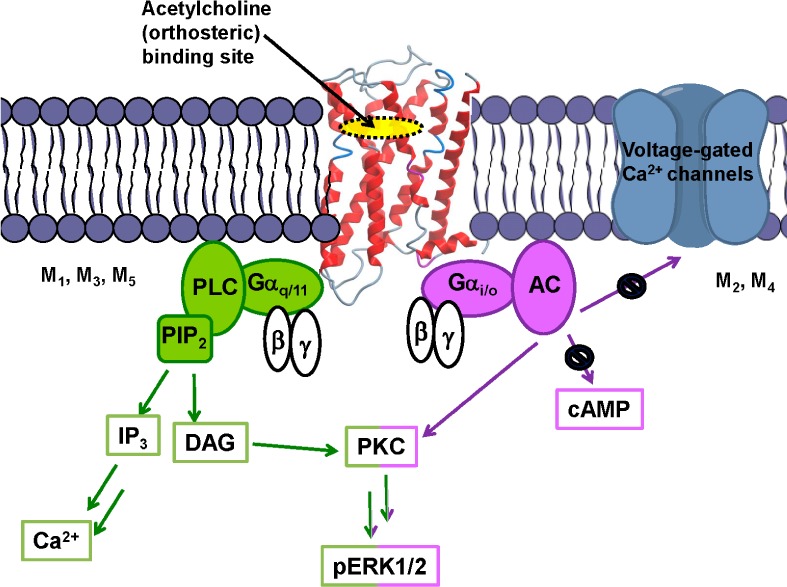
Common signal transduction pathways of the five muscarinic acetylcholine receptors.
The relative distribution of the five mAChR subtypes in both the CNS and peripheral tissues was elucidated by mRNA hybridization and immunocytochemical methods.3,4 The M1 mAChR is predominantly expressed postsynaptically in the CNS, particularly in regions of the hippocampus, prefrontal cortex, and striatum. The M2 and M3 mAChRs are expressed both pre- and postsynaptically in different brain regions, most notably the basal forebrain, the thalamus, and the hippocampus. Furthermore, the peripheral tissues mostly express the M2 and M3 subtypes, particularly in cardiac and smooth muscle tissues. The M4 and M5 mAChRs are almost exclusively expressed in the CNS, the former presynaptically in the striatum and the hippocampus, and the latter in the substantia nigra.
This broad distribution is suggestive of muscarinic receptors mediating a diverse range of biological functions and hence playing potentially critical roles in a number of central and peripheral disease processes. This Review will henceforth focus on the role of the muscarinic system in the regulation of learning and memory in the CNS, specifically, the utility of targeting the M1 mAChR for the treatment of neurodegenerative disorders, such as Alzheimer’s disease (AD) and schizophrenia.
AD is a condition of the CNS with no definitive cause and no known cure. Four key “hallmarks” of this disease are cognitive decline in memory and learning, the dysfunction and eventual death of cholinergic neurons, the accumulation of β-amyloid plaques, and the formation of neurofibrillary tangles as a result of tau-protein hyperphosphorylation.5 Schizophrenia is a debilitating disorder most likely caused by a combination of genetic and environmental factors, though the biological origin is unknown. The disease manifests as four “symptom domains”:6 positive (hallucinations, delusions), negative (social withdrawal, lack of initiative), cognitive (attention deficit and impaired memory), and affective (anxiety, depression, aggression, and suicidal tendencies).
A poorly treated symptom of both disorders is cognitive impairment, which manifests as dysfunctions in memory consolidation, learning, and higher-order processing. While disruption of multiple neurotransmitter systems may be implicated in these deficits,7 considerable evidence suggests that impaired cortical-hippocampal cholinergic signaling plays an integral part in the manifestation of these conditions.6 The advent of research tools such as receptor knockout (KO) mice and subtype-selective muscarinic ligands has afforded significant advances in our understanding of the role of the cholinergic system in the physiology and pathophysiology of cognition.8,9 In particular, M1 mAChR activation by subtype-selective allosteric enhancement has shown considerable promise as a means of ameliorating cognitive decline and treating disease state pathophysiology.10 However, the lack of structural information about the M1 mAChR allosteric binding sites and the complex behavior of allosteric ligands is such that rational drug design and preclinical pharmacological evaluation are fraught with challenges impeding the development of viable therapeutics. This Review will discuss essential considerations in the classification and pharmacological evaluation of allosteric ligands, summarize the M1 mAChR allosteric ligands reported to date, and offer a perspective on the pharmacological methods employed in their development.
The Cholinergic Hypothesis of Memory Dysfunction
The culmination of considerable biochemical, electrophysiological, and pharmacological evidence gained from studies in elderly patients gave rise to the cholinergic hypothesis of memory dysfunction some decades ago.11 Four critical observations that unequivocally validated this hypothesis were as follows:
-
(i)
the substantial loss of presynaptic cholinergic basal forebrain cortical projection neurons in human post-mortem Alzheimer’s-diseased brains;12
-
(ii)
the dysfunction of cholinergic markers, such as acetylcholine (ACh), choline (a precursor of ACh), and choline acetyl transferase (an enzyme for ACh production), observed in the human post-mortem brains of patients suffering from cognitive deficits;13
-
(iii)
the behavioral cognitive impairments observed upon pharmacological disruption of cholinergic activity;11 and
-
(iv)
the improvement in cognition observed in elderly patients upon artificial enhancement of cholinergic activity11
Accordingly, cholinergic enhancement via inhibition of the enzyme responsible for ACh degradation, cholinesterase, was pursued, giving rise to the four anticholinesterase drugs currently approved for the treatment of the various stages of AD: tacrine, donepezil, rivastigmine, and galantamine. While widely used, these drugs have a propensity for nonselective activity, leading to a swathe of side effects associated with excessive cholinergic stimulation, such as diarrhea, hypersalivation, and bradycardia. The failing of the anticholinesterases is unsurprising considering that they target an enzyme present at the synapses of all cholinergic neurons, likely resulting in broad spectrum, abrupt muscarinic activation in whatever tissue compartment that these drugs are distributed. Furthermore, these therapies have been shown to elicit little to no therapeutic efficacy in up to 75% of the patient population,14 which is possibly due to the progressive degeneration of presynaptic cholinergic nerve terminals as the disease state progresses.
Xanomeline: An Important Proof-of-Concept Compound
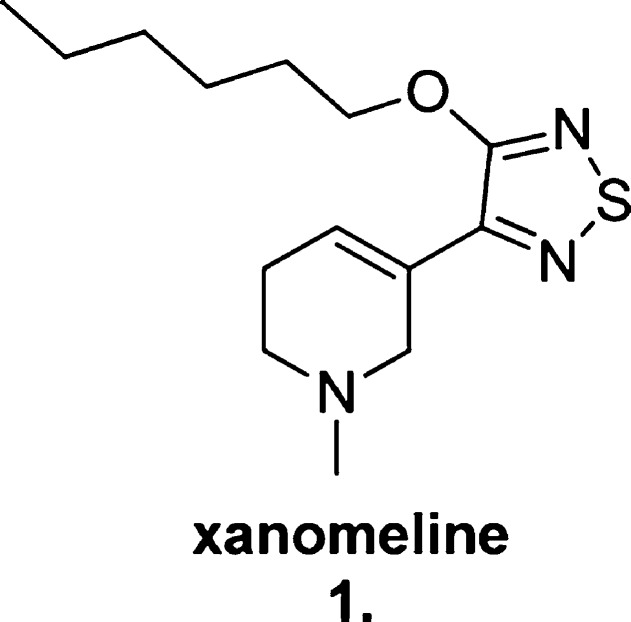
An alternative method by which cholinergic enhancement may be achieved is to mimic the actions of ACh, namely, by selective mAChR activation (and nAChR activation, which is beyond the scope of this Review). Important proof-of-concept for selective mAChR activation as a potential therapy for the cognitive deficits associated with neurodegenerative conditions came with the discovery of the compound xanomeline (1, Figure 2), a M1/M4-preferring agonist (see Mirza et al.15 for a comprehensive review). This compound produced increased brain ACh levels, demonstrated a reversal of antagonist-induced memory block following IP administration in mice, and attenuated cognitive decline in a 6 month clinical trial in human AD patients following oral administration.16
Figure 2.
Structure of the M1/M4 mAChR-preferring agonist xanomeline (1).
Studies in M1–M5 muscarinic receptor KO mice directly linking the absence of each receptor subtype to specific biochemical, physiological, and behavioral changes have enabled assignment of each receptor with likely biological functions.8 M1 mAChR KO mice exhibited deficits in tasks most likely involving neural communication between the cerebral cortex and the hippocampus. Moreover, activation of the M1 mAChR was found to be the predominant mediator of the effects on cognition, attention, and learning that were observed following xanomeline administration;17 these effects were attenuated in M1 mAChR KO mice (but not M4 mAChR KOs).
In addition to validating the therapeutic utility of M1 mAChR targeting, xanomeline also validated M4 mAChR activation as a potential treatment for psychosis, demonstrating in vivo efficacy in both animal18 and human19 clinical trials, and exhibiting a similar therapeutic profile to the atypical antipsychotics clozapine and olanzapine.15 The attenuation of amphetamine-induced psychotic behaviors observed with administration of xanomeline was absent in M4 mAChR KO mice (but remained present in M1 mAChR KOs).20 This antipsychotic action is postulated to occur via indirect modulation of dopamine levels; the M4 mAChR is highly expressed within the mesolimbic dopaminergic system, commonly associated with positive symptoms of schizophrenia.21
Unfortunately, while xanomeline exhibits some degree of selectivity as a M1/M4 mAChR agonist, binding studies in cloned human muscarinic receptors revealed no preferential binding affinity for these two subtypes in relation to the others,22 a factor that likely contributed to the peripheral cholinergic side effects that prompted the withdrawal of 52% of AD subjects in a phase III trial of xanomeline.9 This, combined with its vastly different efficacy and selectivity profiles depending on the in vitro experimental paradigm utilized,15 its off-target activity at dopaminergic and 5-HT receptor subtypes,23 and the compound’s metabolic instability and high hepatic first pass effect leading to <1% oral bioavailability in both animals and humans,15 has rendered xanomeline an insufficient clinical candidate. Despite this clinical failure, the M1 mAChR target validation afforded by xanomeline sparked considerable research interest into deconvoluting the mechanisms by which this receptor mediates cognitive processing.
The Role of the M1 mAChR in Cognition
M1 mAChRs are most abundantly expressed postsynaptically in regions of the forebrain such as the hippocampus, striatum, and cerebral cortex.3 Stimulation of M1 mAChRs activates multiple signaling pathways leading to the production of numerous downstream effector molecules, each of which is likely to contribute to the overall physiological functioning of M1 mAChRs, as well as the utility of targeting these receptors for the treatment of cognitive deficits. One such example is the protein kinase C (PKC) family, important signaling enzymes that are produced following Gαq/11 pathway activation and are significantly reduced in the Alzheimer’s diseased brain24 (for a more detailed summary of the potential effects of PKC in cognition and Alzheimer’s disease, see a recent publication by Fisher25). Additionally, it has been demonstrated that activation of M1 mAChRs leads to potentiation of currents through the N-methyl-d-aspartate (NMDA) receptor in hippocampal pyramidal cells,26 a receptor shown to play a critical role in synaptic plasticity and memory consolidation.27
Herein lies one of the key theoretical advantages of postsynaptic M1 mAChR activation. Unlike the anticholinesterases, this mode of treatment does not rely on the presence of intact presynaptic cholinergic nerve terminals to be effective, neurons that are significantly compromised in neurodegenerative disorders.28 Additionally, it has been shown that, in AD cortical tissue, postsynaptic M1 mAChR density is unaltered29 (and in a more recent study significantly increased30), establishing the availability of the target in the most critically affected brain region of patients, regardless of the extent of cognitive decline.
The Role of the M1 mAChR in Modifying AD Pathophysiology
A significant advantage of M1 mAChR activation for the treatment of Alzheimer’s disease is the potential for positive modification of the underlying pathophysiology of the condition that leads to cognitive symptoms in the first place, affording a prospective dual action treatment for AD unable to be accomplished by anticholinesterase therapies. Two widely acknowledged pathophysiological hallmarks of AD are the accumulation of β-amyloid plaques and the formation of neurofibrillary tangles as a result of tau-protein hyperphosphorylation,5 “vicious cycles” that both lead to substantive CNS cholinergic neural death in the cortical and hippocampal regions of the basal forebrain (flowchart depicted in Figure 3).
Figure 3.
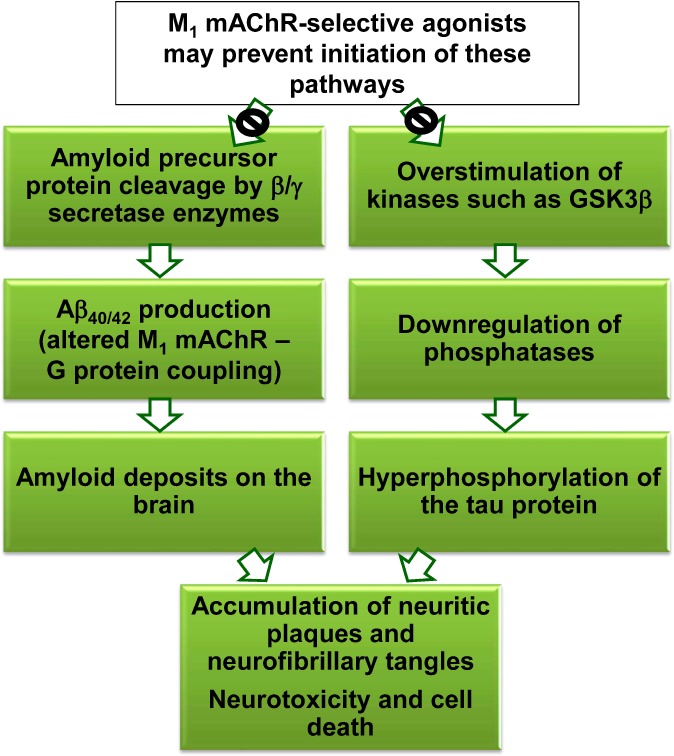
Two pathophysiological pathways underlying Alzheimer’s disease.
Amyloid precursor protein (APP) has been linked to functions such as regulation of synaptic interactions and neuroplasticity.31 APP can be differentially cleaved by secretase enzymes into amyloidogenic and nonamyloidogenic metabolites which forms the basis for the pathophysiology of Alzheimer’s disease and the prospect of M1 mAChR activation as a potential treatment. The amyloidogenic pathway is characterized by β/γ secretase cleavage of APP, resulting in peptides such as Aβ40 and Aβ42, the primary constituents of the aggregated amyloid neuritic plaques observed in the AD brain.32 Accumulation of Aβ peptides is also postulated to impair mAChR coupling to G proteins,33 which is likely to further perpetuate the disease state. In contrast, the nonamyloidogenic pathway is stimulated by the action of the α-secretase enzyme, which cleaves APP within the Aβ peptide sequence, leading to harmless metabolites. The question of which pathway predominates at any given time depends on the relative expression of the secretase enzymes, which is largely dictated by the actions of cellular second messenger systems downstream of GPCRs. Specifically, M1 mAChR activation and initiation of Gq/11-mediated activation of PKC has been linked to the increased expression of α-secretase,34 and consequently promotion of the nonamyloidogenic pathway and a reduction in Aβ peptide levels, which could potentially slow or halt the formation of amyloid plaques and hence, the progression of AD.
Further validation of the critical role of the M1 mAChR in modulating this process was obtained when the treatment of human AD patients with a selective M1 mAChR agonist, talsaclidine (2, Figure 4), led to an observed reduction in Aβ42 levels in their cerebrospinal fluid (CSF).32 Additionally, in vivo studies in 3xTg mice (injected with transgenes for β-amyloid and tau, and therefore a model for AD) reported increased CSF levels of α-secretase-producing enzyme, ADAM17, and a corresponding decrease in β-secretase formation following treatment with another M1 mAChR-selective agonist, AF267B (3, Figure 4).35
Figure 4.
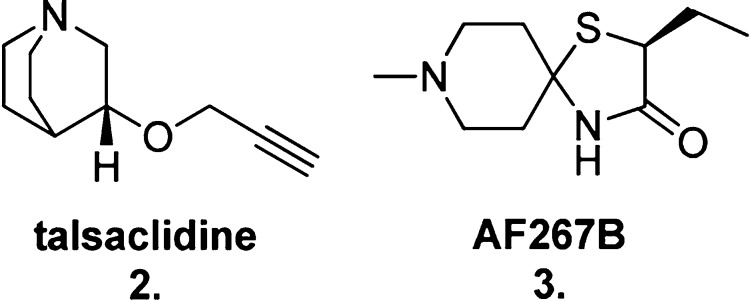
Structures of two M1 mAChR selective agonists, talsaclidine (2) and AF267B (3).
The second “vicious cycle” referred to earlier is the formation of neurofibrillary tangles that occurs when the protein essential for the growth of microtubules in the neuronal cytoskeleton, tau, becomes hyperphosphorylated. Both in vitro and in vivo studies33,35 have demonstrated that activation of the M1 mAChR has the potential to decrease tau phosphorylation by PKC-mediated inhibition of kinases (particularly GSK3β) and upregulation of phosphatases, subsequently decreasing the likelihood of fibrous tangle formation.
A comparative analysis of M1 mAChR expression and response in the post-mortem cortical tissue of humans observed to have no cognitive impairment, mild cognitive impairment, or Alzheimer’s disease was conducted to further understand the link between activation of this receptor and AD pathophysiology.30 [3H]-Oxotremorine-M radioligand binding studies found M1 mAChR expression to be elevated in the AD cortical tissue, indicative of compensatory upregulation in response to a reduction in ACh levels. It was also found that while there was no correlation between function of M1 mAChRs and the degree of cognitive impairment, a negative correlation between M1 mAChR functional activity and severity of neuropathology (based on post-mortem neurofibrillary tangle analysis) was observed, adding further weight to the argument for selective targeting of this receptor in the treatment of AD.
The Role of the M1 mAChR in Treating Schizophrenia
Currently available pharmaceutical treatments for schizophrenia largely target dopamine (D) receptors or a combination of dopamine and serotonin (5-HT) receptors. First generation antipsychotics, such as haloperidol and chlorpromazine, are predominantly D2R antagonists, treating the positive symptoms of the disorder but also eliciting Parkinsonian-like motor dysfunction (extrapyramidal side effects (EPS)).36 Second generation antipsychotics, such as clozapine and olanzapine, treat positive symptoms by acting as D2R antagonists but also partially treat the negative symptoms and reduce EPS by acting as cortical 5-HT2AR antagonists. However, these therapies still demonstrate poor efficacy and a lack of selectivity, resulting in a range of potentially dangerous side effects, including prolongation of QT interval, agranulocytosis, and severe weight gain.37 Aripiprazole is a third generation antipsychotic exhibiting a unique mechanism of action: D2R partial agonism, 5-HT1AR partial agonism, and 5-HT2AR antagonism, the combination of which results in increased and decreased neurotransmission in hypodopaminergic and hyperdopaminergic areas, respectively.38 Thus, it is considered to be the first dopamine–serotonin system stabilizer. Despite reports of an excellent safety and tolerability profile, aripiprazole still demonstrates the potential to cause the same side effects as its first- and second-generation predecessors.
A major deficit of these three classes is that none were designed to treat, nor show substantial efficacy against, the cognitive deficits experienced as part of schizophrenia. This is despite the observation that the degree of cognitive impairment is the strongest predictor of clinical outcome for sufferers of this debilitating mental disorder,39 and that cognitive symptoms are often evident in patients before the onset of psychosis.
The notion of treating schizophrenia by targeting the cholinergic system predates the widely regarded dopamine hypothesis,40 the latter having given rise to the majority of schizophrenia medications to date. Decades-old clinical observations of muscarinic agonists exhibiting modest antipsychotic properties and anticholinergic exacerbation of schizophrenic symptoms formed the foundation of this theory, and more recent studies have demonstrated decreases in muscarinic receptor binding in both the prefrontal cortex and the hippocampus in schizophrenic patients,41,42 regions rich in the M1 mAChR subtype. Taken together, this suggests that the M1 mAChR may represent a therapeutically beneficial target for both positive and cognitive symptom domains. Furthermore, the direct and indirect interactions between the dopaminergic and cholinergic neurotransmitter systems in the brain implicate a role for the M1 mAChR (and indeed other muscarinic receptor subtypes) in modulating the complex underlying pathophysiology of schizophrenia.43 However, while a muscarinic hypothesis of schizophrenia certainly warrants further investigation, particularly the roles of the M1 and M4 mAChR subtypes, it must be acknowledged that schizophrenia is a collection of syndromes likely to be dictated by multiple neurotransmitter systems and ultimately requiring combination drug therapy if all symptoms are to be alleviated.
Caveats of Targeting the M1 mAChR
In principle, selective M1 mAChR agonists are less likely to exhibit side effects than anticholinesterases as they possess a more specific cholinergic objective, a single mAChR subtype. Despite this, there are presently no M1 mAChR agonists approved as neurodegenerative therapies. While several ligands have demonstrated sufficient M1 mAChR selectivity in in vitro studies supporting target validation, these compounds either show poor pharmacokinetic profiles and limited in vivo efficacy or fail to exhibit sufficient receptor subtype selectivity to avoid peripheral muscarinic side effects, such as gastrointestinal disruption, nausea, bradycardia, and excessive exocrine gland secretion.44 This latter issue arises from their targeting the endogenous agonist-binding (orthosteric) site of the receptor, for which a high degree of sequence homology and structural conservation exists across the five mAChRs. This selectivity problem commonly manifests in therapeutics targeting GPCRs with multiple receptor subtypes such as the adenosine, dopamine, and serotonin GPCRs. Another notable caveat of M1 mAChR activation is that it may not necessarily improve coupling between the receptor and the G protein, a critical event that is significantly compromised in the AD prefrontal cortex.45
The Utility of Targeting M1 mAChR Allosteric Sites
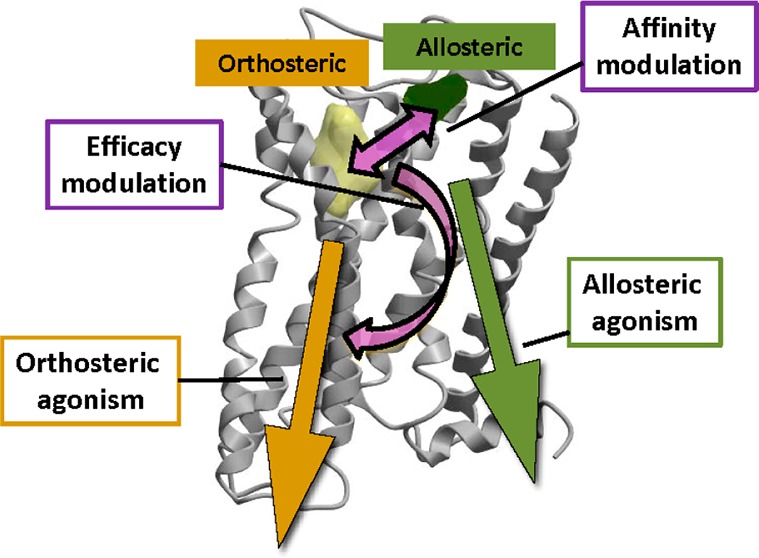
Allosteric ligands bind to a site topographically distinct from the orthosteric site of the receptor and are able to enhance or reduce the effect of the concomitantly bound orthosteric ligand.
The three mechanisms by which allosteric ligands exert their effects,46 as depicted in Figure 5, are as follows:
-
(i)
by modulating the binding affinity of the orthosterically bound ligand;
-
(ii)
by modulating the downstream efficacy of the orthosterically bound ligand; and
-
(iii)
by activating the receptor in their own right, acting as allosteric agonists
Figure 5.
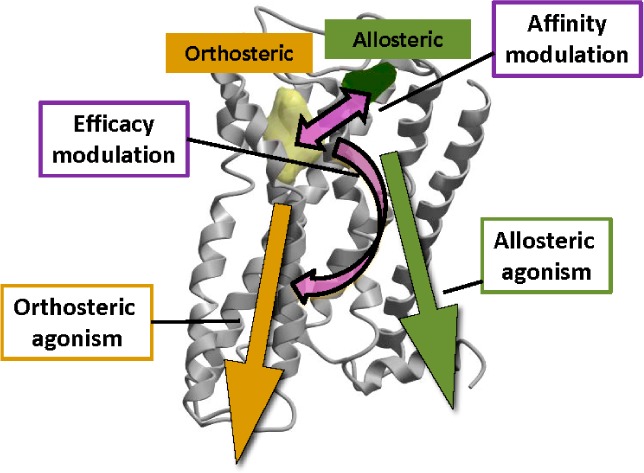
Three modes by which allosteric ligands exert their effects. (1) Orthosteric ligand affinity modulation; (2) orthosteric ligand efficacy modulation; and (3) direct allosteric agonism.
Conformational changes in the receptor induced by allosteric ligand binding are the source of their modulatory activity. Localized alterations to the orthosteric site topography may either enhance or reduce the binding affinity of the orthosteric ligand, while a broader conformational shift in the GPCR may either enhance or reduce the overall signaling efficacy of the receptor. These modulatory effects, combined with any agonism conferred by the allosteric ligand alone, dictate the global change in receptor activity observed. For an elegant representation of the distinct allosteric ligand profiles that may arise from the combination of these three effects, see a recent review by Kenakin.47 To gain an understanding of how the complex multidirectional interplay between an orthosteric ligand, allosteric ligand, and receptor (ternary) complex can be modeled and quantified, see the review by May et al.48
Recently marketed allosteric drugs afford practical validation for the development of allosteric ligands as therapeutic solutions. Cinacalcet49 acts as an allosteric potentiator at the calcium-sensing receptor for the treatment of hyperparathyroidism, maraviroc50 is an anti-HIV drug that acts as an allosteric inhibitor of the chemokine receptor CCR5, and the benzodiazepines,51 used for the treatment of anxiety disorders, are allosteric modulators at GABAA receptors.
Allosteric ligands offer notable advantages over classic orthosteric drugs.52 First, allosteric sites can have a less conserved amino acid sequence between receptor subclasses,53 potentially allowing for selective targeting and minimization of the side effects evident in the binding of pure orthosteric agonists, such as the undesirable peripheral muscarinic effects of xanomeline. Another advantage is the saturability of their effect. Drugs targeting the receptor orthosteric site frequently elicit an all-or-nothing response upon interaction, leading to complete activation or perturbation of downstream signaling and the associated biological function(s). The intensity of action of these ligands is directly proportional to their concentration in the receptor compartment;54 so, depending on the dose that reaches the target receptor and the off-rate of the ligand from the receptor, the duration of effect of orthosteric drugs may carry the risks of side effects, overdose, or desensitization and tolerance.55 In contrast, the effect of a positive or negative allosteric modulator is dictated by the degree of cooperativity they possess with the ligand occupying the orthosteric site,56 such that the intensity of action reaches a limit once all allosteric binding sites are occupied; creating a “ceiling” for their biological effect and potentially avoiding the aforementioned risks.
A somewhat more contentious advantage is the precision of their effect. It is thought that “pure” allosteric modulators (that is, those devoid of agonist activity in their own right) afford the advantage of spatial and temporal specificity52,56 in that they require the presence of the orthosteric ligand to exert their effect, confining their activity to the location and time in which physiological activity is occurring. However, it is now evident that an observed lack of allosteric agonism may also be a function of the ligand concentration, the degree of signal amplification of the pharmacological assay, and the coupling efficiency of the receptor for the signaling pathway being measured.57,58 That is to say that if an allosteric ligand is assessed in an assay with minimal signal amplification that measures a pathway poorly coupled to the receptor of interest, any direct allosteric agonism of the ligand may go undetected. Indeed, it can be reasonably hypothesized that all positive allosteric modulators may display allosteric agonism and all negative allosteric modulators may display inverse agonism, under the right assay conditions.2 Therefore, in order to more accurately predict the functional profile of an allosteric ligand in preclinical animal studies and subsequent clinical trials, in vitro cellular assays that follow initial hit detection ought to resemble the cellular background, native receptor expression levels, local neurotransmitter concentration, and biochemical outcomes of the human pathophysiological tissue of interest as closely as possible. This is likely to pose complex challenges, especially for CNS disorders in which multiple brain regions (with different receptor expression levels and neurotransmitter concentrations) are being targeted to achieve in vivo efficacy.
M1 mAChR allosteric ligands may provide solutions to the problems hindering the development of an optimized cholinergic treatment for cognitive dysfunction. Their subtype selectivity will facilitate further investigation into the physiological and pathophysiological roles of this receptor in the brain, as well as offer the prospect of neurodegenerative disorder therapies devoid of side effects. Additionally, while their ability to modulate endogenous neurotransmitter activity affords the prospect of improved cholinergic tone, the potential for allosteric activation of the M1 mAChR in their own right becomes all the more critical as functional ACh levels decrease in tandem with neurodegeneration and disease state progression. Moreover, the ability of such ligands to stabilize distinct receptor conformations may lead to the discovery of functional profiles that bias the receptor toward neurotrophic and pro-cognitive pathways while disfavoring pathophysiological and neurotoxic pathways.
Structural Studies of M1 mAChR Orthosteric and Allosteric Sites
Until recently, modeling of the muscarinic GPCR family has been largely based on the rhodopsin,59 β-adrenergic,60 and A2A adenosine61 GPCR crystal structures, other Family A GPCRs with which they share common structural motifs.62 However, recent publication of the inactive human M2 and the rat M3 mAChR crystal structures63,64 will undoubtedly provide unparalleled insight into the structures of the other muscarinic receptor subtypes going forward.
Studies of the cloned M1–M5 mAChRs have revealed a high degree of sequence homology between the subtypes, particularly in transmembrane (TM) units 2–7, a region containing a highly structurally conserved hydrophilic cavity.65 Within this cavity projects a TM3 negatively charged aspartate residue common to all subtypes, which has been shown by binding and point-mutation studies to be critical for orthosteric ligand binding,66 forming an ionic interaction with the polar, positively charged (or ionizable) headgroup of both agonists, such as acetylcholine, and antagonists, such as atropine (4 and 5, Figure 6). A combination of computational and molecular biological techniques have identified additional residues extending into this pocket as having key roles in orthosteric ligand binding, kinetics, and receptor activation.67
Figure 6.
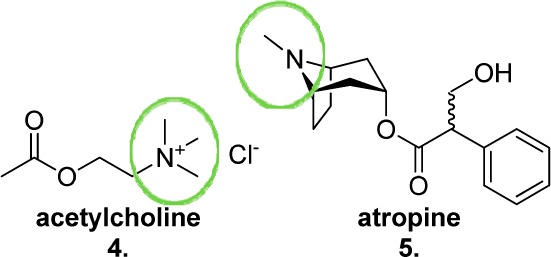
Structures of the muscarinic orthosteric agonist acetylcholine (4) and the muscarinic orthosteric antagonist atropine (5), highlighting the positively charged and ionizable head groups, respectively.
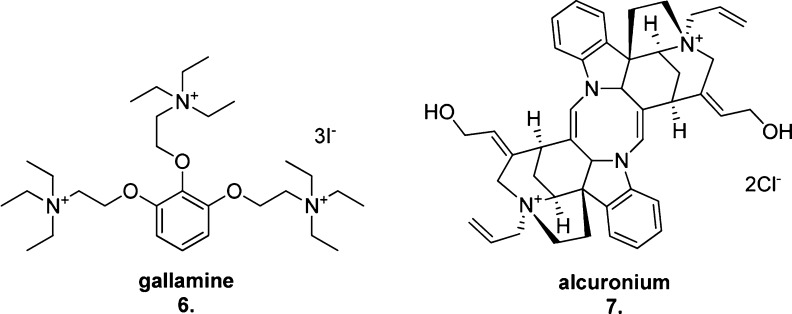
Similar approaches have been adopted to identify the key residues involved in the interaction of allosteric ligands with the M1 mAChR. To date, two M1 mAChR allosteric binding sites have been pharmacologically characterized. Early binding and site-directed mutagenesis studies using the allosteric muscarinic ligands gallamine (6) and alcuronium (7) provided evidence for the first site, an extracellular (EC) allosteric binding domain involving residues from EC loops 2 and 3 adjoining TMs 5, 6, and 7.68 This site is often referred to as the “common” allosteric binding site69 for mAChRs as its topographical location is preserved across the subtypes, while the nonconserved residues in this region confer allosteric ligand mAChR subtype selectivity. More specifically at the M1 mAChR, the positive charges present on both gallamine and alcuronium (Figure 7) are thought to be important for interaction with negatively charged EC3 residues (S388, D393, and E397) extending into the hydrophilic EC space, while the hydrophobic portions of these molecules are thought to form interactions with tryptophan residues (W101 and W400) within upper TM units.70
Figure 7.
Structures of the two “common” muscarinic allosteric site binders, gallamine (6) and alcuronium (7).
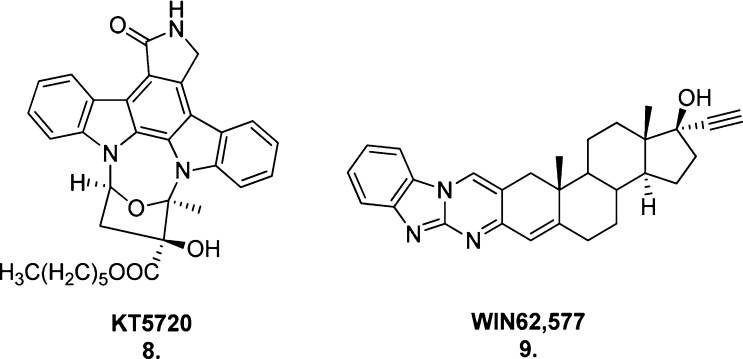
The existence of a second allosteric site was proposed when the ability of the indolocarbazole KT5720 (8, Figure 8) to allosterically enhance the binding of both the agonist ACh and the antagonist N-methylscopolamine (NMS) at the M1–M4 mAChRs was unchanged in the presence of the allosteric ligand gallamine.71 This is indicative of two separate allosteric binding sites and, as neither allosteric ligand altered the potency or relative effectiveness of the other when co-bound, they exhibit neutral cooperativity with one another.72 Additional muscarinic allosteric ligands reported to bind to this second site are WIN62,577 (9) and its analogues (Figure 8).73
Figure 8.
Structures of the second muscarinic allosteric site binders, KT5720 (8) and WIN62,577 (9).
Using a rhodopsin-based homology model of the M1 mAChR, this second allosteric site was later postulated to be located on the intracellular (IC) face of the receptor close to the G protein-coupling domain, involving residues within TMs 2, 3, and 7, the C-terminal helix 8 and IC loop 3, a proposition that is yet to be experimentally validated.74
The future elucidation of the M1 mAChR structure by X-ray crystallography (and eventual cocrystallization with proposed allosteric ligands) will hopefully further validate the homology model-based structural studies undertaken so far and greatly enhance our understanding of ligand–receptor interactions. Regardless, the existence of multiple allosteric sites on the M1 mAChR adds another layer of complexity to the already complicated field of subtype-selective allosteric ligand design.
Important Considerations in Allosteric Ligand Classification and Evaluation
Positive Allosteric Modulators (PAMs) Possessing Intrinsic Agonism
As alluded to earlier, it may be the case that all positive allosteric modulators may display allosteric agonism in their own right if evaluated in a sufficiently amplified pharmacological assay. Therefore, classifying a ligand as a “pure” allosteric modulator should be done tentatively and with due consideration to the context in which the ligand has been tested.
Estimating Ligand Potency
The M1 mAChR PAM literature regularly reports ligand potency by determining an EC50 value. This value is determined by first generating a concentration response curve (CRC) for an orthosteric agonist of the M1 mAChR (e.g., ACh) using the cellular background and functional assay of choice (e.g., calcium mobilization in CHO cells). From this curve, a single concentration is selected depending on the type of allosteric modulation expected to be observed. If positive modulation is expected, as is the case with the M1 mAChR PAM studies described here, an EC20 of ACh may be selected (Figure 9a), the concentration at which 20% of the maximal system response to the ligand is elicited, affording a suitable “window” in which to detect the anticipated modulatory activity.
Figure 9.
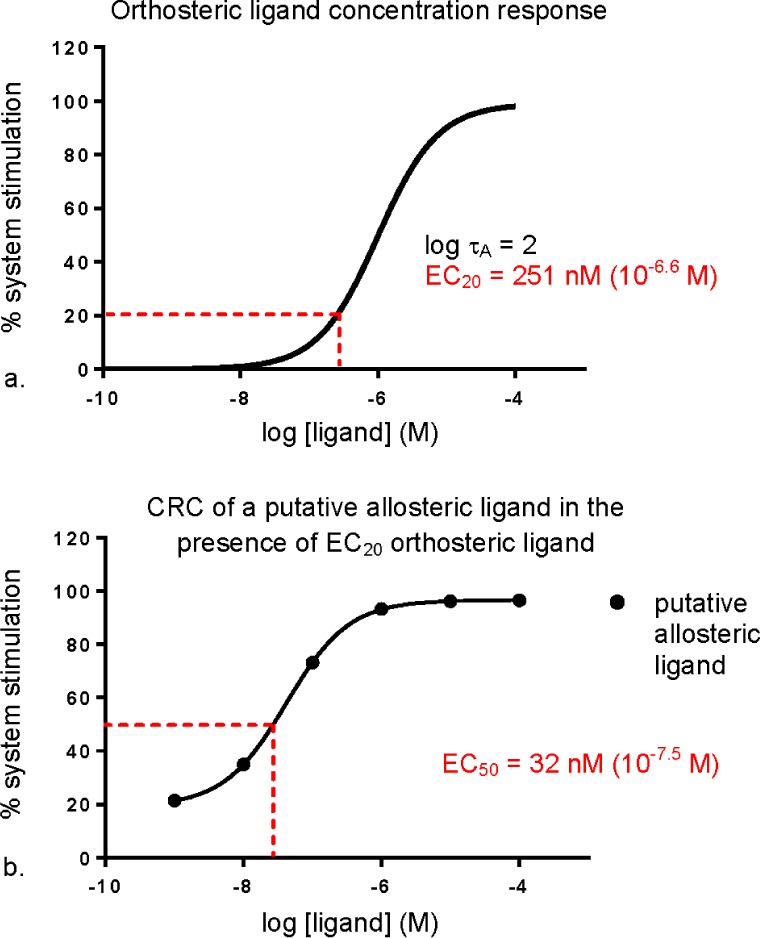
Simulated example of a common screening method for putative allosteric ligands. (a) A concentration–response curve of a known orthosteric ligand (e.g., ACh) used to derive an EC20 concentration value; log τA is the operational efficacy parameter of the orthosteric ligand (the higher the value, the greater the efficacy). (b) How a concentration response curve of a putative allosteric ligand might look in the presence of the derived EC20 concentration of orthosteric ligand.
This predetermined concentration of orthosteric ligand is then coadded with increasing concentrations of the test allosteric ligand in the same cellular background and functional assay, and, assuming the predicted modulation is observed, the potency of the allosteric modulator is equated to the EC50 value, the concentration at which 50% of the maximal system response to the allosteric ligand in the presence of a fixed concentration of orthosteric ligand is elicited (the point of inflection of the titration curve, see Figure 9b).
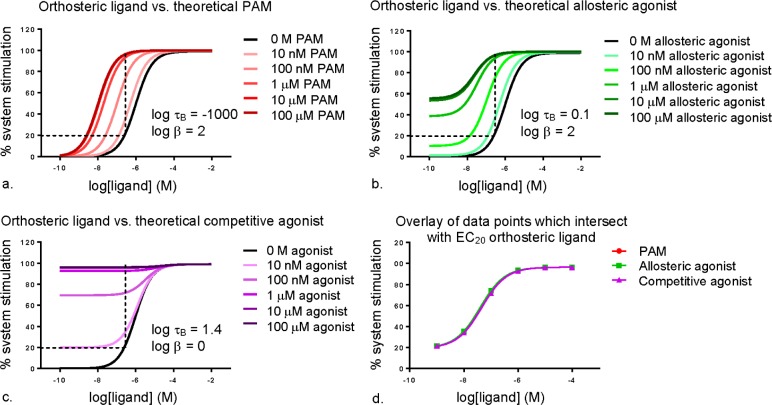
This is a typical and efficient method for screening for activity of putative ligands, and can provide preliminary information about the mode of action of the ligand. However, by virtue of screening against a single concentration of orthosteric ligand, this method examines a one-dimensional cross section of the putative ligand’s pharmacological profile, a cross section that may not necessarily capture the true behavior of the ligand. An interaction study in which the full CRC of the orthosteric ligand is assessed against increasing concentrations of the putative allosteric ligand is necessary to confidently classify the mode of action of the ligand. As demonstrated in Figure 10, a PAM, an allosteric agonist, and a competitive orthosteric agonist would all appear the same when coadded with a single orthosteric ligand concentration (note that Figure 9b and Figure 10d are indistinguishable). It is not until interaction studies over a range of orthosteric ligand concentrations are performed that the mode of action of the test ligands can begin to be deduced (Figure 10a–c).
Figure 10.
Simulated example of interaction studies between an orthosteric agonist and (a) a positive allosteric modulator (PAM), (b) an allosteric agonist, and (c) a competitive agonist, with broken lines indicating the intersection of the concentrations of test ligand with the EC20 of the orthosteric ligand. (d) An overlay of the data points from graphs (a)–(c) which intersect with the EC20 of the orthosteric ligand; log τB is the operational efficacy parameter of the allosteric ligand (the higher the value, the greater the efficacy; the high negative value represents a complete absence of allosteric ligand efficacy); log β is the functional cooperativity factor between the orthosteric and allosteric ligands (log β > 0 = positive cooperativity, log β = 0 = neutral cooperativity or, in this instance, direct competition between two orthosteric ligands).
A combination of functional and radioligand binding assays is necessary to determine whether the allosteric ligand’s modulatory activity is elicited primarily through modulation of binding affinity, signaling efficacy, or a combination of the two. These experiments will also delineate its modulatory effects on the orthosteric ligand from any agonistic response in its own right. These parameters can be estimated via application of the allosteric ternary complex model for binding experiments and the operational model of allosterism for functional experiments.48 It is important to note that, even if this more detailed pharmacological characterization of the allosteric ligand is performed, the estimates of affinity, efficacy, and cooperativity obtained are only applicable in the context of the orthosteric probe utilized, the functional assays performed, and the cellular background under investigation.
Acknowledging Unique Allosteric Properties
Allosteric ligands possess a number of inherent properties that necessitate broader pharmacological evaluation than their orthosteric counterparts. Two fundamental pharmacological properties that can be engendered by GPCR allosteric ligands are stimulus bias75 and probe dependence.76 These terms refer to the differing allosteric modulatory effects observed depending on the cellular signaling pathway being assessed by the assay, and the orthosteric ligand cobound to the receptor, respectively.
The foundation of stimulus bias is the stabilization of distinct receptor conformations. In the same way that different orthosteric agonists and antagonists stabilize multiple active and inactive receptor states, respectively, allosteric ligands may stabilize diverse conformations that result in differential activation of receptor signaling pathways, or collateral efficacy.54 Such molecular tools may afford a greater understanding of the complex relationship between receptor conformations, discrete signaling profiles, and particular physiological and pathophysiological outcomes.
Probe dependence is an important property to consider during the processes of hit identification and lead optimization of allosteric ligands. If the orthosteric probe used to screen and elucidate the structure–activity relationships of novel allosteric chemical scaffolds is not the pathophysiologically relevant endogenous neurotransmitter, the risk is that the novel ligand may exhibit a different, potentially undesirable functional profile (or no function at all) in an in vivo setting.
An excellent mAChR exemplar of these two properties is the M4 mAChR allosteric ligand LY2033298 (10, Figure 11).77 Leach et al.57 performed radioligand binding, [35S]GTPγS, calcium mobilization, ERK1/2 phosphorylation, GSK-3β phosphorylation, and receptor internalization assays to fully assess the allosteric capabilities of LY2033298. They found that the degree of allosteric potentiation conferred by LY2033298 varied widely depending on the signaling pathway being examined, ranging from modest positive modulation of ACh activity in [35S]GTPγS assays to robust enhancement of ACh activity in receptor internalization studies. Estimates of the degree of allosteric agonist activity also varied, further illustrating the property of stimulus bias. This also disproved the original classification of the ligand as a positive allosteric modulator with no agonist activity in its own right.77 Radioligand binding assays were used to highlight the probe dependence of LY2033298; its allosteric agonism and modulatory effects were only detected when cobound with an orthosteric agonist, not an antagonist.
Figure 11.
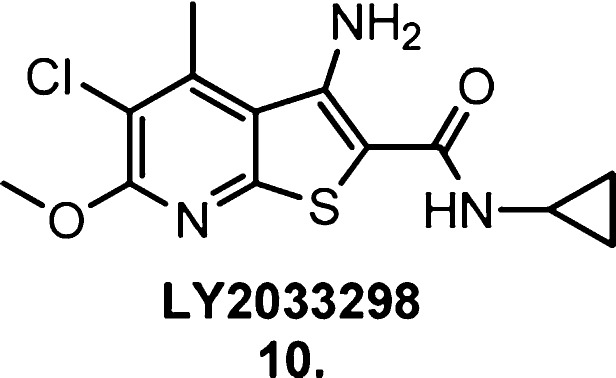
Structure of the M4 mAChR allosteric ligand LY2033298 (10).
LY2033298 also revealed the ability of allosteric ligands to exhibit species-dependent differences in cooperativity.78 This later led to the hypothesis that the proposed subtype selectivity of the modulator may arise from differential cooperativity, rather than variability of the allosteric pockets between receptor subtypes.76 Despite LY2033298 exhibiting selective ACh potentiation at the M4 mAChR, extensive evaluation of LY2033298 at wild-type and mutant M2 mAChRs revealed marked degrees of probe dependence with a range of agonists and antagonists in radioligand binding assays, as well as both positive and negative cooperativity in two functional assays, [35S]GTPγS binding and pERK1/2.
These findings highlight the highly contextual nature of allosteric effects and the importance of assessing binding, multiple functional pathways, different receptor subtypes, and a broad range of probes to fully characterize allosteric ligands.
Identifying a Purely Allosteric Mode of Action
For a ligand to be classified as an allosteric agonist at a particular receptor, it must possess intrinsic efficacy in its own right, regardless of whether an orthosteric ligand is co-bound to the receptor.46 Its agonistic effect must be elicited solely via an allosteric mechanism, and the absence of direct activation via orthosteric site binding must be established.
Comprehensive radioligand binding and functional assays unequivocally demonstrated that the agonistic and modulatory behavior of LY2033298 at the M4 mAChR was elicited via a purely allosteric mechanism;57 with four key observations confirming this hypothesis.
In radioligand binding assays:
-
(a)
LY2033298 exhibited a lack of significant affinity modulation of the muscarinic orthosteric antagonists NMS and QNB (3-quinuclidinyl benzilate), indicating no overlap with the orthosteric binding site
In [35S]GTPγS binding assays:
-
(b)
coaddition of the mAChR antagonist atropine caused a substantial reduction in the maximal effect of LY2033298 with a minimal change in potency, characteristic of noncompetitive negative cooperativity
-
(c)
LY2033298 demonstrated a competitive interaction with the mAChR negative allosteric modulator C7/3-phth, known to bind the “common” muscarinic allosteric site79
-
(d)
LY2033298 exhibited neutral cooperativity upon coaddition with WIN51708, a known “second allosteric site” mAChR ligand,73 confirming the allosteric agonist’s site of action to be the “common” allosteric site on the M4 mAChR
The Prospect of Bitopic Interactions
While the M1 mAChR-selective ligand literature reviewed herein provides substantial evidence to suggest purely allosteric modes of action, there exist several examples where this evidence is inconclusive. Such ligands have hence been termed “putative bitopic M1 mAChR agonists” in this Review. It has been noted that these ligands exhibit unique binding modes and divergent functionality when compared to M1 mAChR PAMs.80,81 Depending on the experimental paradigm, many of these molecules have exhibited degrees of antagonistic interaction with different orthosteric mAChR ligands. While they may be acting as pure allosteric agonists possessing very high negative cooperativity with orthosterically bound ligands, it is equally if not more likely that they are interacting with the orthosteric site and an allosteric site simultaneously, behaving as “bitopic” ligands (also termed “dualsteric” or “multivalent”) (Figure 12).
Figure 12.
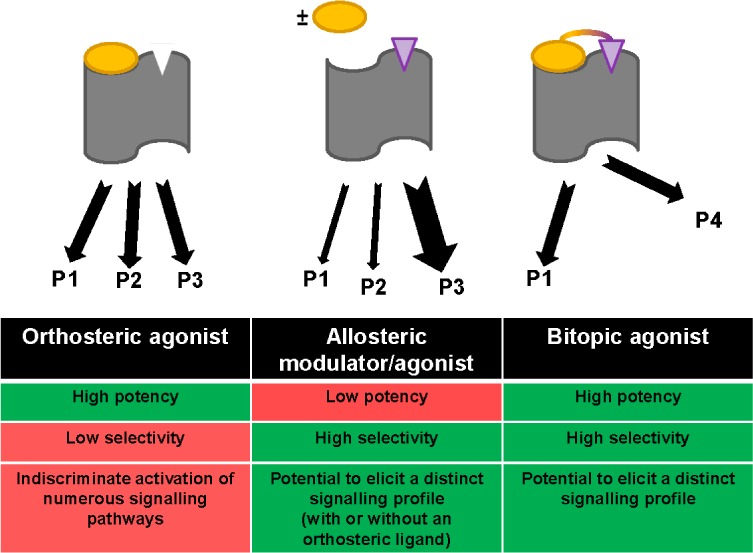
Advantages and disadvantages of orthosteric and allosteric ligands, and the theoretical fusion of their respective advantages by bitopic ligands: high potency, high subtype selectivity, and the potential to elicit distinct signaling profiles (Ps) that may be of therapeutic value.
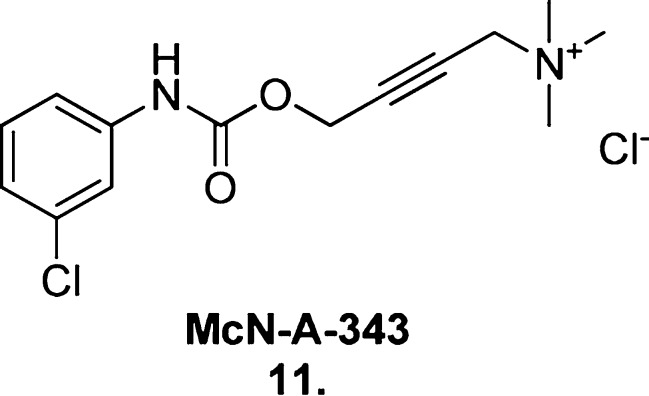
This unique mode of ligand–receptor interaction has been validated82 by a number of proof-of-concept studies (see Valant et al.83 for a review). Notably, the conclusive classification of McN-A-343 (11, Figure 13) as a bitopic ligand,84 initially thought to be an M2 mAChR allosteric partial agonist,85 highlights the detailed level of synthetic manipulation and pharmacological characterization necessary to appropriately classify the mode of action of a bitopic ligand. Just as McN-A-343 was truncated to form small molecular derivatives that were used to identify the orthosteric and allosteric portions of the molecule, similar methods will need to be employed to confirm the true nature of the putative bitopic M1 mAChR agonists described within.
Figure 13.
Structure of the M2 mAChR bitopic ligand McN-A-343 (11).
M1 mAChR-Selective Ligands
Over the past two decades, a structurally diverse array of M1 mAChR-selective ligands have been identified and characterized. Two distinct groups of these ligands will be reviewed herein: M1 mAChR PAMs and putative bitopic M1 mAChR agonists. Orthosteric M1 mAChR ligands are beyond the scope of this Review. The methods used and the implications for the detection, optimization, and development of novel treatments for cognitive deficits of neurodegenerative conditions, and indeed any allosterically derived treatment, will also be discussed.
M1 mAChR PAMs
Brucine
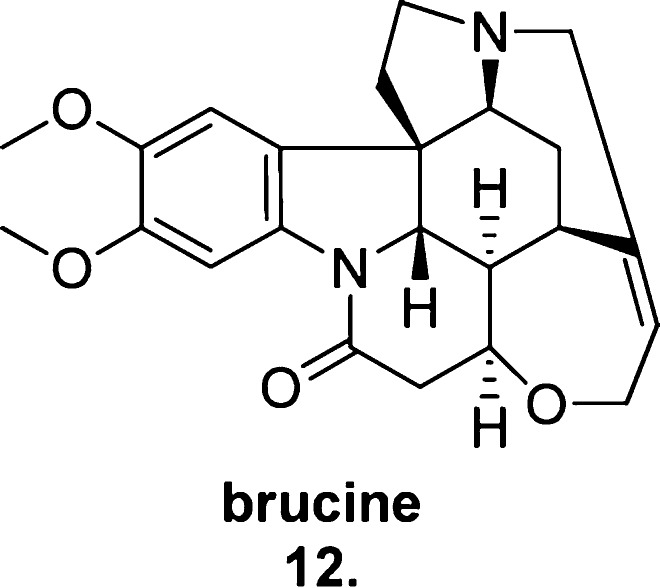
In 1995, Lazareno and Birdsall86 identified the naturally occurring alkaloid strychnine as an M1-, M2-, M4-preferring allosteric ligand shown to possess low negative cooperativity with ACh, prompting the researchers to investigate a modified series of analogues with the intent of identifying ligands that possessed positive cooperativity with ACh as well as greater subtype selectivity.
The result was the discovery that the structurally related natural product brucine, 10,11-dimethoxy strychnine (12, Figure 14), exhibits modest positive cooperativity with ACh in both equilibrium binding experiments and functional assays (such as the [35S]-GTPγS assay and the calcium mobilization assay) at the M1 mAChR.87 Unfortunately, brucine exhibited low affinity (0.1 mmol for the ACh-occupied receptor), minimal cooperativity (1.5–2-fold enhancement of ACh activity) and required high micromolar concentrations to produce any detectable activity. This, combined with its poor pharmacological profile, rendered the compound insufficient for use in native tissue and animal studies, and off-target activity suggested that the scaffold was unfavorable for further M1 mAChR PAM analogue development. However, brucine provided important validation for selective allosteric modulation of M1 mAChR activity as a viable field of therapeutic research.
Figure 14.
Structure of the M1-preferring allosteric ligand brucine (12).
Benzyl Quinolone Carboxylic Acid, BQCA
An exciting development in the pursuit of selective M1 mAChR allosteric potentiators was Merck’s discovery of BQCA (13, Figure 15),88 by far the most actively investigated scaffold in this field to date. BQCA is the first reported orally bioavailable M1 mAChR allosteric ligand exhibiting absolute subtype selectivity; no activity was detected at the other four muscarinic receptor subtypes in calcium mobilization assays (concentrations up to 100 μM). A notable feature of BQCA is its high degree of cooperativity; 100 μM of BQCA induced a robust 129-fold leftward shift in the ACh CRC (estimated from fluorometric imaging plate reader (FLIPR) calcium mobilization assays). Furthermore, BQCA produced an inflection point value of 845 nM in the presence of 3 nM ACh. A combination of molecular modeling, site-directed mutagenesis, and radioligand binding studies revealed the residues Y179 and W400 to be important to the BQCA–receptor interaction, which is suggestive, but not conclusive, of the modulator exerting its effects via the “common” allosteric site.
Figure 15.
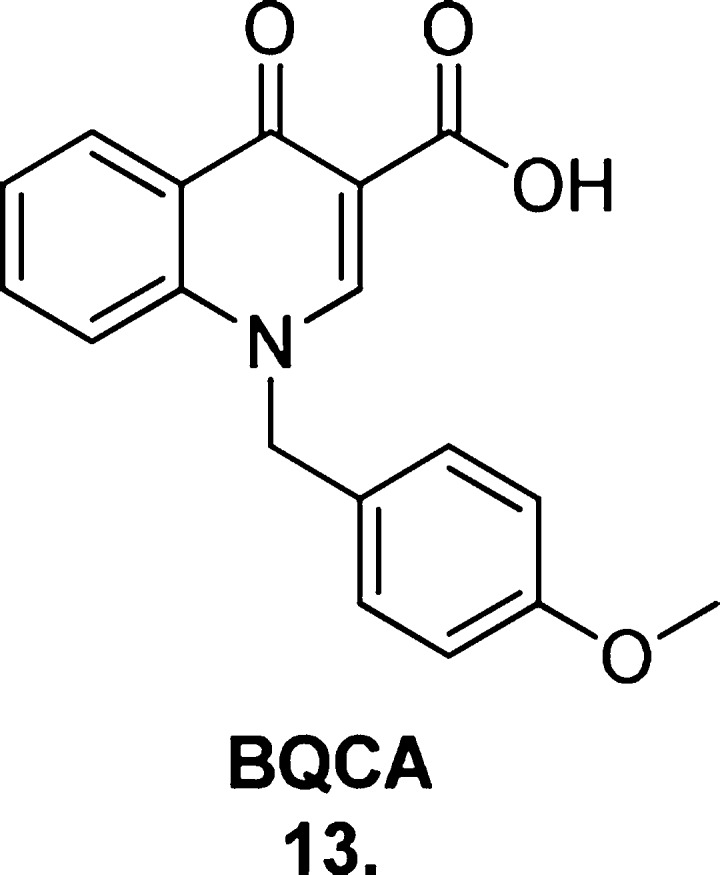
Structure of the breakthrough M1-selective mAChR PAM BQCA (13).
Interestingly, it was recently reported that BQCA is the first allosteric GPCR ligand proven to abide by a strict two-state Monod-Wyman-Changeux (MWC) model of receptor activation,2 in that its degree of modulation tracks with both the intrinsic efficacy of the cobound orthosteric ligand and the coupling efficiency of the receptor for a given intracellular signaling pathway.58 Therefore, BQCA displays no stimulus bias, but still shows probe dependence by stabilizing the active receptor state favored by orthosteric agonists. As a result, it exhibits positive modulation when co-bound with orthosteric agonists and negative modulation when co-bound with orthosteric antagonists. It has been postulated that the inability of BQCA to stabilize a discrete receptor conformation arises from the ligand possessing few chemical functionalities capable of forming strong receptor interactions. Furthermore, the same study demonstrated that BQCA behaves as both a positive allosteric modulator and an allosteric agonist.58
BQCA possesses a sufficient pharmacological profile for analysis in both native tissue assays and in vivo animal studies. BQCA potentiated phosphorylated ERK production and RNA expression of the neuronal activation markers c-fos and arc in critical forebrain regions of the mouse brain where cholinergic degeneration in human AD patients is most pronounced.
Importantly, BQCA displayed efficacy in a contextual fear conditioning (CFC) mouse model of cognitive dysfunction. Mice dosed with 15–20 mg/kg BQCA exhibited behaviors suggesting a reversal of the scopolamine-induced block of memory formation.88 As BQCA was shown to have no effect on [3H]NMS affinity in radioligand binding assays (at concentrations up to 30 μM), this reversal of induced memory deficits was proposed to be solely due to BQCA’s allosteric enhancement of endogenous ACh activity, decreasing the concentration of agonist required to displace the antagonist.
The short-term memory of acquired fear (as observed in the CFC model) is linked to the hippocampus, in which the M1 mAChR is expressed in abundance. However, M1 mAChR KO mice have shown intact cognitive processes associated with hippocampal-dependent learning89 and no impairment in mAChR-mediated hippocampal pyramidal cell excitation.90
Shirey et al.91 reported evidence indicating the M1 mAChR is more likely to have a role in prefrontal cortex (PFC)-dependent learning, so the ability of BQCA to potentiate the carbachol (muscarinic agonist)-induced inward current (excitability) of medial (m)PFC pyramidal cells in rat brain slices was examined. BQCA increased the intensity and frequency of carbachol-induced spontaneous excitatory postsynaptic currents (sEPSCs), effects that were not observed in brain slices of M1 mAChR KO mice. In support of these observations, another electrophysiology study demonstrated that coapplication of BQCA enhanced synaptic stimulation by carbachol in mPFC pyramidal cells, subsequently producing long-term depression (LTD), a critical process that, in conjunction with long-term potentiation (LTP), mediates synaptic plasticity.92 These findings translated well to in vivo electrophysiology studies in which BQCA induced an elevation in the spontaneous firing rate of mPFC neurons in rats.91
In complement to these findings, further in vivo analysis using a mouse model of Alzheimer’s disease (Tg2576 mice; genetically modified to overexpress an amyloidogenic familial AD mutant form of APP) indicated that BQCA improved the performance of these mice in a discrimination reversal learning test, a PFC-dependent learning task.91 The propensity for error was almost 7 times lower in BQCA-treated Tg2576 mice compared to controls. BQCA was found to be taken up into the brain between 30 min and 1 h after dosing, and maintained a constant level for 4 h; however, concentrations in the brain were found to be substantially lower than that in systemic circulation. Furthermore, BQCA (in the presence of carbachol) promoted the nonamyloidogenic pathway of APP cleavage in PC12 cells overexpressing human APP and M1 mAChRs, as measured by increased levels of the protein fragments released by α-secretase cleavage of APP.
Overall, these studies validate the hypothesis that selective enhancement of M1 mAChR activity in the PFC may lead to improved cognitive function in human patients, whether by directly enhancing PFC function and/or by modulating hippocampal function via cortical projection neurons. BQCA has shown efficacy in additional animal models of cognition that lend support to this hypothesis. A 10 mg/kg dose of BQCA attenuated scopolamine-induced memory deficit in a spontaneous alternation task93 and prevented natural forgetting94 in rats, suggestive of enhancement of both hippocampal- and PFC-dependent memory processes. Taken together, these studies endorse the investigation of M1 mAChR PAMs as therapeutics for the symptoms and pathophysiology of AD.
Despite its many advantageous properties, critical deficits of BQCA include its low affinity (105 μM as estimated by Canals et al.58) and low free fraction (FF) of 1–4% (percentage of compound unbound by blood plasma proteins). However, the BQCA structure is highly amenable to optimization, affording the same benefits to medicinal chemists as conventional fragment hits do: a promising core scaffold with affinity for the target of interest, multiple points for diversification facilitating thorough structure–activity relationship (SAR) studies, and the ability to add functionalities for physicochemical optimization without exceeding a MW of 500 Da. This latter consideration is especially important for compounds like BQCA which require blood-brain barrier (BBB) permeability to reach their therapeutic target; a modified version of the Rule of Five suggests a MW < 450 Da for BBB passage.95
The multiple points for diversification on the BQCA scaffold have since resulted in a plethora of SAR studies that have generated a wide variety of analogues, some of which display substantial improvement on the characteristics of the parent compound. It is important to note however that all subsequent BQCA analogues described here were evaluated in only one functional assay against a single orthosteric ligand concentration, equating compound potencies to the EC50 (point of inflection) of the compound’s CRC in the presence of an EC20 of ACh at human M1 mAChR-expressing CHO cells using FLIPR calcium mobilization assays. Hence, the allosteric properties of stimulus bias and probe dependence remain untested.
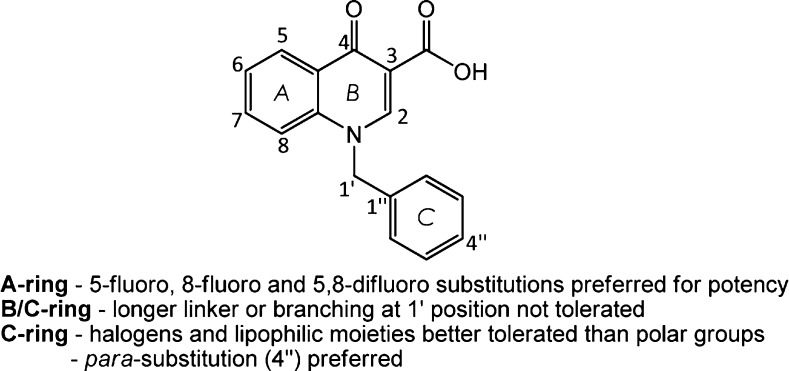
After examining substituents with differing properties such as size, lipophilicity, and polarity, Yang et al.96 derived some fundamental features they found to be critical to the scaffold’s M1 mAChR potency, summarized in Figure 17. Note that A-, B-, C-, and D-ring labeling will be used for ease of communication from this point onward.
Figure 17.
Summary of the pharmacophoric features reported by Yang et al.96
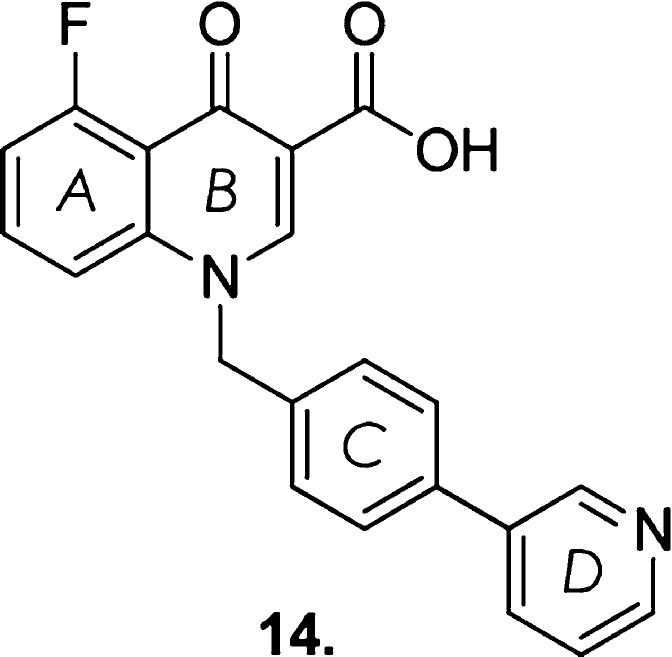
Of the ∼100 analogues synthesized and tested, the compound possessing the best balance of potency and FF was compound 14 (Figure 16); EC50 = 61 nM, hFF = 5.5%, rFF = 5.1%. However, subsequent evaluation of compound concentration in CSF after oral dosing in rats (a surrogate measure for CNS exposure) yielded poor results, and was most likely the result of compound 14 being later confirmed as a P-glycoprotein (P-gp) substrate, one of the efflux transporters present in the BBB.
Figure 16.
Structure of compound 14.
The most extensive BQCA analogue studies to date have been reported by Kuduk and co-workers.97−105 Table 1 provides a summary of the most notable compounds from each series of structural modification; including their potency, favorable pharmacological properties and notable caveats.
Table 1. Summary of the BQCA Analogues Possessing the Best Balance of Properties, and Any Notable Caveats (as reported by Kuduk et al.).
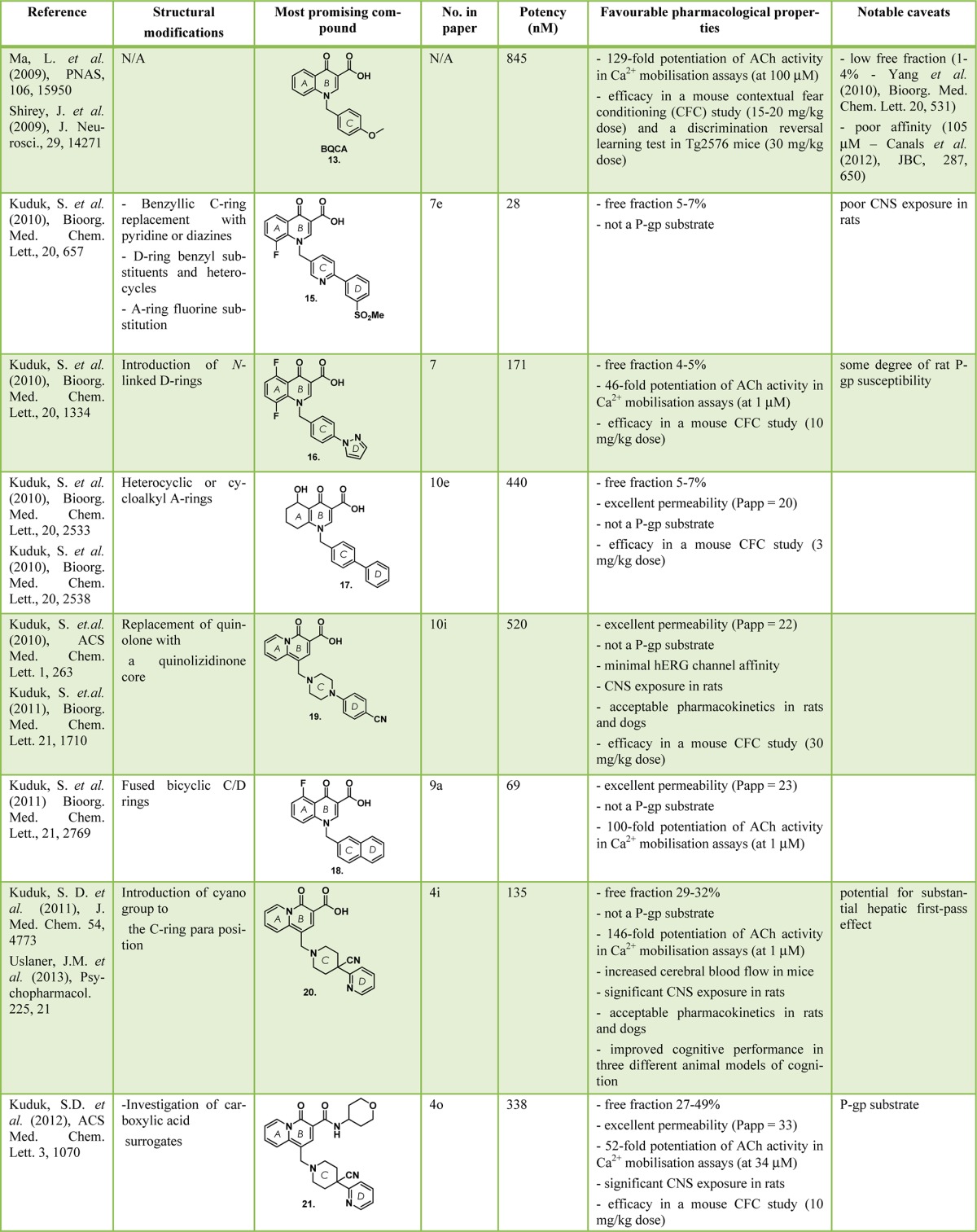
Figure 18 provides a summary of the key SAR findings of these same publications. Challenging issues faced during these studies included the often confounding SAR, and difficulties in obtaining an optimal combination of pharmacological and pharmacokinetic properties. Additional patented structures discovered by this group not included in this Review are summarized in a recent publication.106
Figure 18.
Summary of the pharmacophoric features reported by Kuduk et al.
Vanderbilt University Compounds
Using high-throughput screening, researchers at Vanderbilt University investigated a broad range of chemical space to identify novel, structurally distinct chemical scaffolds that produce substantial allosteric modulatory activity at the M1 mAChR.107
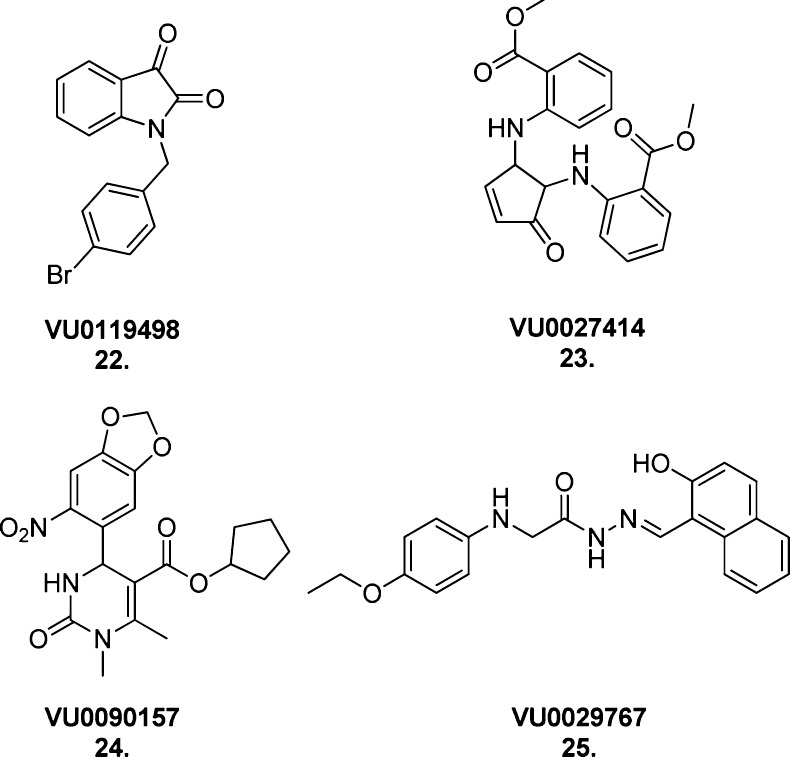
At a concentration of 10 μM, 1634 compounds significantly increased the muscarinic agonist carbachol’s EC20 response above control levels in CHO cells expressing the rat M1 mAChR. Subsequent screens of the more potent ligands confirmed 105 compounds (at 30 μM concentration) as positively modulating an EC20 concentration of ACh by measurement of intracellular calcium mobilization in the same cellular background. Four structurally diverse hits were selected for further analysis (22–25, Figure 19).
Figure 19.
Structures of the structurally diverse muscarinic allosteric ligands VU0119498 (22), VU0027414 (23), VU0090157 (24), and VU0029767 (25).
An allosteric mode of action was confirmed using [3H]NMS binding experiments; none of the test compounds demonstrated a competitive interaction with the orthosteric site-bound radio-labeled ligand. Using M1 mAChR calcium mobilization assays as described previously, all four compounds demonstrated positive allosteric modulation of the ACh-induced receptor response and no observed allosteric agonism in their own right. The most substantial enhancements of ACh potency were reported for VU0029767 (25) (15-fold) and VU0119498 (22) (14-fold). Unfortunately, none of the test compounds showed absolute subtype selectivity for the M1 mAChR. As VU0029767 exhibited minimal modulation at the other four receptor subtypes and VU0090157 (24) showed only weak modulation at the M4 mAChR, these two M1 mAChR-preferring PAMs were further analyzed and found to exert their effects at the M1 mAChR primarily through enhancement of ACh affinity for the orthosteric site (as indicated by shifts in the ACh/[3H]NMS competition binding assay curve). The two compounds were tested at both wild-type M1 mAChRs and M1 mAChRs containing the single point mutation Y381A, which replaces a tyrosine residue critical for orthosteric ACh binding with an alanine residue, thereby reducing ACh affinity and potency.
Interestingly, they found that the two ligands exhibited markedly different profiles. The potentiating ability of VU0029767 was preserved between wild-type and mutated receptors, while VU0090157 exhibited no potentiation of the ACh response at the mutated receptor. This suggests that these structurally diverse ligands may possess different degrees of cooperativity with ACh or two distinct binding modes at the M1 mAChR. A shared characteristic, however, is that both ligands appear to stabilize a receptor conformation that renders ACh uncharacteristically tolerant of the Y381A mutation, as evidenced by the preserved 20% basal ACh activity between wild type and mutated receptors. The actual binding modes and sites of both allosteric compounds are thus far unconfirmed.
Additionally, the two ligands were found to induce different functional profiles in the ACh-mediated response of the wild-type receptor; the modulatory function of VU0090157 was preserved across three downstream signaling pathways linked to intracellular calcium release, phosphoinositide hydrolysis and phospholipase D activation respectively, while the degrees of potentiation of VU0029767 across these same pathways varied substantially. This finding highlights the importance of screening putative allosteric ligands across a broad range of functional assays to obtain thorough pharmacological characterization.
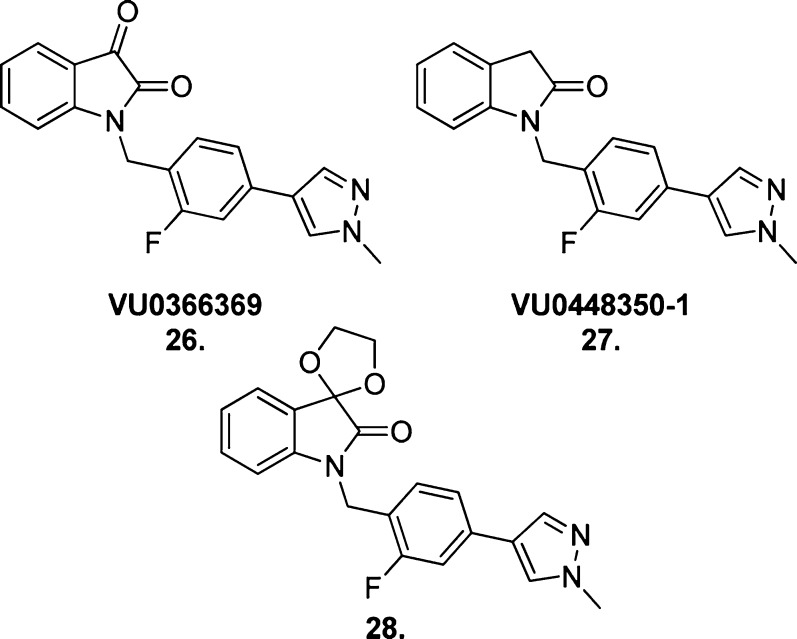
VU0119498 (22) was not pursued in earlier studies107 as it potentiated ACh activity at all three Gq/11-coupled mAChRs, M1, M3, and M5. However, following publication of BQCA,88 to which it bears a resemblance, this compound was pursued as a candidate for further optimization to improve subtype selectivity.108 Previous optimization of this structure to develop an M5-selective PAM demonstrated that modifications to the aromatic left side of the isatin scaffold tended to yield ligands of highly variable activation, potentiation, and selectivity profiles.109 Hence, modifications to the right side, such as chain length alterations, additional benzyl substituents, and introduction of a diverse range of substituted biaryl moieties, were explored.
The result was VU0366369 (26, Figure 20), the second highly selective and potent M1 mAChR PAM reported.108 BQCA and VU0366369 are reported to possess similar M1 mAChR potencies in the same cellular background (EC50 = 845 and 830 nM respectively); however, the latter compound exhibited a markedly low degree of cooperativity (3-fold left shift of ACh CRC at 30 μM) compared to the former (129-fold left shift of ACh CRC at 100 μM). Taking into consideration the known promiscuity of isatin motifs in high-throughput screens, replacement with alternative structures was undertaken.110,111 Replacement with an indolinone (27, Figure 20) resulted in a marked reduction in M1 mAChR potency compared to compound 26 (EC50 = 2.4 μM) but a 10-fold improvement in ACh cooperativity (27-fold left shift of ACh CRC at 30 μM).110 Similarly, spirocyclic replacements for the isatin core largely resulted in potency reductions but improvements in ACh cooperativity, as well as increased subtype selectivity. An exemplar compound from this series was compound 28 (Figure 20).
Figure 20.
Structure of the BQCA-like PAMs VU0366369 (26), VU0448350-1 (27), and compound 28.
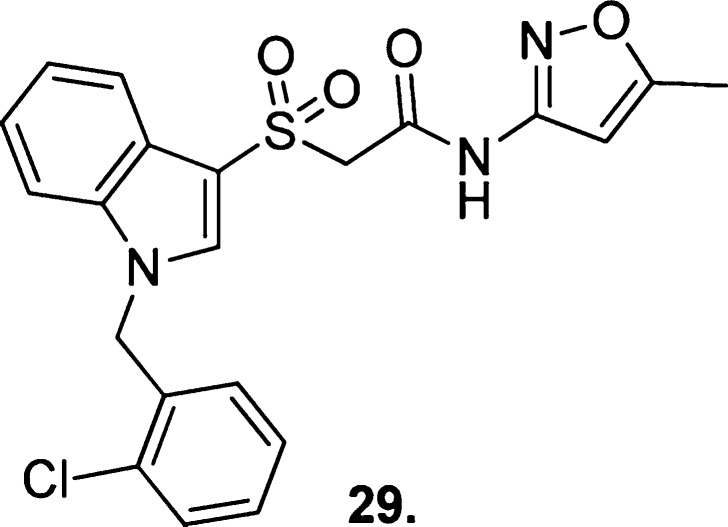
A novel chemical scaffold112 derived from the same high-throughput screen as VU0366369 (26)108 showing weak M1 mAChR PAM activity but high M1 mAChR selectivity was developed into a prospective lead, compound 29 (Figure 21).
Figure 21.
Structure of the novel scaffold compound 29.
After modifications to the large northern portion of compound 29 proved detrimental to activity (evaluated in Ca2+ mobilization assays), synthetic efforts focused on introducing novel substituents to the southern benzyl moiety. 3-Chloro-, 3-bromo-, and 3-pyrazole-substitution gave the most potent analogues (EC50s = 5.8, 3.8, and 2.2 μM, respectively) with highly basic and sterically bulky groups not tolerated at this or any other position.
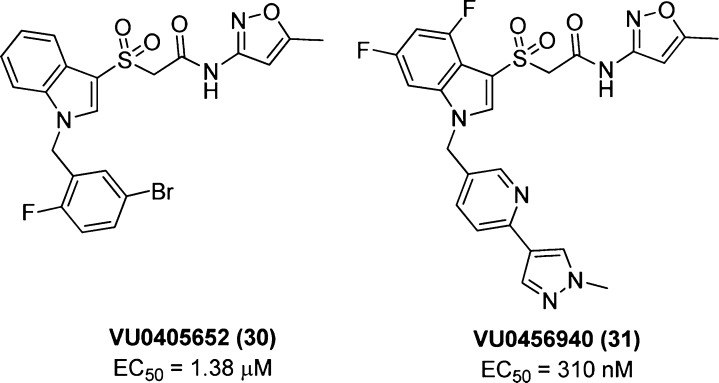
Extensive SAR efforts around this scaffold afforded further pharmacological improvements, resulting in M1 mAChR-selective compounds 30(112) and 31(113) (Figure 22). Compound 30 displayed relatively modest potency (when compared with BQCA and its optimized analogues) but promising positive modulation of ACh activity (at 30 μM). Compound 30 also displayed improved brain penetration compared to that of BQCA, minimal off-target activity and no orthosteric binding at any mAChR subtypes, and was observed to promote nonamyloidogenic processing of APP in conjunction with carbachol. Compound 31 displayed a substantial improvement in potency compared to BQCA and its predecessor compound 30, and potentiated M1 mAChR-mediated, carbachol-induced excitability of striatal medium spiny neurons as well as nonamyloidogenic APP processing.
Figure 22.
Structures of the novel scaffold analogues VU0405652 (30) and VU0456940 (31).
Putative Bitopic M1 mAChR Agonists
ACADIA Pharmaceuticals Compounds
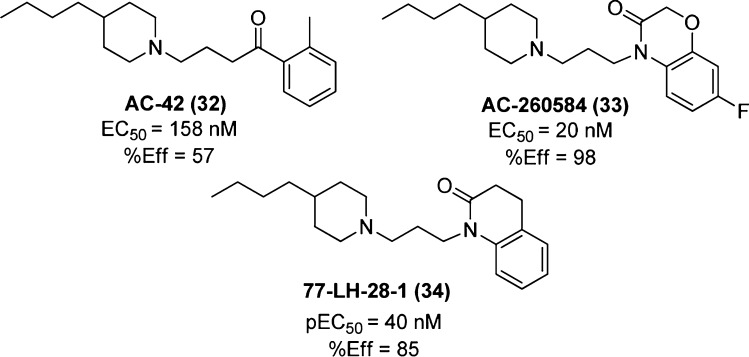
AC-42 (32, Figure 23) selectively activates the M1 mAChR subtype and was shown to bind to a novel combination of M1 mAChR residues within the N-terminus/TM1 and EC3/TM7 regions.114 This novel binding epitope cannot, however, be used as conclusive evidence of a purely allosteric binding mode.
Figure 23.
Structures of AC-42 (32), AC-260584 (33), and 77-LH-28-1 (34); R-SAT functional assays measuring β-galactosidase activity gave estimates of potency, EC50 (converted from pEC50), and efficacy, %Eff (normalized to the maximum response to the muscarinic agonist carbachol).
Extensive pharmacological studies have revealed that AC-42 exhibits characteristics suggestive of both orthosteric and allosteric modes of action.114−118 IP accumulation assays in CHO cells expressing the hM1 mAChR revealed the orthosteric antagonist atropine to decrease the potency of AC-42 as evidenced by a concentration-dependent dextral shift in the AC-42 CRC, characteristic of a competitive interaction.116 In equilibrium binding studies,115 AC-42 almost completely inhibited binding of the radiolabeled [3H]NMS (0.2 nM), indicative of either competitive antagonism via the orthosteric site or a high degree of negative cooperativity via the allosteric site, or perhaps a combination of the two. Further studies confirmed the ability of AC-42 to interact allosterically with the M1 mAChR; the ligand significantly slowed the rate of [3H]NMS dissociation from the M1 mAChR.115
Notably, single point mutations of key orthosteric site residues Y381A and W101A,114,115,117 while reducing the affinity of prototypical orthosteric muscarinic agonists ACh and pilocarpine, actually led to increases in the affinity of AC-42 from that recorded at the wild-type M1 mAChR.115 Furthermore, AC-42 exhibited a divergent signaling and regulatory profile to that of other orthosteric muscarinic agonists such as oxotremorine-M. AC-42 was unable to stimulate M1 mAChR-Gαi1/2 coupling119 and did not result in M1 mAChR internalization after prolonged (24 h) exposure. Rather, it actually upregulated total receptor expression.120 Taken together, these observations support the hypothesis that AC-42 adopts a binding mode distinct from that of traditional nonselective agonists,121 but one which places AC-42 in close proximity to the orthosteric site, implying that its agonistic properties may be derived from interactions with orthosteric residues (as well as allosteric residues); the definition of a bitopic ligand.
AC-42 was superseded by the more potent and efficacious structural analogue, AC-260584 (33, Figure 23), when it was found to exhibit the same high M1 mAChR subtype selectivity as its predecessor but with the added advantages of oral bioavailability in rodents and the exhibition of antipsychotic and pro-cognitive effects in animal models of schizophrenia and AD.122,123 While these results show great therapeutic promise, the ligand’s classification as a purely allosteric agonist is likely premature. AC-260584 exhibited the same potential for binding orthosteric site residues as did AC-42.117
77-LH-28-1 (34, Figure 23), an M1 mAChR-selective compound closely structurally related to the AC compounds, demonstrated agonistic behavior at rat hippocampal M1 mAChRs in vitro and CNS penetration leading to the stimulation of rat hippocampal cell firing in vivo.124 Furthermore, activation of M1 mAChRs by 77-LH-28-1 enhanced NMDA receptor activation in vitro, facilitating long-term potentiation.125 Consistent with the interactions of AC-42, the mAChR orthosteric antagonists pirenzepine and scopolamine both produced a dextral displacement of the 77-LH-28-1 CRC;124 however, 77-LH-28-1 has also been shown to compete with the prototypical muscarinic negative allosteric modulator C7/3-phth (at the NMS-occupied receptor),115 adding weight to the theory that this scaffold interacts with portions of both orthosteric and allosteric sites on the M1 mAChR.
The affinity of 77-LH-28-1, like AC-42, was improved at M1 mAChRs containing the orthosteric mutations Y381A and W101A (pKa = 7.16 and 8.64, respectively) from that recorded at the wild-type (pKa = 6.74). Additionally, a novel M1 mAChR receptor mutation F77I resulted in substantially reduced efficacy of both AC-42 and 77-LH-28-1, while having little effect on the efficacy of traditional orthosteric ligands,115,118 highlighting the importance of this residue in facilitating receptor activation by this scaffold, and the potential utility of this mutated receptor as a detection tool for this novel binding mode. Ligand docking studies performed on an M1 mAChR homology model further support a bitopic binding mode for 77-LH-28-1.115
N-Desmethylclozapine
Clozapine is a second-generation antipsychotic known for its unrivaled efficacy as a dual-action treatment for the positive and negative symptoms of schizophrenia,126 and notorious for promiscuous off-target activity that has rendered it a risky therapeutic option. N-Desmethylclozapine (35, Figure 24) is a biologically active metabolite of clozapine thought to play a role in the parent drug’s unique therapeutic profile.
Figure 24.
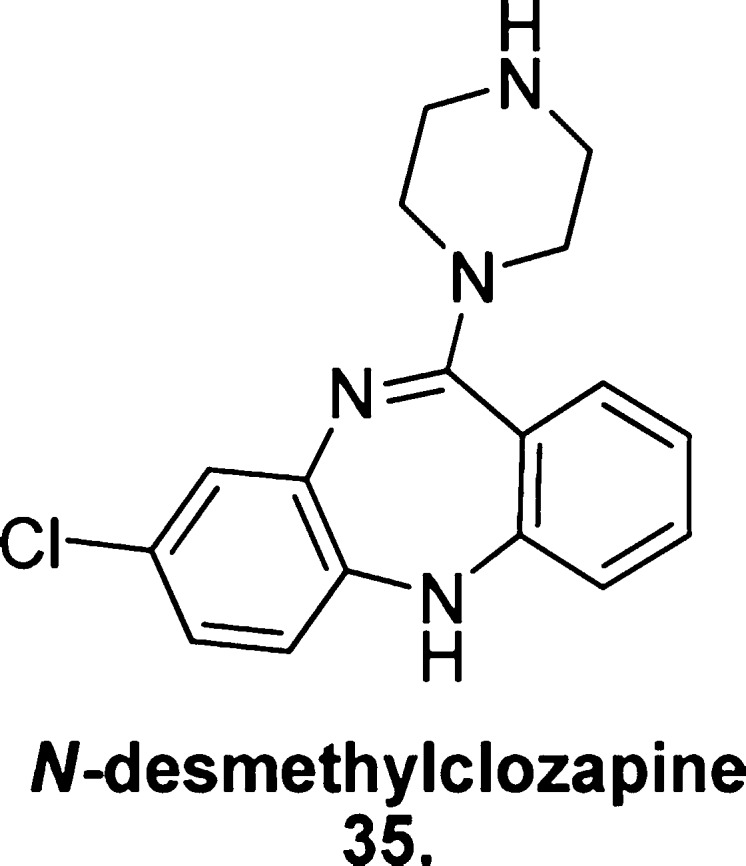
Structure of N-desmethylclozapine (35).
Following an evaluation of the metabolite’s effects at human cloned mAChRs,127N-desmethylclozapine was described as an M1 mAChR-preferring allosteric agonist, indirectly facilitating the potentiation of NMDA receptor activity, shown to be depleted in patients with psychotic disorders. In binding assays, N-desmethylclozapine demonstrated a competitive interaction with the known M1 mAChR allosteric ligand brucine (12), suggesting an allosteric mode of action; however, the activity of N-desmethylclozapine in Ca2+ mobilization assays was completely blocked when coincubated with atropine, suggesting an interaction with the orthosteric site as well. These observations certainly indicate that the ligand may be behaving in a bitopic manner.
N-Desmethylclozapine has since been reported as a human native M1 mAChR antagonist, despite its agonistic activity at human recombinant and rat native M1 mAChRs,128 rendering its therapeutic prospect as a treatment for cognitive deficits obsolete. Disappointing outcomes such as this serve as a lesson for future development, highlighting the importance of evaluating lead compounds not just on rat and human recombinant receptors to assess their viability for in vitro and early in vivo trials, but to examine their activity at human native receptors to ensure their viability as future therapeutics.
Vanderbilt University Compounds
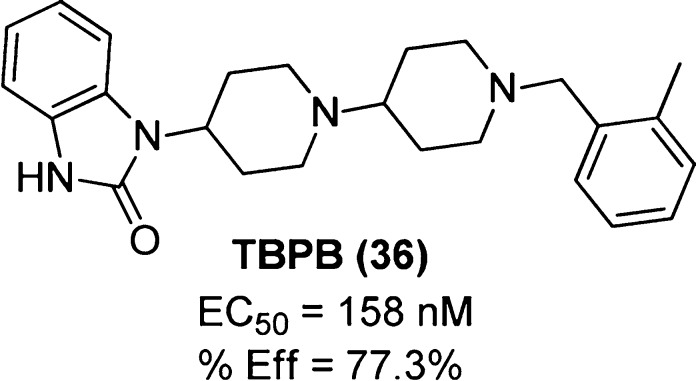
Compared to the scaffolds of previously reported compounds, TBPB ([1-(1′-(2-tolyl)-1,4′-bipiperidin-4-yl)-1H benzo[d]imidazol-2(3H)-one]) (36, Figure 25) represents a structurally unique mAChR ligand that exhibits selectivity for the M1 mAChR at the level of function. TBPB displayed agonistic behavior and potentiates NMDA receptor currents at rat hippocampal M1 mAChRs in vitro and was also shown to promote nonamyloidogenic APP processing in PC12 cells as measured by a decrease in Aβ42 levels and an increase in α-secretase metabolites.129 Furthermore, TBPB elicited antipsychotic-like behavior in rodent models of schizophrenia.
Figure 25.
Structure of TBPB (36). Calcium mobilization assays in rat M1-transfected CHO-K1 cells gave an estimate of potency, EC50, and efficacy, %Eff (normalized to the maximum response to the muscarinic agonist carbachol).
In calcium mobilization assays at the rM1 mAChR, TBPB displayed an apparent noncompetitive interaction with the orthosteric antagonist atropine. However, as this assay does not assess the ligands in an equilibrated state, this is not conclusive of an allosteric mode of action. Indeed the TBPB-mediated modulation of APP processing in PC12 cells expressing the hM1 mAChR was completely blocked by atropine which, as previously explained, could either be the result of a very high degree of negative cooperativity between the two ligands when concomitantly bound at the orthosteric site (atropine) and the allosteric site (TBPB), or evidence of a competitive interaction. Hence, the binding mode of TBPB is unclear and remains unresolved following the observed preservation of TBPB activity at M1 mAChRs bearing a mutation at a residue critical to orthosteric ligand binding (Y381A). Despite confirming that this residue is not essential to the TBPB-M1 mAChR interaction, binding to other orthosteric site residues remains a valid possibility (as in the case of AC-42 and related compounds).
Further evidence for the potential orthosteric site-interaction of TBPB is that the M1 mAChR allosteric modulator VU0029767 (25) elicited a concentration-dependent potentiation of the cellular response to an EC20 concentration of TBPB “with a potency consistent with the potency of this compound for potentiating the response to ACh.”107 While this observation may be the result of positive cooperativity between two ligands binding to two distinct allosteric sites on the M1 mAChR, the possibility that a portion of TBPB is in fact occupying the orthosteric site in this interaction cannot be ignored.
SAR studies on this scaffold to date have proven unfruitful,130,131 with a range of substitutions at both the distal basic piperidine nitrogen and the fused benzyl ring mostly yielding compounds displaying either decreases in M1 mAChR potency, a loss of M1 mAChR selectivity, or undesirable off-target activity. Interestingly, a recent study identified “molecular switches” within the structure of TBPB that convert the ligand from a selective allosteric agonist to a pan-mAChR orthosteric antagonist.132 Surprisingly, addition of a single fluorine atom to the central piperidine ring was sufficient to disrupt an interaction critical for allosteric binding. This result, combined with the finding that TBPB behaves as an orthosteric antagonist at the other mAChR subtypes (in Ca2+ mobilization assays), led to the first definitive classification of TBPB as a bitopic ligand; though additional supportive pharmacological data will add further weight to this classification.
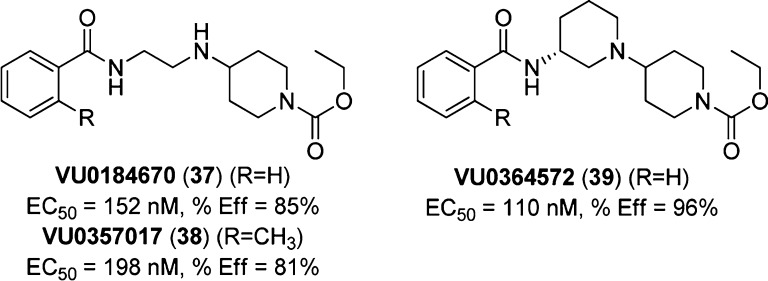
Following high-throughput screening and synthetic optimization, VU0184670 and VU0357017/ML071133 (37 and 38, Figure 26) were identified as potent (EC50 = 152 and 198 nM, respectively) and selective M1 mAChR agonists, producing maximum agonist effects of greater than 80% of the maximal response to ACh (as assessed by calcium mobilization assays). The activity of both VU0184670 and VU0357017 was preserved at receptors containing the mutated orthosteric site residue Y381A, and the ligands also demonstrated noncompetitive interactions with the orthosteric antagonist atropine in calcium mobilization assays. In line with studies of TBPB, these findings suggest, but do not conclusively confirm, an allosteric mode of action for these compounds. While these compounds show minimal perturbation of [3H]NMS binding at concentrations required for activity in calcium mobilization assays, concentrations greater than 1 μM displace radioligand binding, as evidenced by a clear reduction in the percentage of [3H]NMS receptor occupancy, suggestive of an orthosteric site interaction. Hence, a bitopic mode of action for these compounds remains a valid possibility.
Figure 26.
Structures of VU0184670 (37), VU0357017 (38), and VU0364572 (39). Calcium mobilization assays in rat M1-transfected CHO-K1 cells gave an estimate of potency, EC50, and efficacy, %Eff (normalized to the maximum response to the muscarinic agonist ACh).
These compounds demonstrated selective M1 mAChR-mediated potentiation of NMDA receptor currents in vitro and reversal of scopolamine-induced memory impairment in CFC in rats; however the need for improved potency and efficacy, as well as the elimination of functional D2 antagonism, prompted additional synthetic modifications in the form of cyclic constraints to be pursued.134 This study resulted in VU0364572 (39, Figure 26), a compound which produced enantioselective M1 mAChR activation. This compound maintained the favorable in vitro characteristics observed previously but also displayed the desired improvements in activity and clean ancillary pharmacology. Moreover, VU0364572 possessed low plasma protein binding, increased oral bioavailability, and excellent CNS exposure. An alternative approach involving the introduction of a more diverse variety of bicyclic constraints yielded some compounds with low nanomolar potency; however, a corresponding loss of muscarinic subtype selectivity was observed.135 This led the authors to speculate that these compounds may possess a previously unappreciated bitopic mechanism of action, whereby lower potency analogues appear purely allosteric but higher potency analogues result in predominantly orthosteric interactions.
Interestingly, a recent publication demonstrated that VU0357017 and VU0364572 exhibit stimulus bias; both compounds behaved as agonists of M1 mAChR-mediated Ca2+ mobilization and ERK1/2 phosphorylation while having no effect on β-arrestin recruitment.136 Studies in native tissues of M1 mAChR-mediated responses demonstrate that while both compounds potentiate NMDA receptor currents in hippocampal pyramidal cells and induce hippocampal LTP, they are devoid of electrophysiological activity in PFC pyramidal cells. These observations correlate well with the aforementioned study in which VU0357017 demonstrated efficacy in CFC, a hippocampal-dependent animal model of cognition.133 As with TBPB, subtle “molecular switches” were also found within this scaffold,132,137 where modifications such as inversion of chirality, reversed attachment, and benzyl ring replacement converted allosteric agonism to orthosteric antagonism. These observations prompted additional studies that ultimately led to the classification of VU0357017 and VU0364572 as bitopic ligands. Both ligands competitively displaced [3H]NMS binding at all five muscarinic receptor subtypes and behaved as orthosteric partial agonists at the M1 mAChR, but they also exhibited a characteristic allosteric effect of slowing the rate of [3H]NMS dissociation at M1 mAChR-expressing cell membranes.137
Lu AE51090
A more recent addition to the M1 mAChR-selective ligand literature is Lundbeck’s Lu AE51090138 (40, Figure 27). The original hit compound was found to be highly potent though not exclusively selective for the M1 mAChR subtype, so a number of analogues were synthesized to attempt to remedy this. Structural modifications included fluoro- and methoxy-substitution of the tetrahydroquinolinone ring system, replacement of this system with benzoxazolone and benzothiazinone ring systems, and the introduction of a range of alkyl chains and saturated and unsaturated rings at the amide position. The panel of analogues was screened against all five muscarinic receptor subtypes in functional Ca2+ mobilization assays. From this, Lu AE51090 was selected as an ideal lead compound based on both M1 mAChR activity preservation (the compound displayed a potency of 61 nM and a percentage efficacy of 83% compared to ACh) and M1 mAChR selectivity (the compound showed no agonism and negligible binding at the M2–M5 subtypes). The compound demonstrated acceptable absorption, distribution, metabolism, and excretion (ADME) properties for in vivo analysis, and subsequently showed a dose-dependent reversal in delay-induced memory decay evident in mice performing a PFC and hippocampal-dependent task.
Figure 27.
Structure of Lu AE51090 (40).
In a functional interaction assay against the orthosteric antagonist atropine, Lu AE51090 exhibited a saturable, noncompetitive profile suggesting an allosteric mode of action. As with the aforementioned “allosteric agonists”, Lu AE51090 activity was not compromised at Y381A mutated receptors. Once again, this evidence does not unequivocally rule out the possibility of a bitopic mechanism.
Interestingly, when screened against a broad range of 69 receptors, ion channels, transporters, and enzymes, Lu AE51090 exhibited only minor off-target activity. This is in contrast to AC-42 and TBPB, which displayed considerable affinity for other GPCRs including dopamine, serotonin, and adrenergic receptor subtypes, implying that Lu AE51090 may be a more useful molecular tool for exploring M1 mAChR activation in disease-specific tissues and in vivo cognition models.
With a view to designing an antipsychotic drug with pro-cognitive potential, the same group recently reported the in silico screening, synthesis, and evaluation of compounds displaying combined D2 R affinity and M1 mAChR agonism,139 giving rise to compound 41 (Figure 28). While the binding mode and activity profile of this compound at the D2 R was unexamined, the M1 mAChR activity of compound 41 was unaffected by the Y381A orthosteric mutation, consistent with an allosteric or possibly bitopic mode of action at this receptor. A similar mutagenesis approach, or functional interaction studies, would have elucidated a potential binding mode for the compound at the D2 R, be it orthosteric, allosteric, or bitopic. The complete mode of action of this compound hence remains purely speculative. It may be an orthosteric D2/allosteric M1 ligand, a pure allosteric ligand at both receptors, or a bitopic ligand at one or both targets. This ambiguity remains unexplored as, unfortunately, the compound also displayed hERG channel inhibition and synthetic strategies to overcome this adverse property were unsuccessful, resulting in the hit-to-lead campaign being abandoned.
Figure 28.
Structure of compound 41.
GlaxoSmithKline Compounds
GlaxoSmithKline have contributed a number of other subtype-selective M1 mAChR agonists to this body of literature.140−144 While these compounds are not purported specifically as either PAMs or allosteric agonists, their excellent subtype selectivity and striking similarities to some of the ligands previously described make them noteworthy.
Considerable SAR studies were performed around a 2′ biaryl amide scaffold,140,141 ultimately yielding compounds 42 and 43 (Figure 29), which showed favorable pharmacokinetic characteristics in addition to their high potency, intrinsic activity and exceptional selectivity against a panel of targets (as assessed by FLIPR calcium mobilization assays). In other studies,142,143 optimization of a novel N-substituted benzimidazolone hit structure gave the CNS-penetrant, orally active, and subtype-selective compounds 44 and 45 (Figure 29). Recently, the M1 mAChR allosteric agonist GSK1034702, an isomer of compound 44, has shown efficacy in improving episodic memory in humans in a nicotine abstinence model of cognitive dysfunction.144 An 8 mg dose of GSK1034702 significantly improved immediate recall (but not delayed recall) following abstinence-induced impairment. Furthermore, in preclinical studies, the potency of GSK1034702 was sensitive to the M1 mAChR F77I mutation, a mutation that also had a pronounced effect on the efficacy of AC-42 and 77-LH-28-1; suggesting a similar, potentially bitopic, binding mode.
Figure 29.
Structures of GSK compounds 42–45, with the motifs common to other M1 mAChR-selective ligands highlighted in red.
More detailed pharmacological investigation would verify the binding mode and classification of these GSK ligands. However, simple visual comparison of these structures with both a putative bitopic ligand (Lu AE51090) and an acknowledged bitopic ligand (TBPB) reveals conspicuous similarities that support the notion of a shared mode of binding and activation at this receptor (Figure 29).
Perspective
Our knowledge of the intricate behaviors and interactions of M1 mAChR allosteric ligands is rapidly expanding; however, despite the increasing depth of our understanding, researchers are still hampered by a number of difficulties that largely arise from the complexities of screening, optimizing, and classifying allosteric ligands.
With regards to initial library screening, each analogue is typically evaluated in one pharmacological assay and tested in the presence of a single concentration of one orthosteric ligand to gain a measure of ligand potency and degree of allosteric effect. While this is a valid method for the detection of putative ligand activity, the properties of stimulus bias and probe dependence that may be engendered by allosteric ligands remain unassessed. This may potentially reveal only one aspect of the ligand’s pharmacological capabilities, recognizing only one “string” in the putative allosteric ligand’s “bow”. If the test ligand selectively activates different downstream receptor signaling pathways, but not the pathway being assessed by the assay, pharmacologically desirable molecules may go undetected.54 Or, as outlined earlier in Figures 9 and 10, this may lead to misclassification of the ligand itself. Another consideration with regard to screening is the nature of the receptor, be it rat, human recombinant, or human native. As previously highlighted with LY2033298 (10)76,78 and N-desmethylclozapine (35),128 identification of species-dependent variability in both cooperativity and mode of action can lead to the discovery of altered selectivity and functional profiles that may have had deleterious results in a clinical setting if not identified earlier. Furthermore, the potential for allosteric ligands to elicit different physiological and toxicological outcomes in animal and human studies poses considerable challenges to the traditional drug discovery pipeline that will be difficult to resolve. In an ideal world, screening for novel allosteric drug candidates would involve a wide panel of orthosteric probes, assay outputs, and receptor targets. However, factoring in time, economic, and technological constraints, the best approach at present is to bear these considerations in mind and, following hit identification, apply this broader analysis in the hit-to-lead optimization process.
The potential issues surrounding the allosteric ligand screening process carry through into their optimization. While the evaluation of M1 mAChR-selective allosteric ligands frequently encounters “flat” or “shallow” SAR as a challenge impeding the development of allosteric ligands,98,102,103,108,112 it is possible that these observations are a consequence of too narrow a pharmacological analysis, given screening constraints. Broader evaluation of a limited cluster of novel scaffolds in radioligand binding and a variety of functional assays in the presence of multiple classes of orthosteric ligands at this stage of development may deconvolute the “confounding” SAR frequently observed in these studies and potentially reveal the capacity to differentially modulate discrete receptor signaling pathways and affect unique changes in the traditional behavior of orthosteric ligands. Additionally, quantification of properties such as binding cooperativity, functional cooperativity, and allosteric agonism would reduce the dependence on titration curve EC50 values as the key determinant for future structural modifications. This would likely generate more detailed, “textured” SARs, advance our understanding of the relationship between allosteric ligand, orthosteric ligand, and receptor, and more accurately direct drug candidate optimization. Furthermore, as subtle changes in allosteric ligand structure have been shown to dramatically modify receptor subtype selectivity,87 all promising analogues to emerge from the optimization process ought to be rescreened to ensure preservation of the desired selectivity profile.
Finally, the highly contextual (and sometimes premature) classification of allosteric ligands as PAMs, NAMs, and agonists further complicates this field of research. Misclassification typically results from incomplete investigation of the binding mode and functional profile of these ligands. Notable examples already described include the potential over-reliance on specific receptor mutants (e.g., orthosteric site Y381A mutation) as a means of discriminating orthosteric and allosteric binding modes, and the difficulty in distinguishing competitive and noncompetitive interactions, particularly when recording functional response data at a time point prior to the ligands reaching a state of equilibrium (e.g., the calcium mobilization assay). The prospect of bitopic modes of action further confounds this issue.
There is no simple means to overcoming this classification challenge. Confirming a purely allosteric mode of action and further investigating the exact nature of the putative bitopic M1 mAChR agonists already described cannot be achieved using one experimental technique alone, such as a mutagenesis study or a single functional assay. More comprehensive synthetic and pharmacological studies need to be undertaken.57,84 Knowledge of each ligand’s true binding mode with the M1 mAChR will facilitate rational drug design and will likely result in the more productive synthesis of potent, efficacious therapeutic leads. Additionally, adopting a global, more detailed lexicon to describe allosteric ligand activity56 may aid communication between researchers.
Conclusion
The unique properties of allosteric ligands require more thorough pharmacological evaluation if we are to advance our understanding of their interactions and comprehend the complete therapeutic potential of these molecules. Their complex behavior also demands a more detailed classification system that is globally recognized. The sooner these necessities are acknowledged and embraced, the more fruitful our labors will be.
In the field of selective M1 mAChRs specifically, this would translate to an improved ability to map the key binding residues of the multiple allosteric sites present on the receptor, more informed design of putative allosteric ligands, and a greater understanding of the intricate role of this muscarinic receptor subtype in the normal functioning of the CNS and the pathophysiology of CNS diseases. As a consequence, it will be possible to fully appreciate the scope of M1 mAChR activation as a potential neurodegenerative disorder therapy.
Glossary
Abbreviations
- mAChR
muscarinic acetylcholine receptor
- CNS
central nervous system
- GPCR
G protein-coupled receptor
- PLC
phospholipase C
- IP3
inositol trisphosphate
- AC
adenylate cyclase
- cAMP
cyclic adenosine monophosphate
- AD
Alzheimer’s disease
- ACh
acetylcholine
- KO
knockout
- PKC
protein kinase C
- NMDA
N-methyl-d-aspartate
- APP
amyloid precursor protein
- CSF
cerebrospinal fluid
- EPS
extrapyramidal side effects
- TM
transmembrane
- EC
extracellular
- NMS
N-methylscopolamine
- IC
intracellular
- PAM
positive allosteric modulator
- CRC
concentration response curve
- QNB
3-quinuclidinyl benzilate
- BQCA
benzyl quinolone carboxylic acid
- FLIPR
fluorometric imaging plate reader
- MWC
Monod-Wyman-Changeux
- CFC
contextual fear conditioning
- PFC
prefrontal cortex
- sEPSCs
spontaneous excitatory postsynaptic currents
- FF
free fraction
- SAR
structure–activity relationship
- BBB
blood-brain barrier
- P-gp
P-glycoprotein
- TBPB
[1-(1′-(2-tolyl)-1,4′-bipiperidin-4-yl)-1H benzo[d]imidazol-2(3H)-one]
- ADME
absorption, distribution, metabolism, and excretion
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors acknowledge financial support from the Australian Research Council (Discovery Grant DP110100687) and the National Health and Medical Research Council (NHMRC) of Australia (Program Grant 519461). A.C. is a Principal Research Fellow of the NHMRC, and B.J.D. is supported by an Australian Postgraduate Award.
The authors declare no competing financial interest.
References
- van Koppen C. J.; Kaiser B. (2003) Regulation of muscarinic acetylcholine receptor signaling. Pharmacol. Ther. 98, 197–220. [DOI] [PubMed] [Google Scholar]
- Canals M.; Sexton P. M.; Christopoulos A. (2011) Allostery in GPCRs: “MWC” revisited. Trends Biochem. Sci. 36, 663–672. [DOI] [PubMed] [Google Scholar]
- Caulfield M. P. (1993) Muscarinic Receptors-Characterization, Coupling and Function. Pharmacol. Ther. 58, 319–379. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M. (2008) Synthesis, trafficking and localization of muscarinic acetylcholine receptors. Pharmacol. Ther. 119, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. (2008) M1 Muscarinic Agonists Target Major Hallmarks of Alzheimer’s Disease - The Pivotal Role of Brain M1 Receptors. Neurodegener. Dis. 5, 237–240. [DOI] [PubMed] [Google Scholar]
- Langmead C. J.; Watson J.; Reavill C. (2008) Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 117, 232–243. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Yan J.; Zhou P.; Li J.; Gao H.; Xia Y.; Wang Q. (2012) Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 97, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. (2004) Muscarinic Acetylcholine Receptor Knockout Mice: Novel Phenotypes and Clinical Implications. Annu. Rev. Pharmacol. Toxicol. 44, 423–450. [DOI] [PubMed] [Google Scholar]
- Bodick N. C.; Offen W. W.; Levey A. I.; Cutler N. R.; Gauthier S. G.; Satlin A.; Shannon H. E.; Tollefson G. D.; Rasmussen K.; Bymaster F. P.; Hurley D. J.; Potter W. Z.; Paul S. M. (1997) Effects of Xanomeline, a Selective Muscarinic Receptor Agonist, on Cognitive Function and Behavioral Symptoms in Alzheimer Disease. Arch. Neurol. 54, 465–473. [DOI] [PubMed] [Google Scholar]
- Digby G. J.; Shirey J. K.; Conn P. J. (2010) Allosteric activators of muscarinic receptors as novel approaches for treatment of CNS disorders. Mol. BioSyst. 6, 1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus R. T.; Dean R. L.; Beer B.; Lippa A. S. (1982) The Cholinergic Hypothesis of Geriatric Memory Dysfunction. Science 217, 408–417. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J.; Price D. L.; Struble R. G.; Clark A. W.; Coyle J. T.; Delon M. R. (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239. [DOI] [PubMed] [Google Scholar]
- Francis P. T.; Palmer A. M.; Snape M.; Wilcock G. K. (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry 66, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E. (2000) Cholinesterase Inhibitors Stabilize Alzheimer’s Disease. Ann. N.Y. Acad. Sci. 920, 321–327. [DOI] [PubMed] [Google Scholar]
- Mirza N. R.; Peters D.; Sparks R. G. (2003) Xanomeline and the Antipsychotic Potential of Muscarinic Receptor Subtype Selective Agonists. CNS Drug Rev. 9, 159–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster F. P.; Whitesitt C. A.; Shannon H. E.; DeLapp N.; Ward J. S.; Calligaro D. O.; Shipley L. A.; Buelke-Sam J. L.; Bodick N. C.; Farde L.; Sheardown M. J.; Olesen P. H.; Hansen K. T.; Suzdak P. D.; Swedberg M. D. B.; Sauerberg P.; Mitch C. H. (1997) Xanomeline: A Selective Muscarinic Agonist for the Treatment of Alzheimer’s Disease. Drug Dev. Res. 40, 158–170. [Google Scholar]
- Bymaster F. P.; McKinzie D. L.; Felder C. C.; Wess J. (2003) Use of M1-M5 Muscarinic Receptor Knockout Mice as Novel Tools to Delineate the Physiological Roles of the Muscarinic Cholinergic System. Neurochem. Res. 28, 437–442. [DOI] [PubMed] [Google Scholar]
- Shannon H. E.; Rasmussen K.; Bymaster F. P.; Hart J. C.; Peters S. C.; Swedberg M. D. B.; Jeppeson L.; Sheardown M. J.; Sauerberg P.; Fink-Jensen A. (2000) Xanomeline, an M1/M4 preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr. Res. 42, 249–259. [DOI] [PubMed] [Google Scholar]
- Shekhar A.; Potter W. Z.; Lightfoot J.; Lienemann J.; Dube S.; Mallinckrodt C.; Bymaster F. P.; McKinzie D. L.; Felder C. C. (2008) Selective Muscarinic Receptor Agonist Xanomeline as a Novel Treatment Approach for Schizophrenia. Am. J. Psychiatry 165, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Woolley M. L.; Carter H. J.; Gartlon J. E.; Watson J. M.; Dawson L. A. (2009) Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur. J. Pharmacol. 603, 147–149. [DOI] [PubMed] [Google Scholar]
- Tzavara E. T.; Bymaster F. P.; Davis R. J.; Wade M. R.; Perry K. W.; Wess J.; McKinzie D. L.; Felder C.; Nomikos G. G. (2004) M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related central nervous system pathologies. FASEB J. 18, 1410–1427. [DOI] [PubMed] [Google Scholar]
- Wood M. D.; Murkitt K. L.; Ho M.; Watson J. M.; Brown F.; Hunter A. J.; Middlemiss D. N. (1999) Functional comparison of muscarinic partial agonists at muscarinic receptor subtypes hM1, hM2, hM3, hM4 and hM5 using microphysiometry. Br. J. Pharmacol. 126, 1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.; Roth B. L. (2010) Allosteric Antipsychotics: M4Muscarinic Potentiatiors as Novel Treatments for Schizophrenia. Neuropsychopharmacology 35, 851–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S. W. Y.; Pomakian J.; Marshall G. A.; Vinters H. V.; Cummings J. L.; Chen C. P. L.-H.; Wong P. T.-H.; Lai M. K. P. (2007) Disrupted muscarinic M1 receptor signaling correlates with loss of protein kinase C activity and glutamatergic deficit in Alzheimer’s disease. Neurobiol. Aging 28, 1381–1387. [DOI] [PubMed] [Google Scholar]
- Fisher A. (2012) Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer’s disease. J. Neurochem. 120, 22–33. [DOI] [PubMed] [Google Scholar]
- Marino M. J.; Rouse S. T.; Levey A. I.; Potter L. T.; Conn P. J. (1998) Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-d-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc. Natl. Acad. Sci. U.S.A. 95, 11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L.; Volianskis A.; Bannister N.; France G.; Hanna L.; Mercier M.; Tidball P.; Fang G.; Irvine M. W.; Costa B. M.; Monaghan D. T.; Bortolotto Z. A.; Molnar E.; Lodge D.; Jane D. E. (2013) The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 64, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.; Maloney A. J. F. (1976) Selective Loss of Central Cholinergic Neurons in Alzheimer’s Disease. Lancet 2, 1403. [DOI] [PubMed] [Google Scholar]
- Flynn D. D.; Ferrari-DiLeo G.; Mash D. C.; Levey A. I. (1995) Differential Regulation of Molecular Subtypes of Muscarinic Receptors in Alzheimer’s Disease. J. Neurochem. 64, 1888–1891. [DOI] [PubMed] [Google Scholar]
- Overk C. R.; Felder C. C.; Tu Y.; Schober D. A.; Bales K. R.; Wuu J.; Mufson E. J. (2010) Cortical M1 receptor concentration increases without a concomitant change in function in Alzheimer’s disease. J. Chem. Neuroanat. 40, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R.; O’Connor K.; Tate W. P.; Abraham W. C. (2003) Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 70, 1–32. [DOI] [PubMed] [Google Scholar]
- Hock C.; Maddalena A.; Raschig A.; Muller-Spahn F.; Eschweiler G.; Hager K.; Heuser I.; Hampel H.; Muller-Thomsen T.; Oertel W.; Wienrich M.; Signorell A.; Gonzalez-Agosti C.; Nitsch R. M. (2003) Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of Ab42 in patients with Alzheimer’s disease. Amyloid 10, 1–6. [DOI] [PubMed] [Google Scholar]
- Fisher A.; Michaelson D. M.; Brandeis R.; Haring R.; Chapman S.; Pittel Z. (2000) M1Muscarinic Agonists as Potential Disease-Modifying Agents in Alzheimer’s Disease: Rationale and Perspectives. Ann. N.Y. Acad. Sci. 920, 315–320. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. D.; Gandy S. E.; Cicchetti P.; Ehrlich M. E.; Czernik A. J.; Fracasso R. P.; Ramabhadran T. V.; Unterbeck A. J.; Greengard P. (1990) Processing of Alzheimer beta/A4 amyloid precursor protein: Modulation by agents that regulate protein phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 87, 6003–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A.; Oddo S.; Billings L. M.; Green K. N.; Martinez-Coria H.; Fisher A.; LaFerla F. M. (2006) M1 Receptors Play a Central Role in Modulating AD-like Pathology in Transgenic Mice. Neuron 49, 671–682. [DOI] [PubMed] [Google Scholar]
- Farde L.; Nordstrom A.-L.; Wiesel F.-A.; Pauli S.; Halldin C.; Sedvall G. (1992) Positron Emission Tomographic Analysis of Central D1 and D2 Dopamine Receptor Occupancy in Patients Treated With Classical Neuroleptics and Clozapine. Arch. Gen. Psychiatry 49, 538–544. [DOI] [PubMed] [Google Scholar]
- Serretti A.; De Ronchi D.; Lorenzi C.; Berardi D. (2004) New Antipsychotics and Schizophrenia: A Review on Efficacy and Side Effects. Curr. Med. Chem. 11, 343–358. [DOI] [PubMed] [Google Scholar]
- DeLeon A.; Patel N. C.; Crismon M. L. (2004) Aripiprazole: A Comprehensive Review of Its Pharmacology, Clinical Efficacy, and Tolerability. Clin. Ther. 26, 649–666. [DOI] [PubMed] [Google Scholar]
- Minzenberg M. J.; Carter C. S. (2012) Developing treatments for impaired cognition in schizophrenia. Trends Cognit. Sci. 16, 35–42. [DOI] [PubMed] [Google Scholar]
- Felder C. C.; Bymaster F. P.; DeLapp N. (2000) Therapeutic Opportunities for Muscarinic Receptors in the Central Nervous System. J. Med. Chem. 43, 4333–4353. [DOI] [PubMed] [Google Scholar]
- Crook J. M.; Tomaskovic-Crook E.; Copolov D. L.; Dean B. (2000) Decreased Muscarinic Receptor Binding in Subjects with Schizophrenia: A Study of the Human Hippocampal Formation. Biol. Psychiatry 48, 381–388. [DOI] [PubMed] [Google Scholar]
- Crook J. M.; Tomaskovic-Crook E.; Copolov D. L.; Dean B. (2001) Low Muscarinic Receptor Binding in Prefrontal Cortex From Subjects With Schizophrenia: A Study of Brodmann’s Areas 8, 9, 10 and 46 and the Effects of Neuroleptic Drug Treatment. Am. J. Psychiatry 158, 918–925. [DOI] [PubMed] [Google Scholar]
- Raedler T. J.; Bymaster F. P.; Tandon R.; Copolov D.; Dean B. (2007) Towards a muscarinic hypothesis of schizophrenia. Mol. Psychiatry 12, 232–246. [DOI] [PubMed] [Google Scholar]
- Korczyn A. D. (2000) Muscarinic M1 agonists in the treament of Alzheimer’s disease. Expert Opin. Invest. Drug 9, 2259–2267. [DOI] [PubMed] [Google Scholar]
- Flynn D. D.; Weinstein D. A.; Mash D. C. (1991) Loss of High-Affinity Agonist Binding to M1Muscarinic Receptors in Alzheimer’s Disease: Implications for the Failure of Cholinergic Replacement Therapies. Ann. Neurol. 29, 256–262. [DOI] [PubMed] [Google Scholar]
- Langmead C. J.; Christopoulos A. (2006) Allosteric agonists of 7TM receptors: expanding the pharmacological toolbox. Trends Pharmacol. Sci. 27, 475–481. [DOI] [PubMed] [Google Scholar]
- Kenakin T. P. (2012) Biased signalling and allosteric machines: new vistas and challenges for drug discovery. Br. J. Pharmacol. 165, 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T.; Leach K.; Sexton P. M.; Christopoulos A. (2007) Allosteric Modulation of G Protein-Coupled Receptors. Annu. Rev. Pharmacol. Toxicol. 47, 1–51. [DOI] [PubMed] [Google Scholar]
- Dong B. J. (2005) Cinacalcet: An Oral Calcimimetic Agent for the Management of Hyperparathyroidism. Clin. Ther. 27, 1725–1751. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez J.; Rueda P.; Staropoli I.; Kellenberger E.; Alcami J.; Arenzana-Seisdedos F.; Lagane B. (2011) New Insights into the Mechanisms whereby Low Molecular Weight CCR5 Ligands Inhibit HIV-1 Infection. J. Biol. Chem. 286, 4978–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert F. J.; Roeske W. R.; Gee K. W.; Yamamura H. I. (1983) An Allosteric Model For Benzodiazepine Receptor Function. Biochem. Pharmacol. 32, 2375–2383. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. (2002) Allosteric Binding Sites On Cell-Surface Receptors: Novel Targets For Drug Discovery. Nat. Rev. Drug Discovery 1, 198–210. [DOI] [PubMed] [Google Scholar]
- Conn P. J.; Jones C. K.; Lindsley C. W. (2009) Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 30, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2005) New Concepts In Drug Discovery: Collateral Efficacy And Permissive Antagonism. Nat. Rev. Drug Discovery 4, 919–927. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2003) Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol. Sci. 24, 346–354. [DOI] [PubMed] [Google Scholar]
- Keov P.; Sexton P. M.; Christopoulos A. (2011) Allosteric modulation of G protein-coupled receptors: A pharmacological perspective. Neuropharmacology 60, 24–35. [DOI] [PubMed] [Google Scholar]
- Leach K.; Loiacono R. E.; Felder C. C.; McKinzie D. L.; Mogg A.; Shaw D. B.; Sexton P. M.; Christopoulos A. (2010) Molecular Mechanisms of Action and In Vivo Validation of an M4 Muscarinic Acetylcholine Receptor Allosteric Modulator with Potential Antipsychotic Properties. Neuropsychopharmacology 35, 855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M.; Lane J. R.; Wen A.; Scammells P. J.; Sexton P. M.; Christopoulos A. (2012) A Monod-Wyman-Changeux Mechanism Can Explain G Protein-coupled Receptor (GPCR) Allosteric Modulation. J. Biol. Chem. 287, 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K.; Kumasaka T.; Hori T.; Behnke C. A.; Motoshima H.; Fox B. A.; Le Trong I.; Teller D. C.; Okada T.; Stenkamp R. E.; Yamamoto M.; Miyano M. (2000) Crystal Structure of Rhodopsin: A G Protein-Coupled Receptor. Science 289, 739–745. [DOI] [PubMed] [Google Scholar]
- Rasmussen S. G. F.; Choi H.-J.; Rosenbaum D. M.; Kobilka T. S.; Thian F. S.; Edwards P. C.; Burghammer M.; Ratnala V. R. P.; Sanishvili R.; Fischetti R. F.; Schertler G. F. X.; Weis W. I.; Kobilka B. K. (2007) Crystal structure of the human b2 adrenergic G-protein-coupled receptor. Nature 450, 383–388. [DOI] [PubMed] [Google Scholar]
- Jaakola V.-P.; Griffith M. T.; Hanson M. A.; Cherezov V.; Chien E. Y. T.; Lane J. R.; Ijzerman A. P.; Stevens R. C. (2008) The 2.6 Angstrom Crystal Structure of a Human A2A Adenosine Receptor Bound to an Antagonist. Science 322, 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann R.; Schrock K.; Boselt I.; Staubert C.; Russ A.; Schoneberg T. (2011) Evolution of GPCR: Change and continuity. Mol. Cell. Endocrinol. 331, 170–178. [DOI] [PubMed] [Google Scholar]
- Haga K.; Kruse A. C.; Asada H.; Yurugi-Kobayashi T.; Shiroishi M.; Zhang C.; Weis W. I.; Okada T.; Kobilka B. K.; Haga T.; Kobayashi T. (2012) Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 482, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse A. C.; Hu J.; Pan A. C.; Arlow D. H.; Rosenbaum D. M.; Rosemond E.; Green H. F.; Liu T.; Chae P. S.; Dror R. O.; Shaw D. E.; Weis W. I.; Wess J.; Kobilka B. K. (2012) Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482, 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme E. C.; Birdsall N. J. M.; Buckley N. J. (1990) Muscarinic Receptor Subtypes. Annu. Rev. Pharmacol. Toxicol. 30, 633–673. [DOI] [PubMed] [Google Scholar]
- Spalding T. A.; Birdsall N. J. M.; Curtis C. A. M.; Hulme E. C. (1994) Acetylcholine Mustard Labels the Binding Site Aspartate in Muscarinic Acetylcholine Receptors. J. Biol. Chem. 269, 4092–4097. [PubMed] [Google Scholar]
- Goodwin J. A.; Hulme E. C.; Langmead C. J.; Tehan B. G. (2007) Roof and Floor of the Muscarinic Binding Pocket: Variations in the Binding Modes of Orthosteric Ligands. Mol. Pharmacol. 72, 1484–1496. [DOI] [PubMed] [Google Scholar]
- Ellis J. (1997) Allosteric Binding Sites on Muscarinic Receptors. Drug Dev. Res. 40, 193–204. [Google Scholar]
- Wess J. (2005) Allosteric Binding Sites on Muscarinic Acetylcholine Receptors. Mol. Pharmacol. 68, 1506–1509. [DOI] [PubMed] [Google Scholar]
- Gnagey A. L.; Seidenberg M.; Ellis J. (1999) Site-Directed Mutagenesis Reveals Two Epitopes Involved in the Subtype Selectivity of the Allosteric Interactions of Gallamine at Muscarinic Acetylcholine Receptors. Mol. Pharmacol. 56, 1245–1253. [DOI] [PubMed] [Google Scholar]
- Lazareno S.; Popham A.; Birdsall N. J. M. (2000) Allosteric Interactions of Staurosporine and Other Indolocarbazoles with N-[methyl-3H]Scopolamine and Acetylcholine at Muscarinic Receptor Subtypes: Identification of a Second Allosteric Site. Mol. Pharmacol. 58, 194–207. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J. M.; Lazareno S.; Popham A.; Saldanha J. (2001) Multiple allosteric sites on muscarinic receptors. Life Sci. 68, 2517–2524. [DOI] [PubMed] [Google Scholar]
- Lazareno S.; Popham A.; Birdsall N. J. M. (2002) Analogs of WIN 62,577 Define a Second Allosteric Site on Muscarinic Receptors. Mol. Pharmacol. 62, 1492–1505. [DOI] [PubMed] [Google Scholar]
- Espinoza-Fonseca L. M.; Trujillo-Ferrara J. G. (2006) The existence of a second allosteric site on the M1 muscarinic acetylcholine receptor and its implications for drug design. Bioorg. Med. Chem. Lett. 16, 1217–1220. [DOI] [PubMed] [Google Scholar]
- Kenakin T.; Christopoulos A. (2013) Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat. Rev. Drug Discovery 12, 205–216. [DOI] [PubMed] [Google Scholar]
- Valant C.; Felder C. C.; Sexton P. M.; Christopoulos A. (2012) Probe Dependence in the Allosteric Modulation of a G Protein-Coupled Receptor: Implications for Detection and Validation of Allosteric Ligand Effects. Mol. Pharmacol. 81, 41–52. [DOI] [PubMed] [Google Scholar]
- Chan W. Y.; McKinzie D. L.; Bose S.; Mitchell S. N.; Witkin J. M.; Thompson R. C.; Christopoulos A.; Lazareno S.; Birdsall N. J. M.; Bymaster F. P.; Felder C. C. (2008) Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 105, 10978–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratman S.; Leach K.; Sexton P. M.; Felder C. C.; Loiacono R. E.; Christopoulos A. (2011) Impact of species variability and ’probe dependence’ on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br. J. Pharmacol. 162, 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame A.; Christopoulos A.; Mitchelson F. (1997) Three Allosteric Modulators Act at a Common Site, Distinct from that of Competitive Antagonists, at Muscarinic Acetylcholine M2 Receptors. J. Pharmacol. Exp. Ther. 282, 278–285. [PubMed] [Google Scholar]
- May L. T.; Avlani V. A.; Langmead C. J.; Herdon H. J.; Wood M. D.; Sexton P. M.; Christopoulos A. (2007) Structure-Function Studies of Allosteric Agonism at M2 Muscarinic Acetylcholine Receptors. Mol. Pharmacol. 72, 463–476. [DOI] [PubMed] [Google Scholar]
- Antony J.; Kellershohn K.; Mohr-Andra M.; Kebig A.; Prilla S.; Muth M.; Heller E.; Disingrini T.; Dallanoce C.; Bertoni S.; Schrobang J.; Trankle C.; Kostenis E.; Christopoulos A.; Holtje H.-D.; Barocelli E.; De Amici M.; Holzgrabe U.; Mohr K. (2009) Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. FASEB J. 23, 442–450. [DOI] [PubMed] [Google Scholar]
- Lane J. R.; Sexton P. M.; Christopoulos A. (2013) Bridging the gap: bitopic ligands of G-protein-coupled receptors. Trends Pharmacol. Sci. 34, 59–66. [DOI] [PubMed] [Google Scholar]
- Valant C.; Sexton P. M.; Christopoulos A. (2009) Orthosteric/Allosteric Bitopic Ligands - Going Hybrid at GPCRs. Mol. Interventions 9, 125–135. [DOI] [PubMed] [Google Scholar]
- Valant C.; Gregory K. J.; Hall N. E.; Scammells P. J.; Lew M. J.; Sexton P. M.; Christopoulos A. (2008) A Novel Mechanism of G Protein-coupled Receptor Functional Selectivity - Muscarinic Partial Agonist McN-A-343 As A Bitopic Orthosteric/Allosteric Ligand. J. Biol. Chem. 283, 29312–29321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall N. J. M.; Burgen A. S. V.; Hulme E. C.; Stockton J. M.; Zigmond M. J. (1983) The effect of McN-A-343 on muscarinic receptors in the cerebral cortex and heart. Br. J. Pharmacol. 78, 257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareno S.; Birdsall N. J. M. (1995) Detection, Quantitation, and Verification of Allosteric Interactions of Agents with Labeled and Unlabeled Ligands at G Protein-Coupled Receptors: Interactions of Strychnine and Acetylcholine at Muscarinic Receptors. Mol. Pharmacol. 48, 362–378. [PubMed] [Google Scholar]
- Birdsall N. J. M.; Farries T.; Gharagozloo P.; Kobayashi S.; Kuonen D.; Lazareno S.; Popham A.; Sugimoto M. (1997) Selective Allosteric Enhancement of the Binding and Actions of Acetylcholine at Muscarinic Receptor Subtypes. Life Sci. 60, 1047–1052. [DOI] [PubMed] [Google Scholar]
- Ma L.; Seager M. A.; Wittman M.; Jacobson M.; Bickel D.; Burno M.; Jones K.; Graufelds V. K.; Xu G.; Pearson M.; McCampbell A.; Gaspar R.; Shughrue P.; Danziger A.; Regan C.; Flick R.; Pascarella D.; Garson S.; Doran S.; Kreatsoulas C.; Veng L.; Lindsley C. W.; Shipe W.; Kuduk S.; Sur C.; Kinney G.; Seabrook G. R.; Ray W. J. (2009) Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl. Acad. Sci. U.S.A. 106, 15950–15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T.; Yamada M.; Duttaroy A.; Wess J. (2001) Hyperactivity and Intact Hippocampus-Dependent Learning in Mice Lacking the M1 Muscarinic Acetylcholine Receptor. J. Neurosci. 21, 5239–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse S. T.; Hamilton S. E.; Potter L. T.; Nathanson N. M.; Conn P. J. (2000) Muscarinic-induced modulation of potassium conductances is unchanged in mouse hippocampal pyramidal cells that lack functional M1 receptors. Neurosci. Lett. 278, 61–64. [DOI] [PubMed] [Google Scholar]
- Shirey J. K.; Brady A. E.; Jones P. J.; Davis A. A.; Bridges T. M.; Kennedy J. P.; Jadhav S. B.; Menon U. N.; Xiang Z.; Watson M. L.; Christian E. P.; Doherty J. J.; Quirk M. C.; Snyder D. H.; Lah J. J.; Levey A. I.; Nicolle M. M.; Lindsley C. W.; Conn P. J. (2009) A Selective Allosteric Potentiator of the M1 Muscarinic Acetylcholine Receptor Increases Activity of Medial Prefrontal Cortical Neurons and Restores Impairments in Reversal Learning. J. Neurosci. 29, 14271–14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana D. A.; Warburton E. C.; Bashir Z. I. (2011) Induction of Activity-Dependent LTD Requires Muscarinic Receptor Activation in Medial Prefrontal Cortex. J. Neurosci. 31, 18464–18478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon C.; Jatzke C.; Wegener N.; Gravius A.; Danysz W. (2012) Using cholinergic M1 receptor positive allosteric modulators to improve memory via enhancement of brain cholinergic communication. Eur. J. Pharmacol. 697, 73–80. [DOI] [PubMed] [Google Scholar]
- Chambon C.; Wegener N.; Gravius A.; Danysz W. (2011) A new automated method to assess the rat recognition memory: Validation of the method. Behav. Brain Res. 222, 151–157. [DOI] [PubMed] [Google Scholar]
- Reichel A. (2006) The Role of Blood-Brain Barrier Studies in the Pharmaceutical Industry. Curr. Drug Metab. 7, 183–203. [DOI] [PubMed] [Google Scholar]
- Yang F. V.; Shipe W. D.; Bunda J. L.; Nolt M. B.; Wisnoski D. D.; Zhao Z.; Barrow J. C.; Ray W. J.; Ma L.; Wittman M.; Seager M. A.; Koeplinger K. A.; Hartman G. D.; Lindsley C. W. (2010) Parallel synthesis of N-biaryl quinolone carboxylic acids as selective M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 20, 531–536. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Chang R. K.; Di Marco C. N.; Ray W. J.; Ma L.; Wittman M.; Seager M. A.; Koeplinger K. A.; Thompson C. D.; Hartman G. D.; Bilodeau M. T. (2010) Quinolizidinone Carboxylic Acids as CNS Penetrant, Selective M1 Allosteric Muscarinic Receptor Modulators. ACS Med. Chem. Lett. 1, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuduk S. D.; Chang R. K.; Di Marco C. N.; Ray W. J.; Ma L.; Wittman M.; Seager M. A.; Koeplinger K. A.; Thompson C. D.; Hartman G. D.; Bilodeau M. T. (2011) Quinolizidinone carboxylic acid selective M1 allosteric modulators: SAR in the piperidine series. Bioorg. Med. Chem. Lett. 21, 1710–1715. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Di Marco C. N.; Chang R. K.; Ray W. J.; Ma L.; Wittman M.; Seager M. A.; Koeplinger K. A.; Thompson C. D.; Hartman G. D.; Bilodeau M. T. (2010) Heterocyclic fused pyridone carboxylic acid M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 20, 2533–2537. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Di Marco C. N.; Cofre V.; Pitts D. R.; Ray W. J.; Ma L.; Wittman M.; Seager M.; Koeplinger K.; Thompson C. D.; Hartman G. D.; Bilodeau M. T. (2010) Pyridine containing M1 positive allosteric modulators with reduced plasma protein binding. Bioorg. Med. Chem. Lett. 20, 657–661. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Di Marco C. N.; Cofre V.; Pitts D. R.; Ray W. J.; Ma L.; Wittman M.; Veng L.; Seager M. A.; Koeplinger K.; Thompson C. D.; Hartman G. D.; Bilodeau M. T. (2010) N-Heterocyclic derived M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 20, 1334–1337. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Di Marco C. N.; Cofre V.; Ray W. J.; Ma L.; Wittman M.; Seager M. A.; Koeplinger K. A.; Thompson C. D.; Hartman G. D.; Bilodeau M. T. (2011) Fused heterocyclic M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 21, 2769–2772. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; DiPardo R. M.; Beshore D. C.; Ray W. J.; Ma L.; Wittman M.; Seager M. A.; Koeplinger K. A.; Thompson C. D.; Hartman G. D.; Bilodeau M. T. (2010) Hydroxy cycloalkyl fused pyridone carboxylic acid M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 20, 2538–2541. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Chang R. K.; Greshock T. J.; Ray W. J.; Ma L.; Wittmann M.; Koeplinger K. A.; Seager M. A.; Thompson C. D.; Hartman G.; Bilodeau M. T. (2012) Identification of Amides as Carboxylic Acid Surrogates for Quinolizidinone-Based M1 Positive Allosteric Modulators. ACS Med. Chem. Lett. 3, 1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner J. M.; Eddins D.; Puri V.; Cannon C. E.; Sutcliffe J.; Chew C. S.; Pearson M.; Vivian J. A.; Chang R. K.; Ray W. J.; Kuduk S. D.; Wittmann M. (2013) The muscarinic M1 receptor positive allosteric modulator PQCA improves cognitive measures in rat, cynomolgus macaque, and rhesus macaque. Psychopharmacology 225, 21–30. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Beshore D. C. (2012) Novel M1 allosteric ligands: a patent review. Expert Opin. Ther. Pat. 22, 1385–1398. [DOI] [PubMed] [Google Scholar]
- Marlo J. E.; Niswender C. M.; Days E. L.; Bridges T. M.; Xiang Y.; Rodriguez A. L.; Shirey J. K.; Brady A. E.; Nalywajko T.; Luo Q.; Austin C. A.; Baxter Williams M.; Kim K.; Williams R.; Orton D.; Brown H. A.; Lindsley C. W.; Weaver C. D.; Conn P. J. (2009) Discovery and Characterization of Novel Allosteric Potentiators of M1 Muscarinic Receptors Reveals Multiple Modes of Activity. Mol. Pharmacol. 75, 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. M.; Kennedy J. P.; Noetzel M. J.; Breininger M. L.; Gentry P. R.; Conn P. J.; Lindsley C. W. (2010) Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: Development of a potent and highly selective M1 PAM. Bioorg. Med. Chem. Lett. 20, 1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. M.; Kennedy J. P.; Cho H. P.; Breininger M. L.; Gentry P. R.; Hopkins C. R.; Conn P. J.; Lindsley C. W. (2010) Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M5 PAM. Bioorg. Med. Chem. Lett. 20, 558–562.20004578 [Google Scholar]
- Melancon B. J.; Poslusney M. S.; Gentry P. R.; Tarr J. C.; Sheffler D. J.; Mattmann M. E.; Bridges T. M.; Utley T. J.; Daniels J. S.; Niswender C. M.; Conn P. J.; Lindsley C. W.; Wood M. R. (2013) Isatin replacements applied to the highly selective, muscarinic M1 PAM ML137: Continued optimization of an MLPCN probe molecule. Bioorg. Med. Chem. Lett. 23, 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poslusney M. S.; Melancon B. J.; Gentry P. R.; Sheffler D. J.; Bridges T. M.; Utley T. J.; Daniels J. S.; Niswender C. M.; Conn P. J.; Lindsley C. W.; Wood M. R. (2013) Spirocyclic replacements for the isatin in the highly selective, muscarinic M1 PAM ML137: the continued optimization of an MLPCN probe molecule. Bioorg. Med. Chem. Lett. 23, 1860–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. R.; Bridges T. M.; Sheffler D. J.; Cho H. P.; Lewis L. M.; Days E.; Daniels J. S.; Jones C. K.; Niswender C. M.; Weaver C. D.; Conn P. J.; Lindsley C. W.; Wood M. R. (2011) Discovery and optimization of a novel, selective and brain penetrant M1 positive allosteric modulator (PAM): The development of ML169, an MLPCN probe. Bioorg. Med. Chem. Lett. 21, 2697–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr J. C.; Turlington M. L.; Reid P. R.; Utley T. J.; Sheffler D. J.; Cho H. P.; Klar R.; Pancani T.; Klein M. T.; Bridges T. M.; Morrison R. D.; Blobaum A. L.; Xiang Z.; Daniels J. S.; Niswender C. M.; Conn P. J.; Wood M. R.; Lindsley C. W. (2012) Targeting Selective Activation of M1 for the Treatment of Alzheimer’s Disease: Further Chemical Optimization and Pharmacological Characterization of the M1 Positive Allosteric Modulator ML169. ACS Chem. Neurosci. 3, 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding T. A.; Trotter C.; Skjaerbaek N.; Messier T. L.; Currier E. A.; Burstein E. S.; Li D.; Hacksell U.; Brann M. R. (2002) Discovery of an Ectopic Activation Site on the M1 Muscarinic Receptor. Mol. Pharmacol. 61, 1297–1302. [DOI] [PubMed] [Google Scholar]
- Avlani V. A.; Langmead C. J.; Guida E.; Wood M. D.; Tehan B. G.; Herdon H. J.; Watson J. M.; Sexton P. M.; Christopoulos A. (2010) Orthosteric and Allosteric Modes of Interaction of Novel Selective Agonists of the M1 Muscarinic Acetylcholine Receptor. Mol. Pharmacol. 78, 94–104. [DOI] [PubMed] [Google Scholar]
- Langmead C. J.; Fry V. A. H.; Forbes I. T.; Branch C. L.; Christopoulos A.; Wood M. D.; Herdon H. J. (2006) Probing the Molecular Mechanism of Interaction between 4-n-Butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine (AC-42) and the Muscarinic M1 Receptor: Direct Pharmacological Evidence That AC-42 Is an Allosteric Agonist. Mol. Pharmacol. 69, 236–246. [DOI] [PubMed] [Google Scholar]
- Spalding T. A.; Ma J.-N.; Ott T. R.; Friberg M.; Bajpai A.; Bradley S. R.; Davis R. E.; Brann M. R.; Burstein E. S. (2006) Structural Requirements of Transmembrane Domain 3 for Activation by the M1 Muscarinic Receptor Agonists AC-42, AC-260584, Clozapine, and N-Desmethylclozapine: Evidence for Three Distinct Modes of Receptor Activation. Mol. Pharmacol. 70, 1974–1983. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A.; O’Brien J. A.; Pascarella D.; Mallorga P. J.; Scolnick E. M.; Sur C. (2004) Mapping the interaction site of M1 muscarinic receptor allosteric agonists. Soc. Neurosci. Abstr. 30, 846.16. [Google Scholar]
- Thomas R. L.; Mistry R.; Langmead C. J.; Wood M. D.; Challiss R. A. J. (2008) G Protein Coupling and Signaling Pathway Activation by M1 Muscarinic Acetylcholine Receptor Orthosteric and Allosteric Agonists. J. Pharmacol. Exp. Ther. 327, 365–374. [DOI] [PubMed] [Google Scholar]
- Thomas R. L.; Langmead C. J.; Wood M. D.; Challiss R. A. J. (2009) Contrasting Effects of Allosteric and Orthosteric Agonists on M1 Muscarinic Acetylcholine Receptor Internalization and Down-regulation. J. Pharmacol. Exp. Ther. 331, 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G.; Langmead C. J.; Tehan B. G.; Hulme E. C. (2009) Mutagenic Mapping Suggests a Novel Binding Mode for Selective Agonists of M1 Muscarinic Acetylcholine Receptors. Mol. Pharmacol. 75, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. R.; Lameh J.; Ohrmund L.; Son T.; Bajpai A.; Nguyen D.; Friberg M.; Burstein E. S.; Spalding T. A.; Ott T. R.; Schiffer H. H.; Tabatabaei A.; McFarland K.; Davis R. E.; Bonhaus D. W. (2010) AC-260584, an orally bioavailable M1 muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology 58, 365–373. [DOI] [PubMed] [Google Scholar]
- Vanover K. E.; Veinbergs I.; Davis R. E. (2008) Antipsychotic-Like Behavioural Effects and Cognitive Enhancement by a Potent and Selective Muscarinic M1 Receptor Agonist AC-260584. Behav. Neurosci. 122, 570–575. [DOI] [PubMed] [Google Scholar]
- Langmead C. J.; Austin N. E.; Branch C. L.; Brown J. T.; Buchanan K. A.; Davies C. H.; Forbes I. T.; Fry V. A. H.; Hagan J. J.; Herdon H. J.; Jones G. A.; Jeggo R.; Kew J. N. C.; Mazzali A.; Melarange R.; Patel N.; Pardoe J.; Randall A. D.; Roberts C.; Roopun A.; Starr K. R.; Teriakidis A.; Wood M. D.; Whittington M.; Wu Z.; Watson J. (2008) Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br. J. Pharmacol. 154, 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K. A.; Petrovic M. M.; Chamberlain S. E. L.; Marrion N. V.; Mellor J. R. (2010) Facilitation of Long-Term Potentiation by Muscarinic M1 Receptors Is Mediated by Inhibition of SK Channels. Neuron 68, 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki T.; Nagao N.; Nakahara T. (2008) Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesis. Prog. Brain Res. 172, 199–212. [DOI] [PubMed] [Google Scholar]
- Sur C.; Mallorga P. J.; Wittmann M.; Jacobson M. A.; Pascarella D.; Williams J. B.; Brandish P. E.; Pettibone D. J.; Scolnick E. M.; Conn P. J. (2003) N-Desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. Proc. Natl. Acad. Sci. U.S.A. 100, 13674–13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. R.; Dada A.; Jones G. A.; Deisz R. A.; Gigout S.; Langmead C. J.; Werry T. D.; Hendry N.; Hagan J. J.; Davies C. H.; Watson J. M. (2010) N-Desmethylclozapine (NDMC) is an antagonist at the human native muscarinic M1 receptor. Neuropharmacology 58, 1206–1214. [DOI] [PubMed] [Google Scholar]
- Jones C. K.; Brady A. E.; Davis A. A.; Xiang Z.; Bubser M.; Noor Tantawy M.; Kane A. S.; Bridges T. M.; Kennedy J. P.; Bradley S. R.; Peterson T. E.; Ansari M. S.; Baldwin R. M.; Kessler R. M.; Deutch A. Y.; Lah J. J.; Levey A. I.; Lindsley C. W.; Conn P. J. (2008) Novel Selective Allosteric Activator of the M1 Muscarinic Acetylcholine Receptor Regulates Amyloid Processing and Produces Antipsychotic-Like Activity in Rats. J. Neurosci. 28, 10422–10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. M.; Brady A. E.; Kennedy J. P.; Daniels R. N.; Miller N. R.; Kim K.; Breininger M. L.; Gentry P. R.; Brogan J. T.; Jones C. K.; Conn P. J.; Lindsley C. W. (2008) Synthesis and SAR of analogues of the M1 allosteric agonist TBPB. Part I: Exploration of alternative benzyl and privileged structure moieties. Bioorg. Med. Chem. Lett. 18, 5439–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. R.; Daniels R. N.; Bridges T. M.; Brady A. E.; Conn P. J.; Lindsley C. W. (2008) Synthesis and SAR of analogs of the M1 allosteric agonist TBPB. Part II: Amides, sulfonamides and ureas-The effect of capping the distal basic piperidine nitrogen. Bioorg. Med. Chem. Lett. 18, 5443–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler D. J.; Sevel C.; Le U.; Lovett K. M.; Tarr J. C.; Carrington S. J. S.; Cho H. P.; Digby G. J.; Niswender C. M.; Conn P. J.; Hopkins C. R.; Wood M. R.; Lindsley C. W. (2013) Further exploration of M1 allosteric agonists: Subtle structural changes abolish M1 allosteric agonism and result in pan-mAChR orthosteric antagonism. Bioorg. Med. Chem. Lett. 23, 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois E. P.; Bridges T. M.; Lewis L. M.; Dawson E. S.; Kane A. S.; Xiang Z.; Jadhav S. B.; Yin H.; Kennedy J. P.; Meiler J.; Niswender C. M.; Jones C. K.; Conn P. J.; Weaver C. D.; Lindsley C. W. (2010) Discovery and Characterization of Novel Subtype-Selective Allosteric Agonists for the Investigation of M1 Receptor Function in the Central Nervous System. ACS Chem. Neurosci. 1, 104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois E. P.; Digby G. J.; Sheffler D. J.; Melancon B. J.; Tarr J. C.; Cho H. P.; Miller N. R.; Morrison R.; Bridges T. M.; Xiang Z.; Daniels J. S.; Wood M. R.; Conn P. J.; Lindsley C. W. (2011) Development of a highly selective, orally bioavailable and CNS penetrant M1 agonist derived from the MLPCN probe ML071. Bioorg. Med. Chem. Lett. 21, 6451–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon B. J.; Gogliotti R. D.; Tarr J. C.; Saleh S. A.; Chauder B. A.; Lebois E. P.; Cho H. P.; Utley T. J.; Sheffler D. J.; Bridges T. M.; Morrison R. D.; Daniels J. S.; Niswender C. M.; Conn P. J.; Lindsley C. W.; Wood M. R. (2012) Continued optimization of the MLPCN probe ML071 into highly potent agonists of the hM1 muscarinic acetylcholine receptor. Bioorg. Med. Chem. Lett. 22, 3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby G. J.; Noetzel M. J.; Bubser M.; Utley T. J.; Walker A. G.; Byun N. E.; Lebois E. P.; Xiang Z.; Sheffler D. J.; Cho H. P.; Davis A. A.; Nemirovsky N. E.; Mennenga S. E.; Camp B. W.; Bimonte-Nelson H. A.; Bode J.; Italiano K.; Morrison R.; Daniels J. S.; Niswender C. M.; Olive M. F.; Lindsley C. W.; Jones C. K.; Conn P. J. (2012) Novel Allosteric Agonists of M1 Muscarinic Acetylcholine Receptors Induce Brain Region-Specific Responses That Correspond with Behavioural Effects in Animal Models. J. Neurosci. 32, 8532–8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby G. J.; Utley T. J.; Lamsal A.; Sevel C.; Sheffler D. J.; Lebois E. P.; Bridges T. M.; Wood M. R.; Niswender C. M.; Lindsley C. W.; Conn P. J. (2012) Chemical Modification of the M1 Agonist VU0364572 Reveals Molecular Switches in Pharmacology and a Bitopic Binding Mode. ACS Chem. Neurosci. 3, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams A. G.; Hentzer M.; Mikkelsen G. K.; Larsen K.; Bundgaard C.; Plath N.; Christoffersen C. T.; Bang-Andersen B. (2010) Discovery of N-{1-[3-(3-Oxo-2,3-dihydrobenzo[1,4]oxazin-4-yl)propyl]piperidin-4-yl}-2-phenylacetamide (Lu AE51090): An Allosteric Muscarinic M1 Receptor Agonist with Unprecedented Selectivity and Procognitive Potential. J. Med. Chem. 53, 6386–6397. [DOI] [PubMed] [Google Scholar]
- Sams A. G.; Larsen K.; Mikkelsen G. K.; Hentzer M.; Christoffersen C. T.; Jensen K. G.; Frederiksen K.; Bang-Andersen B. (2012) Hit-to-lead investigation of a series of novel combined dopamine D2 and muscarinic M1 receptor ligands with putative antipsychotic and pro-cognitive potential. Bioorg. Med. Chem. Lett. 22, 5134–5140. [DOI] [PubMed] [Google Scholar]
- Budzik B.; Garzya V.; Shi D.; Foley J. J.; Rivero R. A.; Langmead C. J.; Watson J.; Wu Z.; Forbes I. T.; Jin J. (2010) 2′ Biaryl amides as novel and subtype selective M1 agonists. Part I: Identification, synthesis, and initial SAR. Bioorg. Med. Chem. Lett. 20, 3540–3544. [DOI] [PubMed] [Google Scholar]
- Budzik B.; Garzya V.; Shi D.; Walker G.; Lauchart Y.; Lucas A. J.; Rivero R. A.; Langmead C. J.; Watson J.; Wu Z.; Forbes I. T.; Jin J. (2010) 2′ Biaryl amides as novel and subtype selective M1 agonists. Part II: Further optimization and profiling. Bioorg. Med. Chem. Lett. 20, 3545–3549. [DOI] [PubMed] [Google Scholar]
- Budzik B.; Garzya V.; Shi D.; Walker G.; Woolley-Roberts M.; Pardoe J.; Lucas A.; Tehan B.; Rivero R. A.; Langmead C. J.; Watson J.; Wu Z.; Forbes I. T.; Jin J. (2010) Novel N-Substituted Benzimidazolones as Potent, Selective, CNS-Penetrant, and Orally Active M1 mAChR Agonists. ACS Med. Chem. Lett. 1, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. J.; Forbes I. T.; Watson S. P.; Garzya V.; Stevenson G. I.; Walker G. R.; Mudhar H. S.; Flynn S. T.; Wyman P. A.; Smith P. W.; Murkitt G. S.; Lucas A. J.; Mookherjee C. R.; Watson J. M.; Gartlon J. E.; Bradford A. M.; Brown F. (2010) The discovery of a series of N-substituted 3-(4-piperidinyl)-1,3-benzoxazolinones and oxindoles as highly brain penetrant, selective muscarinic M1 agonists. Bioorg. Med. Chem. Lett. 20, 5434–5438. [DOI] [PubMed] [Google Scholar]
- Nathan P. J.; Watson J.; Lund J.; Davies C. H.; Peters G.; Dodds C. M.; Swirski B.; Lawrence P.; Bentley G. D.; O’Neill B. V.; Robertson J.; Watson S.; Jones G. A.; Maruff P.; Croft R. J.; Laruelle M.; Bullmore E. T. (2013) The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int. J. Neuropsychopharmacol. 16, 721–731. [DOI] [PubMed] [Google Scholar]