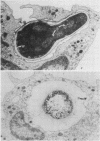
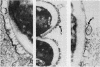
Abstract
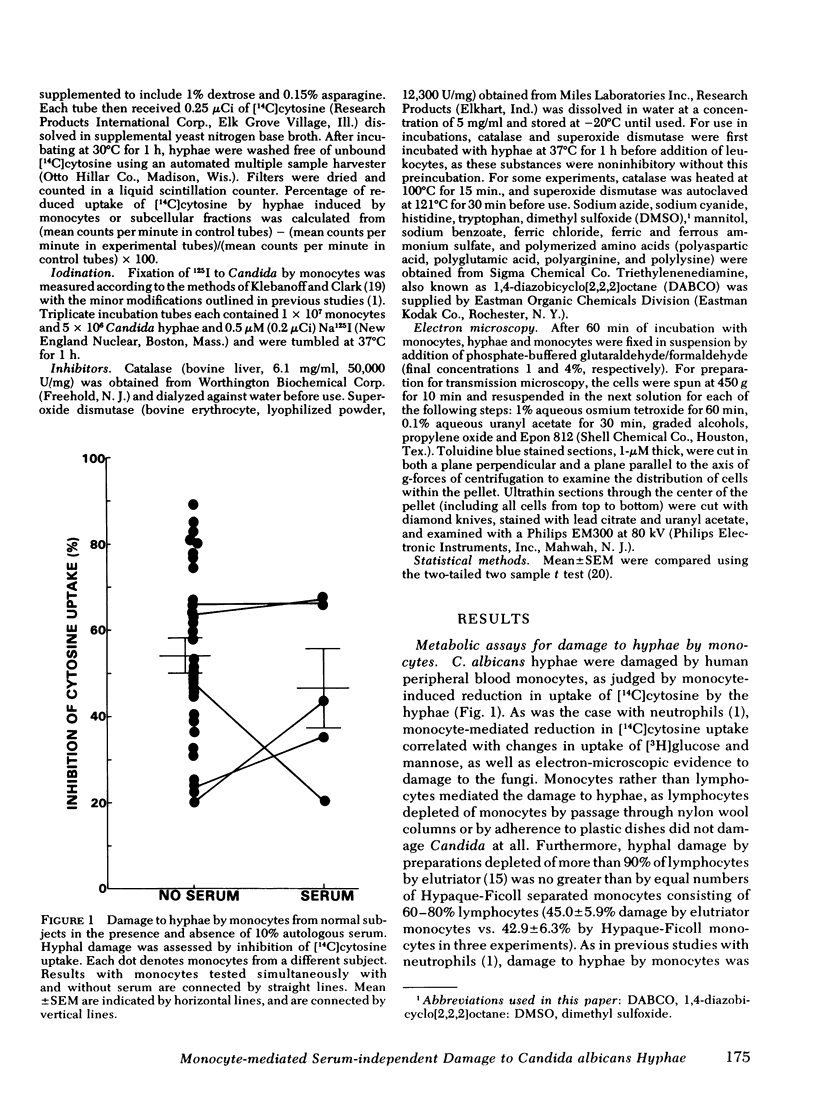
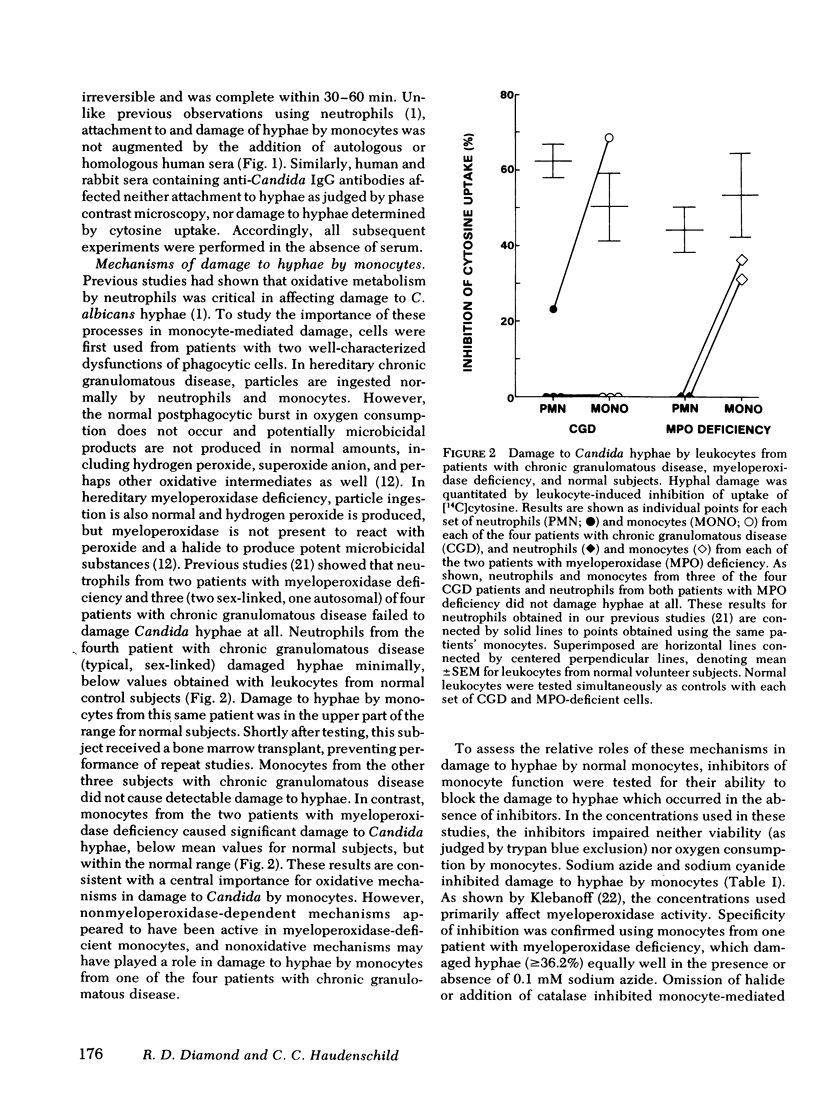
Human peripheral blood monocytes attached to Candida albicans hyphae in the absence of serum and damaged the hyphae without completely ingesting them. Attachment and damage was not augmented by the addition of serum. Damage to hyphae was quantitated by a previously developed metabolic assay that measured leukocyte-induced reduction in uptake of [14C]cytosine by the hyphae. Use of cells from patients with hereditary disorders of leukocyte function, chronic granulomatous disease, and myeloperoxidase deficiency indicated that myeloperoxidase-independent and nonoxidative mechanisms could sometimes damage hyphae where oxidative mechanisms were impaired. Damage to hyphae by normal monocytes was inhibited by concentrations of sodium azide and sodium cyanide that primarily affect myeloperoxidase activity, as well as by halide-free conditions, catalase, and putative antagonists of hypochlorous acid or singlet oxygen. Iodination of hyphae, a myeloperoxidase and hydrogen peroxide-dependent process of monocytes, was similarly inhibited by sodium azide, sodium cyanide, and catalase. Under anaerobic conditions, damage to hyphae was reduced by 64.0-68.4%. In contrast, inhibitors of potential nonoxidative antifungal mechanisms, iron salts to saturate iron chelators, and polyanionic amino acid polymers to neutralize cationic proteins did not block damage to hyphae by monocytes. Preparations rich in lysosomal granules from fractionated normal monocytes also did not damage hyphae. Overall, it appeared that oxidative mechanisms were most important for damage to hyphae by normal monocytes.
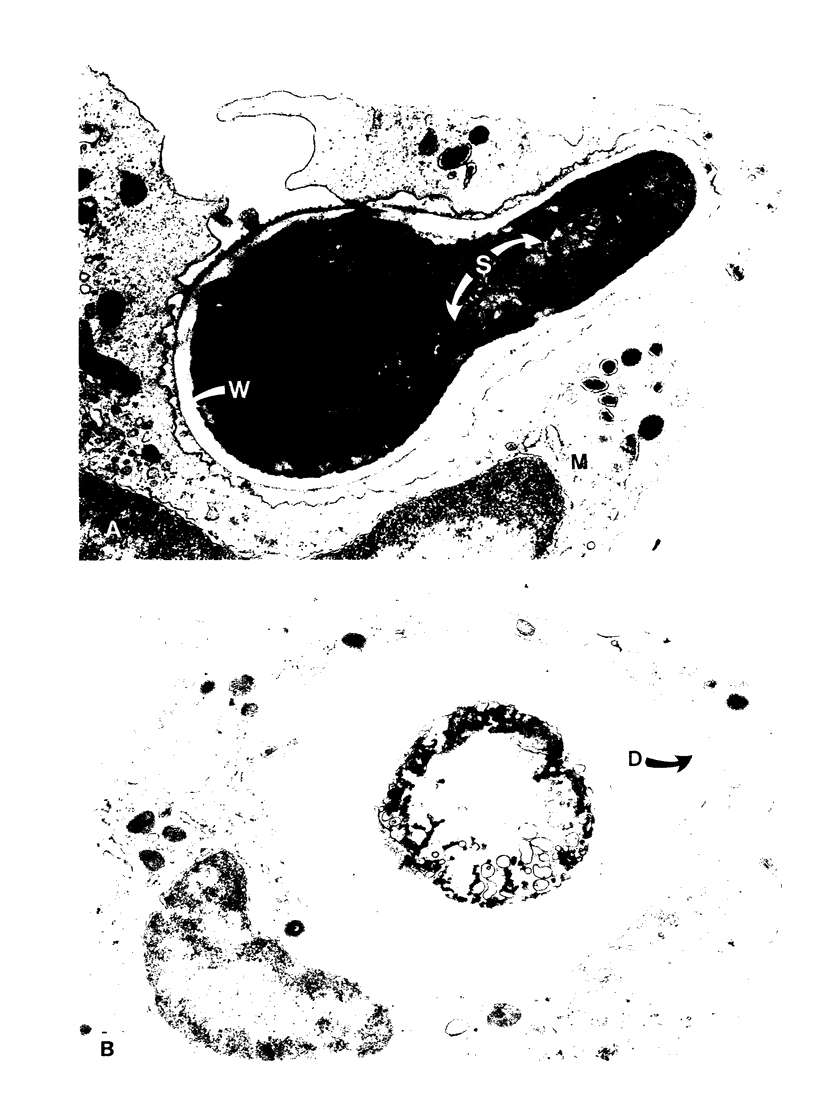
Electron microscopy confirmed that Candida hyphae were damaged and probably killed by monocytes, but monocytes appeared to sustain significant damage in the process. In the absence of serum, monocyte cell membranes became closely approximated to Candida cell walls. It appeared that some Candida could escape this partial engulfment, as they were seen floating free with vesicular trilaminar membrane remnants covering hyphal surfaces. In general, monocytes appeared to be damaged by interactions with Candida hyphae more than neutrophils had been in previous studies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Monocyte function in children with neutropenia and chronic infections. Blood. 1972 Jul;40(1):31–41. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cline M. J. Bactericidal Activity of Human Macrophages: Analysis of Factors Influencing the Killing of Listeria monocytogenes. Infect Immun. 1970 Aug;2(2):156–161. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I., Territo M. C., Golde D. W. UCLA Conference. Monocytes and macrophages: functions and diseases. Ann Intern Med. 1978 Jan;88(1):78–88. doi: 10.7326/0003-4819-88-1-78. [DOI] [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A., Haudenschild C. C. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest. 1980 Nov;66(5):908–917. doi: 10.1172/JCI109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadebusch H. H., Johnson A. G. Natural host resistance to infection with Cryptococcus neoformans. IV. The effect of some cationic proteins on the experimental disease. J Infect Dis. 1966 Dec;116(5):551–565. doi: 10.1093/infdis/116.5.551. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The production of superoxide radical during the decomposition of potassium peroxochromate(V). Biochemistry. 1974 Aug 27;13(18):3811–3815. doi: 10.1021/bi00715a030. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball H. R., Ford G. H., Wolff S. M. Lysosomal enzymes in normal and Chediak-Higashi blood leukocytes. J Lab Clin Med. 1975 Oct;86(4):616–630. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E. Metabolic event involved in the bactericidal activity of normal mouse macrophages. Infect Immun. 1971 Mar;3(3):390–397. doi: 10.1128/iai.3.3.390-397.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson V. L., Hansing R. L., McClary D. O. The role of metabolic energy in the lethal action of basic proteins on Candida albicans. Can J Microbiol. 1977 Feb;23(2):166–174. doi: 10.1139/m77-024. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Growth inhibition of Candida albicans by rabbit alveolar macrophages. Infect Immun. 1977 Mar;15(3):910–915. doi: 10.1128/iai.15.3.910-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Inhibition of specific amino acid uptake in Candida albicans by lysosomal extracts from rabbit alveolar macrophages. Infect Immun. 1978 Aug;21(2):506–513. doi: 10.1128/iai.21.2.506-513.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. J., Sagone A. L., Balcerzak S. P., Ackerman G. A., LoBuglio A. F. Effects of corticosteroid therapy on human monocyte function. N Engl J Med. 1975 Jan 30;292(5):236–241. doi: 10.1056/NEJM197501302920504. [DOI] [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Singh H., Vadasz J. A. Singlet oxygen: a major reactive species in the furocoumarin photosensitized inactivation of E. coli ribosomes. Photochem Photobiol. 1978 Oct-Nov;28(4-5):539–545. doi: 10.1111/j.1751-1097.1978.tb06966.x. [DOI] [PubMed] [Google Scholar]
- Slivka A., LoBuglio A. F., Weiss S. J. A potential role for hypochlorous acid in granulocyte-mediated tumor cell cytotoxicity. Blood. 1980 Feb;55(2):347–350. [PubMed] [Google Scholar]
- Steigbigel R. T., Lambert L. H., Jr, Remington J. S. Phagocytic and bacterial properties of normal human monocytes. J Clin Invest. 1974 Jan;53(1):131–142. doi: 10.1172/JCI107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo M. C., Cline M. J. Monocyte function in man. J Immunol. 1977 Jan;118(1):187–192. [PubMed] [Google Scholar]
- Thalinger K. K., Mandell G. L. Bactericidal activity of macrophages in an anaerobic environment. J Reticuloendothel Soc. 1971 May;9(5):393–396. [PubMed] [Google Scholar]
- Van Der Meer J. W., Leijh P. C., Van Den Barselaar, Van Furth R. Functions of phagocytic cells in chronic mucocutaneous candidiasis. Br Med J. 1978 Jan 21;1(6106):147–148. doi: 10.1136/bmj.1.6106.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Evidence for hydroxyl radical generation by human Monocytes. J Clin Invest. 1977 Aug;60(2):370–373. doi: 10.1172/JCI108785. [DOI] [PMC free article] [PubMed] [Google Scholar]