Abstract
Goals
To determine the efficacy and safety of combination therapy in patients with hepatitis C virus (HCV) and end-stage renal disease (ESRD).
Background
There is little data on the treatment of ESRD patients with pegylated interferon and ribavirin. We designed a pilot study to determine the initial and 12-week posttreatment viral response.
Study
A nonrandomized, prospective observational study of adjusted-dose combination therapy. Twenty patients were enrolled and began pegylated interferon at 135 μg/wk SC, and 4 weeks later ribavirin was started at 200 mg PO weekly, increasing gradually to 3 times a week for a total of 48 weeks.
Results
Twenty patients: M:F 18:2; mean age 52.4 years; genotype 1: 18, non-genotype 1: 2. Of the 20 patients, 5 withdrew before starting treatment. Of the 11 patients who reached 3 months, 6 had early virologic response, defined as at least a 2-log drop in their HCV count (54.5%). Of the 5 patients who were treated for 1-year, only 1 patient had a response 12 weeks after treatment. Side effects included 4 cases of anemia and 1 patient with headache.
Conclusions
The initial response rate in individuals taking 3 months of treatment in our study is comparable with studies in non-ESRD patients with no serious adverse side effects. However, the sustained posttreatment rate was low. This demonstrates that combination therapy is a safe therapeutic option in the ESRD population with HCV infection which needs further testing to determine if increasing the length of treatment and/or the dose of ribavirin will affect posttreatment rates.
Keywords: hepatitis C virus, end stage renal disease, combination therapy, pegylated interferon, ribavirin
Approximately 250,000 individuals in the United States have end-stage renal disease (ESRD), and the most common etiologies for this are hypertension and diabetes. The majority of these patients are dependent on hemodialysis (HD) and require this 2 to 3 times per-week. Patients with ESRD without other significant comorbidity are candidates for renal transplantation (RT), with approximately 15,000 such transplants being performed nationally every year. Patient survival is approximately 98% to 100% at 1 year posttransplant and 85% to 90% 5 years posttransplant.
Patients with ESRD have a high prevalence of infection with hepatitis C. Transmission of hepatitis C virus (HCV) in the HD population is thought to be via nosocomal routes, and there is a positive association between duration of HD and HCV seroprevalance, as well as prevalence of HCV in the general population and incidence of HCV seropositivity in HD units.1,2 Recent data, however, suggest that HCV infection is often acquired before developing ESRD.3 Also, HCV is known to be an independent risk factor for progression of renal disease in diabetic patients.4 Internationally reported prevalence rates of HCV infection in ESRD patients have ranged widely from 3% to 80%, with data from the Center of Diseases Control (CDC) estimating the prevalence at 8.5%.5,6 This may be an underestimation as the false negative rate for second generation enzyme-linked immunosorbent assays was approximately 2.6%.7 Using the conservative CDC estimate, the number of patients with ESRD and hepatitis C infection is approximately 22,000.
Histologic assessment of the severity of liver disease also corroborates the impression that HCV is a cause of significant morbidity and mortality in the ESRD population. Anti-HCV positivity was independently associated with death (relative risk: 1.57, 95% confidence interval: 1.23-2.00).8 Mild-to-moderate necroinflammatory activity is present in almost all patients studied. The incidence of bridging fibrosis or cirrhosis has ranged between 22% and 32%.9 The low serum aminotransferases in the ESRD population is well recognized, but still contributes to the false impression of a subclinical course of HCV infection in the ESRD population.1
Patients with ESRD and HCV should ideally be treated early as post-RT HCV titers increase by 1 to 2 log compared with pretransplant levels.5 Histologic progression has also been documented in HCV-infected patients post-RT.10 A prospective study of histologic progression comparing liver histology at a mean of 45 and 81 months post-RT demonstrated significant progression in 36% of patients.11 HCV infection has been identified as an independent risk factor for new-onset diabetes mellitus post-RT.12 Also, due to the high risk of rejection of the transplanted kidney it is not possible to treat with interferon after RT. Long-term survival analysis is available only from retrospective studies, but this does demonstrate a significantly reduced patient survival at 10 years or longer time points after transplantation as compared with non–HCV-infected patients.13–15
Trials of interferon-α monotherapy in ESRD patients have been published,16 but there are few reports of combined pegylated interferon and ribavirin therapy in these patients. Previous trials to treat these patients using interferon-α and ribavirin were aborted due to high incidence of hemolysis.17 A comprehensive review of the literature revealed a few recently published studies with promising results.18,19 Bruchfeld et al18 had 6 patients in their study and Mousa et al19 had a total of 20 treated patients. There have been no large-scale trials regarding combination therapy in ESRD patients.
Sustained virologic response rates in the ESRD patients treated with monotherapy have ranged between 19% and 64%.5 A recent study performed in a non-North American population (with predominantly HCV genotype 4) using combination interferon-α and low-dose ribavirin showed a sustained viral response rate of 50%.19 As interferon-α monotherapy is clearly inferior to pegylated interferon-α monotherapy and to combination therapy with pegylated interferon-α and ribavirin, there is an urgent need to bring the management of ESRD with hepatitis C infection up to date with current management of the general population with HCV infection.
MATERIALS AND METHODS
Study Design
This is a prospective nonrandomized trial of pegylated interferon α-2a and adjusted-dose ribavirin combination therapy for ESRD patients chronically infected with hepatitis C. Informed consent was obtained from all participating subjects. Twenty patients were recruited in the Section of Digestive Diseases at Yale University, and the guidelines for human-experimentation of the US Department of Health and Human Services, Roche pharmaceuticals, as well as the Yale University Human Investigation Committee were thoroughly adhered to.
Eligibility Criteria
Inclusion and exclusion criteria were defined as in Table 1. After meeting the criteria, patients underwent screening procedures including history and physical examination, liver function tests and chem-7, HCV titer, HCV genotype, electrocardiogram, liver imaging (ultrasound, computed tomography, or magnetic resonance imaging), iron studies, autoantibodies, cryoglobulins, haptoglobin, lactate dehydrogenase (LDH), and serum electrophoresis if not performed in the last 6 months. A transjugular liver biopsy was obtained and read by a single pathologist who was blinded to the clinical data.
TABLE 1.
Inclusion/Exclusion Criteria
| Inclusion criteria |
| Age 18 y or above |
| Male or female |
| Presence of hepatitis C virus (HCV) RNA (with or without anti-HCV) in serum |
| Any genotype of HCV |
| Any serum alanine (ALT) or aspartate (AST) aminotransferase |
| Must be able to provide written informed consent |
| No hemolysis at baseline based on serum haptoglobin and LDH |
| ESRD as defined by requiring dialysis |
| Exclusion criteria |
| Decompensated liver disease, as marked by bilirubin > 4 mg/dL, albumin < 3.0 g/dL, prothrombin time > 2 s prolonged, or history of bleeding esophageal varices, ascites, or hepatic encephalopathy |
| Significant systemic or major illnesses other than renal failure including active cardiac disease, reversible defects on cardiac imaging, organ transplantation, serious psychiatric disease or depression, human immunodeficiency virus (HIV) infection, and angina pectoris will be excluded |
| Immunosuppressive therapy with either corticosteroids or major immunosuppressive agents |
| Patients with active excessive alcohol intake (> 50 g/d) |
| Evidence of another form of liver disease in addition to viral hepatitis (eg, autoimmune liver disease, Wilson disease, alcoholic liver disease, hemochromatosis, α-1-antitrypsin deficiency) |
| Evidence of hepatocellular carcinoma; either alphafetoprotein (AFP) levels > 50 ng/mL and/or ultrasound (or other imaging study) demonstrating a mass suggestive of liver cancer will be excluded |
| Use of antiviral therapy within the last 6 mo |
| Evidence of coronary artery disease or cerebral vascular disease, including abnormalities on exercise stress testing in patients with defined risk factors who will be screened for evidence of underlying coronary artery disease |
| Patients with active, serious autoimmune disease such as lupus erythematosis, ulcerative colitis, Crohn's disease, or rheumatoid arthritis that in the opinion of the investigators might be exacerbated by therapy with α interferon will be excluded |
| Patients enrolled in other therapeutic trials |
| Pregnancy will be a contraindication. All females will have a urine pregnancy test and before starting ribavirin, and all sexually active patients will have to use 2 forms of contraception to ensure pregnancy does not occur |
| Any medical condition or medication, which increases the likelihood of hemolysis |
| Anemia with a hemoglobin below 9.5 g/dL of any etiology |
Study Treatment
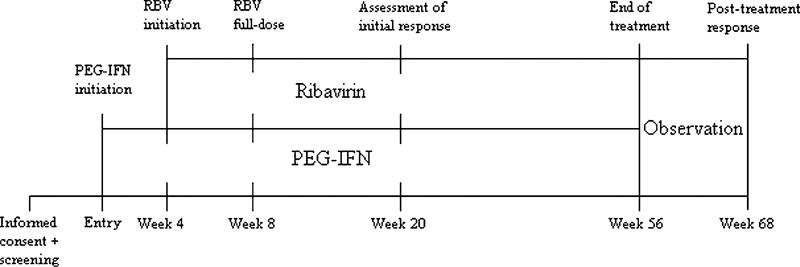
Eligible patients were started on pegylated interferon-α2a (Roche-Nutley, NJ) administered once weekly by SC injection at a dose of 135 μg (Fig. 1). Pegylated interferon is an ideal choice for use in the ESRD population because detailed pharmokinetic data are available for this molecule from patients undergoing HD.20 Four weeks after starting pegylated interferon-α2a, all patients were started on ribavirin 200 mg PO once a week. At week 6 this was increased to 200 mg twice a week and at week 8 to 200 mg 3 times a week. This became the maximum dose and provided an average of 85 mg a day. This is significantly below 175 to 300 mg a day that has been used without complications in a similar ESRD population.21 Subjects were then treated for 48 weeks. Patients having side effects from ribavirin discontinued the medication and restarted when their hemoglobin (Hgb) returned to above 10 mg/dL.
FIGURE 1.
Study timeline.
Study Evaluations
Patients were tested for adverse events using clinical visits and laboratory tests as described at regular intervals. These tests included HCV titer, complete blood count, haptoglobin and LDH, and liver function tests. If the patient did not have a scheduled visit, these results were obtained from their respective dialysis centers and/or primary care physicians.
RESULTS
Study Subjects
Twenty patients were enrolled in the study from September 2003 to December 2004 at the Yale New Haven Hospital. The distribution of age, sex, race, liver pathology, renal etiology, type of dialysis, and HCV genotype are shown in Table 2. Of note are the distributions of the sex (90% male) and HCV genotype (90% type 1).
TABLE 2.
Patient Demographics
| Age | |
| Mean | 52.1 |
| SD | 7.8 |
| Sex | |
| Male | 18 |
| Female | 2 |
| Dialysis | |
| Peritoneal | 4 |
| Hemo | 16 |
| Race | |
| Black | 15 |
| Hispanic | 2 |
| White | 3 |
| Liver path | |
| Stage I | 4 |
| Stage II | 8 |
| Stages II-III | 2 |
| Stage III | 6 |
| Renal etiology | |
| HTN | 9 |
| DM | 1 |
| Both | 7 |
| Other | 3 |
| Hepatitis C virus genotype | |
| 1 (no subtype) | 7 |
| Type 1a | 1 |
| Type 1b | 10 |
| Type 2 | 2 |
DM indicates diabetes mellitus; HTN, hypertension.
Study Disposition
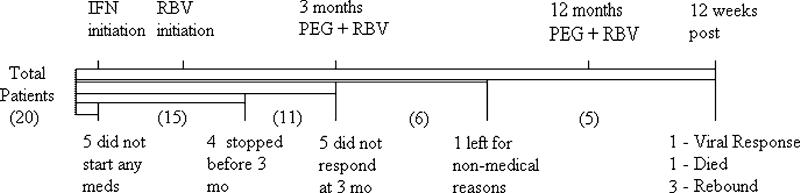
Of the 20 subjects enrolled, 11 reached the 3-month time period. Five from the initial 20 withdrew from the study before starting treatment. Four of the 15 patients who started medications discontinued before 3 months of treatment; 1 for moderate fatigue (no. 2), 1 for recurrence of a previous condition of hematuria (no. 19), 1 for pending operation on peritoneal dialysis site and not wanting to continue (no. 18), and 1 patient had difficulty tolerating pegylated interferon without meeting protocol criteria for discontinuation (no. 17).
One patient (no. 15) did not start ribavirin at any time due to patient's concern for anemia. Only 1 patient (no. 11) after reaching the 3-month time point became noncompliant with his medications. A simplified flow sheet describes the outcomes of the 20 patients who enrolled in Figure 2.
FIGURE 2.
Patient flow sheet.
Adverse Events
Malaise/fatigue was present in all patients to some degree and was typically worse for the first 3 to 4 weeks. This resulted in discontinuation of treatment for 1 patient. One patient complained of mild headaches that did not require stopping treatment. There were minimal arthralgias and myalgias, no reported depression, and no leukocytopenia.
Anemia was the most serious side effect associated with treatment. Although all patients had increased erythropoietin doses, there were 3 subjects whose Hgb dropped below 9 g/dL. One patient had to delay increasing ribavirin until their Hgb stabilized. One patient had to discontinue ribavirin until Hgb reached 10.5 g/dL. One patient preferred to discontinue all medications until anemia resolved and later decided not to continue treatment. It is important to note that none of the cases of anemia were associated with any hemolysis as demonstrated by normal LDH levels.
Virologic Responses
Of the 11 patients who reached the 3-month time point, 5 were determined to be nonresponders defined as having less than a 2-log drop in HCV titer levels (Table 3). Two of the nonresponders decided to continue therapy for 3 more months to assess a possible late response. Of these two, one patient (no. 12) did have a later significant drop in HCV titer and continued therapy for 1 year; whereas, the other patient (no. 5) discontinued his medication.
TABLE 3.
Individual Patient Results
| Number | Sex | Age | Ethnic | Renal Etiology | Liver Biopsy | Hepatitis C Virus Type | Viral Load | Interferon | Ribavirin | 4 wk | 12 wk | 52 wk | 64 wk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 44 | Black | CRF, kidney donation | G II S II | 1a | 667 k | Y | Y | N | N | — | — |
| 2 | F | 57 | Black | HTN, cocaine | G I S III | 1b | > 500 k | Y | N | — | — | — | — |
| 3 | M | 48 | White | HTN, kidney ttx | G I S II | 2 | > 500 k | Y | Y | N | Y | Y | Y |
| 4 | M | 47 | Black | HTN | G I S II | 1b | > 500 k | N | N | — | — | — | — |
| 5 | M | 63 | Black | HTN, DM2, R nephrectomy | G I S I | 1 | 88 k | Y | Y | N | N | — | — |
| 6 | M | 62 | Black | HTN, DM2 | G I S II | 1b | > 500 k | N | N | — | — | — | — |
| 7 | M | 58 | Black | HTN | G II S III | 1 | > 850 k | Y | Y | Y | Y | Y | Died |
| 8 | M | 52 | Hispanic | HTN, DM2 | G I S III | 1b | Unknown | N | N | — | — | — | — |
| 9 | M | 53 | Black | HTN, DM2 | G I S I | 1b | > 500 k | N | N | — | — | — | — |
| 10 | M | 37 | Black | Congenital aplasia | G I S II | 1b | 201 k | N | N | — | — | — | — |
| 11 | M | 44 | Black | HTN | G I S I | 1 | 480 k | Y | Y | N | Y | N | — |
| 12 | M | 56 | Hispanic | Unknown | G I S III | 1b | 27 k | Y | Y | N | N | Y | N |
| 13 | M | 35 | Black | HTN | G I S II | 1 | 281 k | Y | Y | N | Y | Y | N |
| 14 | M | 60 | White | HTN, chronic GNP | G I S II-III | 1b | 362 k | Y | Y | N | Y | Y | N |
| 15 | M | 55 | White | HTN, R nephrectomy | G I S II-III | 2 | 1460 k | Y | N | Y | Y | Y | N |
| 16 | M | 54 | Black | HTN | G I S II | 1 | 6660 k | Y | Y | N | N | — | — |
| 17 | M | 55 | Black | HTN, DM2 | Stage III | 1 | > 500 k | Y | N | — | — | — | — |
| 18 | M | 53 | Black | DM2 | GISI | 1 | 777 k | Y | N | — | — | — | — |
| 19 | M | 56 | Black | HTN, DM2 | G I S III | 1b | 160 k | Y | N | — | — | — | — |
| 20 | F | 60 | Black | HTN, DM2 | G I S II | 1b | 636 k | Y | Y | N | N | — | — |
CRF indicates chronic renal failure; DM, diabetes mellitus; GNP, glomerulonephritis; HTN, hypertension.
A rapid viral response, a 4-week significant result (greater than a 2-log drop in HCV titer levels) was seen in 2/11 patients. At the 3-month assessment, 6/11 patients (54.5%) had a significant drop in their HCV levels. These patients continued on 1 year of therapy. One of these patients (no. 11) was lost to follow-up and stopped taking medication for no stated reason. His HCV titer rebounded, and although he restarted his medication, there was no response after 3 months after reinitiating his treatment.
Patient (no. 18) discontinued medications at 3 weeks because he required surgery on his dialysis site and decided not to continue his treatment postsurgery. He did however have a biologic response at week 3 into therapy.
Overall, out of the 15 patients who began treatment, 8 (53.3%) had a significant drop in their HCV levels at some point during treatment. This offset by the fact that only 1 patient had a posttreatment response (6.7% of those who began treatment). The results are detailed in Table 3.
Five patients reached the 48-week time point for evaluation and were taken off medication to assess the 12-week posttreatment response. Of these patients, 2 were HCV genotype 2. One patient (no. 15) with type 2 did not take ribavirin during the study because of his concerns for anemia, and his HCV levels rebounded. The other patient (no. 3) had a sustained response at 12 weeks posttreatment (68 wk total).
From the 4 genotype 1 patients who took medications for 48 weeks, 1 (no. 7) died of nonrelated causes before reaching the 12-week posttreatment assessment. The other 3 had rebounding of their HCV titers. Thus in our study, only 1/15 (6.7%) patients who began treatment, or 1/5 patients (20%) who reached the 1-year time point had a sustained response at 12 weeks posttreatment. This is significantly lower than recently published reports on sustained viral responses (34%) using only interferon-α therapy.22
DISCUSSION
This pilot study found that the treatment of patients having ESRD and HCV infection with low-dose pegylated interferon and ribavirin is a safe therapeutic option. There was a discontinuation rate of 20% of all patients (26.7% of patients who started) before week 20. Only 1 patient stopped medication after the 3-month assessment, and this was not due to side effects. He later resumed treatment but was unresponsive to the second round. Of those who discontinued, only 1 patient stated withdrawing due to side effects of the medication, and the others had nonmedical reasons.
From the subjects who began treatment, our study found a high rate of initial response to therapy (53.3%). The lack of an adequate 12-week posttreatment response in our patient population (6.7%) is disappointing. The patient who did respond was also type 2 HCV, which is known to be more amiable to treatment. The low response may be due to the suboptimal levels of ribavirin in the serum of our patients due to either noncompliance or inadequate dosing.
Another possibility of the lack of long-term efficacy could be due to these patients’ comorbid diseases, and thus they will need to be treated longer than the standard 48-week protocol to have a sustained response. This is further highlighted by recent studies which document patients with type 1 HCV benefiting from longer treatment.23,24
Another interesting aspect of our study is the observed need of 100% of subjects requiring an increase in their dose of erythropoietin, which would suggest that patients beginning therapy should have an automatic increase.
Our study has several important limitations. The sample size was relatively small (15 patients initiating treatment). We used an adjusted-dose protocol that is lower than published uses in this population. Serum ribavirin levels have not been assessed and could be suboptimal. A repeat liver biopsy was not performed to determine effects of treatment on necroinflammation and fibrosis. HCV titers have not been assessed 1 year after patient undergoing RT to see if treatment had an effect on the 2-log increase in HCV levels between pretransplantation and posttransplantation. The difference between our results and the recently published data could be resolved by comparing ribavirin levels and thus adding to the optimal treatment regimen for these complicated patients.
In summary, the treatment of patients having ESRD and HCV with low-dose pegylated interferon and ribavirin is safe and associated with minimal side effects and low rates of discontinuation. Although the initial response was adequate, the lack of a significant sustained response at 12 weeks posttreatment would suggest that the study be repeated with greater numbers, a potentially higher dose of ribavirin, and increasing the length of treatment. Patients will also need to be antecedently dosed with higher levels of erythropoietin.
Acknowledgments
Supported by Roche Pharmaceuticals.
Footnotes
Conflict of interest disclosure: there are no conflicts of interest for any of the authors.
REFERENCES
- 1.Fabrizi F, Martin P, Dixit V, et al. Acquisition of hepatitis C virus in hemodialysis patients: a prospective study by branched DNA signal amplification assay. Am J Kidney Dis. 1998;31:647–654. doi: 10.1053/ajkd.1998.v31.pm9531181. [DOI] [PubMed] [Google Scholar]
- 2.Pujol H, Ponce IG, Lema MG, et al. High incidence of hepatitis C virus infection in hemodialysis patients in units with high prevalence. J Clin Microbiol. 1996;34:1633–1636. doi: 10.1128/jcm.34.7.1633-1636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman S, Accortt N, Turner A, et al. Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis. 2005;45:684–689. doi: 10.1053/j.ajkd.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Crook ED, Penumalee S, Gavini B, et al. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care. 2005;28:2187–2191. doi: 10.2337/diacare.28.9.2187. [DOI] [PubMed] [Google Scholar]
- 5.Fabrizi F, Poordad FF, Martin P. Hepatitis C infection and the patient with end-stage renal disease. Hepatology. 2002;36:3–10. doi: 10.1053/jhep.2002.34613. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Recommendations for preventing transmission of infections among chronic hemodialysis patients. Morb Mor Weekly Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 7.Bukh J, Wantzin P, Krogsgaard K, et al. High prevalence of hepatitis C virus (HCV) RNA in dialysis patients: failure of commercially available antibody tests to identify a significant number of patients with HCV infection. J Infect Dis. 1993;168:1343–1348. doi: 10.1093/infdis/168.6.1343. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama E, Akiba T, Marumo F, et al. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11:1896–1902. doi: 10.1681/ASN.V11101896. [DOI] [PubMed] [Google Scholar]
- 9.Sterling RK, Sanyal AJ, Luketic VA, et al. Chronic hepatitis C infection in patients with end stage disease characterization of liver histology and viral load in patients awaiting transplantation. Am J Gastroenterol. 1999;94:3576–3582. doi: 10.1111/j.1572-0241.1999.01649.x. [DOI] [PubMed] [Google Scholar]
- 10.Izopet J, Rostaing L, Sandres K, et al. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infec Dis. 2000;181:852–858. doi: 10.1086/315355. [DOI] [PubMed] [Google Scholar]
- 11.Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–263. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 12.Crook ED, Penumalee S, Gavini B, et al. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Am J Transplant. 2005;5:2433–2440. doi: 10.2337/diacare.28.9.2187. [DOI] [PubMed] [Google Scholar]
- 13.Hanafusa T, Ichikawa Y, Kishikawa H, et al. Retrospective study on the impact of hepatitis C virus infection on kidney transplant patients over 20 years. Transplantation. 1998;66:471–476. doi: 10.1097/00007890-199808270-00010. [DOI] [PubMed] [Google Scholar]
- 14.Morales J, Campo C, Castellano G, et al. Impact of hepatitis C in long functioning renal transplant: a clinicopathological followup. Transplant Proc. 1993;25:1450. [PubMed] [Google Scholar]
- 15.Periera B, Wright T, Schmid C, et al. The impact of pretransplantation hepatitis C infection on the outcome of renal transplantation. Transplantation. 1995;60:799. [PubMed] [Google Scholar]
- 16.Kokoglu OF, Ucmak H, Hosoglu S, et al. Efficacy and tolerability of pegylated-interferon alpha-2a in hemodialysis patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:575–580. doi: 10.1111/j.1440-1746.2005.04008.x. [DOI] [PubMed] [Google Scholar]
- 17.Tan AC, Brouwer JT, Leusen RV, et al. Safety of interferon and Ribavirin in dialysis patients with chronic hepatitis C: results of a pilot study.. Program and abstracts of the 50th Annual Meeting and Postgraduate Courses of the American Association for the Study of Liver Diseases; Dallas, TX. November 5–9, 1999; Abstract 813. [Google Scholar]
- 18.Bruchfeld A, Lindahl K, Reichard O, et al. Pegylated interferon and ribavirin in hemodialysis patients. Nephrol Dial Transplant. 2006;21:1444–1445. doi: 10.1093/ndt/gfl072. [DOI] [PubMed] [Google Scholar]
- 19.Mousa DH, Abdalla AH, Al-Shoail G, et al. Alpha-interferon with ribavirin in the treatment of hemodialysis patients with hepatitis C. Transplant Proc. 2004;36:1831–1834. doi: 10.1016/j.transproceed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Lamb MW, Marks MI, Wynohradnyk L, et al. 40 KD Peginterferon Alfa 2a (Pegasys) can be administered safely in patients with end-stage renal disease.. Poster presentation AASLD Annual Meeting; 2001. [Google Scholar]
- 21.Bruchfeld A, Stahle L, Andersson J, et al. Ribavirin treatment in dialysis patients with chronic hepatitis C virus infection—a pilot study. J Viral Hep. 2001;8:287–292. doi: 10.1046/j.1365-2893.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 22.Rocha CM, Perez RM, Ferreira AP, et al. Efficacy and tolerance of interferon-alpha in the treatment of chronic hepatitis C in end-stage renal disease patients on hemodialysis. Liver Int. 2006;26:305–310. doi: 10.1111/j.1478-3231.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 23.Mangia A, Minerva N, Bacca D, et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology. 2008;47:43–50. doi: 10.1002/hep.22061. [DOI] [PubMed] [Google Scholar]
- 24.Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1-infected slow responders. Hepatology. 2007;46:1671–1674. doi: 10.1002/hep.21919. [DOI] [PubMed] [Google Scholar]