Abstract
Thyroid hormones play an essential role in lipid mobilization, lipid degradation, and fatty acid oxidation. Hypothyroidism has been associated with nonalcoholic steatohepatitis; however, the association between thyroid diseases and hepatocellular carcinoma (HCC) in men and women has not been well established. We investigated the association between hypothyroidism and HCC risk in men and women in a case-control study, which included 420 eligible patients with HCC and 1104 healthy controls. We used multivariate unconditional logistic regression models to control for the confounding effects of established HCC risk factors. A long-term history of hypothyroidism (> 10 years) was associated with a statistically significant high risk of HCC in women; after adjusting for demographic factors, diabetes, hepatitis, alcohol consumption, cigarette smoking, and family history of cancer, the odds ratio (OR) was 2.9 (95% confidence interval [CI], 1.3–6.3). Restricted analyses among hepatitis virus—negative subjects, nondrinkers, nondiabetics, nonsmokers, and nonobese individuals indicated a significant association between hypothyroidism and HCC, with an approximate two-fold to three-fold increased risk of HCC development. We observed risk modification among women with diabetes mellitus (OR = 9.4; 95% CI = 2.7–32.7) and chronic hepatitis virus infection (OR = 31.2; 95% CI = 6.3–153.2). A history of hyperthyroidism was not significantly related to HCC (OR = 1.7; CI = 0.6–5.1). We noted significant elevated risk association between hypothyroidism and HCC in women that was independent of established HCC risk factors. Experimental investigations are necessary for thorough assessment of the relationship between thyroid disorders and HCC.
The incidence of hepatocellular carcinoma (HCC), the fifth most common cancer worldwide,1 has been steadily increasing over the past two decades in the United States.2 Chronic hepatitis C virus (HCV) infection and its underlying cirrhosis were suggested to be the reasons for this increase.3,4 Several extrahepatic conditions, however, have been associated with HCV infection, including mixed cryoglobulinemia, porphyria cutanea tarda, and membranoproliferative glomerulone-phritis.5 In addition, patients with HCV are susceptible to autoimmune thyroid diseases before and after completion of interferon therapy,6,8 suggesting an association between thyroid diseases and HCV.
Hypothyroidism has been observed in 5%–12% of patients with HCV8 and is the most common thyroid disorder in the adult population, particularly among older women. A recent report by the National Health and Nutrition Examination Survey indicated that the prevalence of hypothyroidism is 7.9% for those aged 12–79 years and 12.1% for those aged 80 years or older.9
Thyroid hormones play an essential role in lipid mobilization, lipid degradation, and fatty acid oxidation.10 Accordingly, thyroid hormone deficiency may cause hyperlipidemia and may play a role in the pathogenesis of nonalcoholic steatohepatitis (NASH).11 In addition, patients with hypothyroidism may experience a 15% to 30% weight gain12 and associated insulin resistance,13,14 which are significant factors in NASH. In one study,11 the prevalence of hypothyroidism was significantly higher in patients with NASH (15.0%) than in controls (7.2%) (P = 0.001). This finding was later supported by Reddy and colleagues15 at the Mayo Clinic, who reported an odds ratio (OR) of 6.8 (95% confidence interval [CI], 1.1 to 42.1) of hypothyroidism in patients with HCC with unknown etiology (n = 54) compared to patients with HCC with underlying HCV infection or alcoholic liver diseases (n = 116) after adjusting for several confounding factors.
Overall, these studies have suggested a clinical association between hypothyroidism and chronic liver diseases. In an ongoing case-control study, our goal was to determine the association between hypothyroidism and HCC development in the United States by comparing HCC patients with healthy controls. We also sought to describe the relationship between hypothyroidism and established risk factors for HCC, particularly chronic hepatitis virus infection, alcohol consumption, diabetes, and obesity.
Patients and Methods
Study Design and Population
The current investigation is part of an ongoing hospital-based case-control study, which was approved by the institutional review board at The University of Texas M. D. Anderson Cancer Center. Written informed consent for participation was obtained from each study participant.
Case patients were recruited from the population of patients with newly diagnosed HCC who were evaluated and treated at the M. D. Anderson Cancer Center gastrointestinal medical oncology and surgical oncology outpatient clinics. The inclusion criteria were as follows: pathologically confirmed diagnosis of HCC, U.S. residency, and the ability to communicate in English. The exclusion criteria were the presence of other types of primary liver cancer (such as cholangiocarcinoma or fibrolamellar hepatocarcinoma), unknown primary tumors, and concurrent or past history of cancer at another organ site.
From January 2000 through July 2008, 652 patients with suspected HCC were identified, 518 of whom were eligible for this study. We enrolled 420 eligible patients with HCC; 98 eligible patients (18.9%) were not recruited because of patient refusal, patient sickness, or inadequate time to complete the interview. Statistical analyses indicated that the eligible patients who were not recruited did not differ from the recruited patients in terms of demographic, epidemiologic, or clinical factors (retrieved from patients’ medical records).
The control subjects were healthy and genetically unrelated family members (i.e., spouses and in-laws) of patients at the M. D. Anderson Cancer Center who had cancers other than liver, gastrointestinal, lung, or head and neck cancer. The reason for excluding family members and spouses of patients with these cancers as controls was to prevent the introduction of selection bias connected with shared environmental and genetic factors that are highly associated with HCC, e.g., alcohol consumption, smoking, family history of cancer, and hepatitis virus infection.
The eligibility criteria for controls were the same as those for patients, except for having a cancer diagnosis. Control subjects were recruited from diagnostic radiology clinics of the M. D. Anderson Cancer Center, where cancer patients and their companions are sent to receive the initial cancer diagnosis or treatment follow-up examination. A short structured questionnaire was used to screen for potential controls on the basis of the eligibility criteria. Analysis of the answers received on the short questionnaire indicated that 83.6% of those questioned agreed to participate in clinical research. A comparison of those recruited as controls and those who refused to participate in the research revealed no significant differences in age, sex, race/ethnicicy, educational level, personal history of cancer, or the accompanied patient’s type of cancer.
We sought to confirm the control subjects’ reasons for coming to the hospital with cancer patients and whether these reasons could have been related to the risk factors for HCC. We found that the underlying causes for the controls’ companionship were care and altruism. Moreover, all spouses of patients with other cancers who served as control subjects reported that they would have chosen to be referred to M. D. Anderson Cancer Center if they had been diagnosed with cancer during the same time period because they tended to share the same family physician, had the same health insurance coverage, and lived in the same geographic location. All of the above-mentioned results indicated that the patients and controls had the same catchments, which further supported the idea that the control subjects were representative of the M. D. Anderson Cancer Center population from which patients with HCC were selected.16,18 A total of 1286 eligible control subjects were ascertained in the current study. However, 172 control subjects were excluded due to limited blood samples for testing hepatitis B virus (HBV) and HCV markers. An extra 10 control subjects were excluded for living outside the United States. Total of 1104 control subjects were analyzed in this study.
Patients with HCC and controls were recruited simultaneously and were personally interviewed for approximately 25–30 minutes. No proxy interviews were conducted. The interviewers used a structured and validated questionnaire19 to collect information on demographic features and HCC risk factors such as personal smoking history, alcohol consumption, medical history, occupational history, and family history of cancer. The definitions used for smokers, alcohol drinkers, and individuals with a family history of cancer were previously reported.20,21
Cases and controls were interviewed for prior history of thyroid disorders including hypothyroidism, hyperthyroidism, and inflammatory and autoimmune thyroid diseases. Subjects with a history of any thyroid condition were asked for age at diagnosis, treatment exposure, and duration of the disorder.
A detailed questionnaire about obesity was initiated later in the study. From April 2004 through July 2008, a total of 184 patients with HCC and 648 controls were interviewed about their prior history of obesity. The questionnaire used by the interviewers was structured to obtain information about participants’ body weight before cancer diagnosis (patients with HCC) or before recruitment (controls). The body mass index (BMI) was estimated from the participants’ body weight (kg) and height (m) at different ages (<20, 20, 30, 40, and 50 years). The BMI was then classified as a four-level categorical variable: underweight (<18.5 kg/m2), normal -weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (≥30 kg/m2).
Blood samples from cases (n = 420) and controls (n = 1104) were tested for HBV and HCV. HCV antibodies (anti-HCV), hepatitis B surface antigen (HBsAg), and antibodies to hepatitis B core antigen (anti-HBc) were detected by use of a third-generation enzyme-linked immunosorbent assay (Abbott Laboratories, North Chicago, IL). Positive results prompted repeated confirmatory enzyme-linked immunosorbent assay testing.
Statistical Methods
Stata software (Stata Corp., College Station, TX) was used for statistical analysis. Univariate analyses were done by using the χ2 or Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables. We performed multivariate unconditional logistic regression analyses by using all variables that were significant at P < 0.05 in the univariate analyses. For each factor, we calculated the adjusted odds ratio (AOR) and 95% CI values, using maximum likelihood estimation. All AORs were adjusted for age, sex, race, educational level, cigarette smoking, alcohol consumption, diabetes mellitus, thyroid diseases, family history of cancer, and HBV/HCV infection. The final model was chosen on the basis of biologic plausibility and the lowest −2 log likelihood function. Additional adjustment was made for prior history of obesity at different ages (<20, 20, 30, 40, and 50 years) among 184 HCC cases and 648 controls. However, because correlation may exist between BMIs at the specified ages (multicollinearity), prior history of obesity at the different ages were included separately in different logistic regression models.
We used multiple logistic regression models to investigate possible interactions on an additive scale22 of hypothyroidism with HCV/HBV infection and with diabetes mellitus. To assess deviation from the additive model (which assumes no interaction between variables), we calculated the synergism index, S = (OR11 − l)/(OR01 + OR10) − 2, where OR11 = OR of the joint effect of two risk factors, and OR10 and OR01 = OR of each risk factor in the absence of the other. A value of S equal to unity was interpreted as indicative of additivity, whereas a value greater than unity was indicative of superadditivity and synergism.23
Results
Participants’ Characteristics
Table 1 shows the demographic characteristics of case patients and control subjects. Most study subjects were non-Hispanic white males. Overall, case patients were slightly older than control subjects; the mean age ± standard error was 63.0 ± 0.6 years for patients with HCC and 60.0 ± 0.3 years for controls. However, men and women with HCC had a similar age distribution (63.1 ± 0.7 versus 62.5 ± 1.1 years, respectively; P = 0.6). Higher education (≥ college degree) was more frequent among control subjects than among patients with HCC. Cases and controls had a similar distribution of geographical region (U.S. state of residency).
Table 1.
Participants’ Characteristics
| Demographic Variables | HCC Patients N = 420 (%) | Controls N = 1104 (%) | P Value | ||
|---|---|---|---|---|---|
| Sex | 0.001 | ||||
| Male | 299 | 71.2 | 636 | 57.6 | |
| Female | 121 | 28.8 | 468 | 42.4 | |
| Age (years) | 0.001 | ||||
| ≤40 | 15 | 3.6 | 50 | 4.5 | |
| 41–50 | 46 | 10.9 | 181 | 16.4 | |
| 51–59 | 119 | 28.3 | 336 | 30.4 | |
| 60–69 | 117 | 27.9 | 358 | 32.4 | |
| ≥70 | 123 | 29.3 | 179 | 16.2 | |
| Ethnicity | 0.001 | ||||
| Non-Hispanic white | 294 | 70 | 973 | 88.1 | |
| Hispanics | 56 | 13.3 | 84 | 7.6 | |
| African Americans | 40 | 9.5 | 39 | 3.5 | |
| Asians | 30 | 7.1 | 8 | .7 | |
| Educational level | 0.001 | ||||
| ≤High school | 198 | 47.1 | 316 | 28.6 | |
| Some colleges | 94 | 22.4 | 287 | 26.0 | |
| ≥College degree | 128 | 30.5 | 501 | 45.4 | |
| State of residency | 0.5 | ||||
| TX, LA, AK, NM.OK * | 308 | 73.3 | 809 | 73.3 | |
| Other states | 112 | 26.7 | 295 | 26.7 | |
States of Texas, Louisiana, Arkansas, New Mexico, and Oklahoma.
HCC Risk Factors
Multivariate logistic regression analyses indicated that having a history of HCV, HBV, cigarette smoking, heavy consumption of alcohol, or having a family history of cancer (including liver cancer), or diabetes mellitus was significantly associated with HCC development (Table 2). Results were consistent with those of our previous reports about risk factors of HCC.20,21
Table 2.
Risk Factors of Hepatocellular Carcinoma: Multivariate Logistic Regression Analyses
| Risk Factor | Cases N (%) | Controls N (%) | AOR (95% CI)* |
|---|---|---|---|
| Hepatitis virus | |||
| None | 232 (55.2) | 1066 (96.6) | 1 (reference) |
| anti-HCV+ | 94 (22.4) | 6 (0.5) | 61.0 (26.9–138.4) |
| HBsAg+/anti-HBc+ | 30 (7.1) | 4 (0.4) | 47.1 (14.6–151.7) |
| HBsAg−/anti-HBc+ | 24 (5.7) | 25 (2.3) | 3.7 (1.9–6.9) |
| Both HCV and HBV | 40 (9.5) | 3 (0.3) | 52.8 (15.4–180.4) |
| Cigarette smoking | |||
| No | 126 (30.0) | 582 (52.7) | 1 (reference) |
| Yes† | 294 (70.0) | 522 (47.3) | 1.8 (1.3–2.4) |
| ≤ 20 pack year | 115 (27.4) | 258 (23.4) | 1.4 (0.9–2.1) |
| > 20 pack years | 176 (41.9) | 264 (23.9) | 2.0 (1.4–2.9) |
| Alcohol consumption | |||
| No | 137 (32.6) | 485 (43.9) | 1 (reference) |
| Yes‡ | 283 (67.4) | 619(56.1) | 1.3 (1.0–1.9) |
| < 60 mL ethanol/day | 192 (45.7) | 551 (49.9) | 1.1 (0.8–1.6) |
| ≥ 60 ml ethanol/day | 89 (21.2) | 65 (5.9) | 3.2 (1.9–5.3) |
| Family history of cancer | |||
| No | 132 (31.4) | 355 (32.2) | 1 (reference) |
| Yes§ | 264 (62.9) | 740 (67.0) | 1.4 (1.1–1.9) |
| Liver cancer (first-degree) | 24 (5.7) | 9 (0.8) | 3.0 (1.2–8.3) |
| Prior history of diabetes | |||
| No | 280 (66.7) | 989 (89.6) | 1 (reference) |
| Yes | 140 (33.3) | 115 (10.4) | 4.2 (3.0–5.9) |
Logistic model included age, sex race, educational level, HCV, HBV, smoking, alcohol consumption, diabetes mellitus, and family history of cancer.
Duration of smoking was missing for three cases.
Duration of drinking was missing for two cases and three controls.
Any cancer in first- and second-degree relatives.
Thyroid Disorders
A total of 63 patients with HCC (15.0%) and 134 controls (12.1%) recalled a history of thyroid disease (Table 3). Among all study subjects, significantly more cases (11.7%) than controls (8.0%) reported hypothyroidism (P = 0.03), and those subjects with a history of hypothyroidism had a two-fold higher risk of HCC than did subjects with no history of thyroid disorders. This association was determined among patients with long-term hypothyroidism (>3 years). However, a significant relationship between hypothyroidism and HCC was observed only for women, which indicates effect measure modification (heterogeneity) of gender. The estimated χ2 for homogeneity between AORs in men and women24 was 7.86 (one degree of freedom), P < 0.05. In addition, including an interaction term of gender and hypothyroidism in the logistic regression model along with the main effects of gender and hypothyroidism revealed a significant interaction between gender and hypothyroidism on HCC development (P = 0.02).
Table 3.
Thyroid Diseases and Risk for Hepatocellular Carcinoma Development: Multivariate Logistic Regression Analyses
| All |
Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Thyroid Variable | Case N = 420 | Control N = 1104 | AOR (95% CI)* | Case N = 299 | Control N = 636 | AOR (95% CI)† | Case N = 121 | Control N = 468 | AOR (95% CI)‡ |
| Thyroid condition | |||||||||
| None | 357 | 970 | 1 (reference) | 277 | 590 | 1 (reference) | 80 | 380 | 1 (reference) |
| Hypothyroidism | 49 | 88 | 1.9 (1.2–3.1) | 16 | 28 | 0.9 (0.4–6.4) | 33 | 60 | 2.8 (1.6–5.1) |
| Hyperthyroidism | 8 | 14 | 1.7 (0.6–5.1) | 5 | 8 | 1.5 (0.3–6.4) | 3 | 6 | 2.4 (0.5–12.1) |
| Other thyroid conditions | 6 | 32 | 0.7 (0.3–1.9) | 1 | 10 | 0.3 (0.1–4.4) | 5 | 22 | 1.0 (0.3–3.2) |
| Hypothyroidism duration§ | |||||||||
| ≤ 2 | 5 | 13 | 1.4 (0.4–5.1) | 3 | 5 | .8 (0.1–5.2) | 2 | 8 | 2.6 (0.5–14.5) |
| 3–10 years | 16 | 29 | 2.1 (1.0–4.7) | 6 | 9 | 1.4 (0.3–5.6) | > 10 | 20 | 2.6 (1.0–7.2) |
| > 10 years | 25 | 44 | 2.1 (1.1–3.9) | 6 | 12 | 0.7 (0.1–3.2) | 19 | 32 | 2.9 (1.3–6.3) |
Model 1 (All) to estimate AOR for age, sex, race, educational level, diabetes, smoking, alcohol consumption, HCV, HBV, and family history of cancer.
Model 2 (Men) to estimate AOR for age, race, educational level, diabetes, smoking, alcohol consumption, HCV, HBV, and family history of cancer.
Model 3 (Women) to estimate AOR for age, race, educational level, diabetes, smoking, alcohol consumption, HCV, HBV, and family history of cancer.
Hypothyroidism duration (All) was missing for three hepatocellular carcinoma cases and two control subjects; hypothyroidism duration (Men) was missing for one hepatocellular carcinoma case and two control subjects; hypothyroidism duration (Women) was missing for two hepatocellular carcinoma cases.
Women who had a prior history of hypothyroidism for > 10 years had a three times higher risk of HCC than did women without a history of thyroid disorders (P= 0.001) (Table 3). Hyperthyroidism and other thyroid conditions were reported less frequently than hypothyroidism by patients with HCC and control subjects, yielding a nonsignificant association with HCC development in all subjects and in men and women when analyzed separately.
We adjusted for obesity at various ages of subjects before HCC development or control recruitment. We found that such adjustment did not meaningfully change the observed significant association between hypothyroidism and HCC (Fig. 1A). Moreover, restricted analyses among nondrinkers, non-HCV/HBV–infected subjects, subjects without diabetes, and nonsmokers indicated a significant association between hypothyroidism and HCC, with an approximate two-fold to three-fold increased risk of HCC development (Fig. 1B).
Fig. 1.
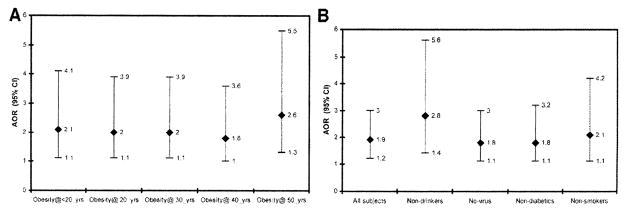
(A) The estimated AOR (95% CI) for the association between hypothyroidism and HCC after adjustment for ail established HCC risk factors and for prior history of obesity at different ages. (B) Estimated AOR (95% CI) for the association between hypothyroidism and HCC after adjustment for all established HCC risk factors in all subjects and in subgroups of nondrinkers, non-virus infected subjects, nondiabetics, and nonsmokers.
Synergism of Hypothyroidism with HBV/HCV and Diabetes
Table 4 shows that the relative excess risk for patients having hypothyroidism and HBV/HCV or diabetes mellitus exceeded the sum of the relative excess risks for each risk factor alone. The risk modification of hypothyroidism by hepatitis virus infection or diabetes was significantly observed in women; AORs were 31.2 (95% CI = 6.3–153.2) for combined hypothyroidism and hepatitis infection and 9.4 (95% CI = 2.7–32.7) for combined hypothyroidism and diabetes mellitus. The estimated S index (95% CI) indicated a significant departure from additivity in the joint effect of hypothyroidism and hepatitis virus infection (S = 2.1 [95% CI = 1–3.28]) and in the joint effect of hypothyroidism and diabetes mellitus (S = 2.3 [95% CI = 1–3.65]).
Table 4.
Risk Modification of Hypothyroidism by Hepatitis Virus Infection and Diabetes Mellitus: Multivariate Logistic Regression Analyses
| All |
Men |
Women |
|||||
|---|---|---|---|---|---|---|---|
| Variables | Cases/Controls | AOR (95% CI) | Cases/Controls | AOR (95% CI) | Cases/Controls | AOR (95% CI) | |
| HCV/HBV | Hypothyroidism | 420/1104 | Model (1)* | 299/636 | Model (2)† | 121/468 | Model (3)‡ |
| No | No | 202/981 | 1 (reference) | 145/588 | 1 (reference) | 57/393 | 1 (reference) |
| Yes | No | 169/35 | 22.1 (14.5–33.8) | 138/20 | 31.5 (18.0–54.4) | 31/15 | 13.3 (6.4–27.8) |
| No | Yes | 30/85 | 2.0 (1.2–3.3) | 7/27 | 0.7 (0.3–1.9) | 23/58 | 2.9 (1.6–5.3) |
| Yes | Yes | 19/3 | 34.3 (9.6–122.5) | 9/1 | 44.2 (5.4–377.2) | 10/2 | 31.2 (6.3–153.2) |
| Diabetes | Hypothyroidism | 420/1104 | Model (4)§ | 299/636 | Model (5)|| | 121/468 | Model (6)¶ |
| No | No | 247/911 | 1 (reference) | 180/534 | 1 (reference) | 67/377 | 1 (reference) |
| Yes | No | 124/105 | 4.1 (2.8–5.8) | 103/74 | 4.7 (3.1–7.2) | 21/31 | 2.8 (1.3–5.9) |
| No | Yes | 33/78 | 1.9 (1.2–3.3) | 7/24 | 0.6 (0.2–7.2) | 26/54 | 2.8 (1.5–5.9) |
| Yes | Yes | 16/10 | 7.9 (3.1–20.1) | 9/4 | 5.9 (1.5–22.9) | 7/6 | 9.4 (2.7–32.7) |
Model 1 (All) to estimate AOR for age, race, sex, educational level, smoking, alcohol consumption, diabetes, and family history of cancer.
Model 2 (Men) to estimate AOR for age, race, educational level, smoking, alcohol consumption, diabetes, and family history of cancer.
Model 3 (Women) to estimate AOR for age, race, educational level, smoking, alcohol consumption, diabetes, and family history of cancer.
Model 4 (All) to estimate AOR for age, race, sex, educational level, smoking, alcohol consumption, HCV, HBV, and family history of cancer.
Model 5 (Men) to estimate AOR for age, race, educational level, smoking, alcohol consumption, HCV, HBV, and family history of cancer.
Model 6 (Women) to estimate AOR for age, race, educational level, smoking, alcohol consumption, HCV, HBV, and family history of cancer.
Discussion
To our knowledge, no other U.S. study published to date has contained a larger population of newly diagnosed patients with pathologically confirmed HCC. The study revealed a high prevalence of hypothyroidism among patients with HCC (11.7%), yielding a two-fold higher risk of HCC for subjects with hypothyroidism than for those with no history of thyroid disorders. The association was significant in women and not significant in men. Our findings are supported by early results from the Mayo Clinic, which showed a prevalence of hypothyroidism among patients with HCC of 11.3%.15
The discovery of a relationship between hypothyroidism and HCC should not be surprising for two reasons. First, hypothyroidism has been associated with the pathogenesis of NASH,11 one of the most common chronic liver diseases characterized by liver inflammation and fibrosis and considered a predisposing condition for HCC development.25,26
The second reason that a relationship between hypothyroidism and HCC should not be surprising is the presence of thyroid disorders in conjunction with the major HCC risk factors. For example, hypothyroidism was previously reported to be more common in patients with HCV infection, with a higher prevalence in patients with HCV who had severe fibrosis than in those with mild fibrosis.8,27 Moreover, the association between thyroid cancer and HCV was previously reported.28 In addition, patients with diabetes, another risk factor for HCC, tend to have a high prevalence of thyroid disorders.29–32 Endogenous glucose production and utilization rates are enhanced by thyroid hormones and could be affected by thyroid hormone deficiency.33 Hypothyroidism has also been associated with insulin resistance and was observed in pediatric patients with type-131 or type-2 diabetes.30 The reasons for the occurrence of hypothyroidism among patients with diabetes have not been well studied. It is possible that an individual’s age29,34 and susceptibility for autoimmune diseases may explain the concurrence of thyroid disorders with diabetes.35 However, as shown in Table 4 and Fig. 1B, restricted analyses among subjects without serological evidence for HCV infection (anti-HCV−) or HBV infection (anti-HBsAg−/anti-HBc−) and subjects without diabetes mellitus did not meaningfully change the observed association between hypothyroidism and HCC.
Whether and why hypothyroidism causes HCC is not clear. However, the association between hypothyroidism and NASH can be explained by the underlying hyperlipidemia, decreased fatty acid oxidation insulin resistance, and lipid peroxidation in patients with hypothyroidism. All of these conditions may enhance the susceptibility to chronic inflammation, DNA damage, and HCC development. Moreover, concurrent thyroid dysfunction among patients with diabetes may exacerbate the coexisting diabetes-induced dyslipidemia and may explain our observation of HCC risk modification among patients with hypothyroidism and diabetes.
Hypothyroidism-induced obesity may have promoted the development of steatohepatitis, fibrosis, and cirrhosis in these patients and increased their susceptibility to HCC development. Moreover, obesity and hyperinsulinemia may increase level of insulin-like growth factor 1, which in turn may reduce hepatic synthesis and blood concentration of sex hormone–binding globulin (SHBG),36,37 a glycoprotein produced in the liver with high binding affinity for testosterone and lower affinity for estradiol. Independent of obesity, there is sufficient evidence that thyroid hormones have a positive effect on hepatic SHBG synthesis and that patients with hypothyroidism may experience a lower level of SHBG.38 Thus, a decreased level of SHBG may lead to increased plasma testosterone and estradiol, both of which may promote cellular proliferation and inhibit apoptosis. Elevated levels of serum testosterone and testosterone-to-estradiol ratio have been proposed to be predictive of HCC development in Japanese men with cirrhosis.39 Nevertheless, the fact that the association between hypothyroidism and HCC continued to be significant after adjustment for prior history of obesity at different ages (Fig. 1A) suggested that other mechanisms of hepatocarcinogenesis were involved, especially among women.
There is evidence suggesting that the liver is a major target tissue for the proliferative effect of growth hormones, growth hormone receptors, and growth hormone binding protein, and that such hormones and hormone receptors are associated with hepatocellular carcinoma.40 Meanwhile, an experimental study in rats with hypothyroidism41 showed that the levels of hepatic growth hormone receptors and growth hormone binding protein messenger RNA were increased in the female group and decreased in the male group as compared to euthyroid controls (P < 0.001); this may partially explain the susceptibility of females with hypothyroidism to develop HCC than were males with hypothyroidism.
To ensure that the observed association between HCC and hypothyroidism was not related to the cancer diagnosis,42 patients with HCC with prior or concurrent head and neck cancer or cancer in other sites were excluded to minimize hypothyroidism misclassification due to head and neck radiotherapy. Interview data indicated no prior history of radiation exposure on head and neck among HCC cases or control subjects. In addition, we considered the duration of hypothyroidism before cancer diagnosis during the analyses. Nevertheless, we observed no HCC risk escalation with increasing the duration of hypothyroidism. We believe that other parameters like severity of hypothyroidism and response to treatment should be accounted for their effect while testing for HCC risk escalation among patients with hypothyroidism.
Another possible limitation was that thyroid diseases were self-reported by patients and controls during personal interviews. Therefore, patients with HCC might be more prone to recall previous diseases than are healthy controls. However, HCC cases were newly diagnosed and prospectively enrolled in the study where both cases and controls were personally and simultaneously interviewed, using a structured-validated questionnaire. Upon reviewing the medical records of patients with HCC, we found no discrepancy between interview information and physicians’ notes.
Questions of prior history of thyroid diseases along with other chronic medical conditions were part of a long list of questions. The questionnaire included year and age at diagnosis, duration, and treatment intake. It is reasonable to assume that subjects who had received a definite diagnosis and had been treated could accurately reported their prior history of medical conditions and recalled the condition duration. All study subjects were blinded for the current study hypothesis and its specific aims.
Control subjects were selected to represent the population from which the patients with HCC were ascertained. Only U.S. patients and controls were included, and the geographic distribution of their residential states was similar. Therefore, it is unlikely that our finding of the positive relationship between hypothyroidism and HCC was confounded by selection bias of cases or controls. The prevalence of hypothyroidism in the control group was consistent with that in the general U.S. population.9
In summary, our results suggested that long-term hypothyroidism is associated with HCC, independent from other major HCC risk factors, and this association was significant only among women. Although hypothyroidism-associated weight gain (overweight or obesity) may partially explain the association between hypothyroidism and HCC, hypothyroidism independent from obesity can also contribute to HCC development. Screening and proper management of thyroid diseases in patients with diabetes or HCV infection may help prevent HCC. Further studies among different populations are warranted to confirm the association between hypothyroidism and HCC and to identify the underlying biological mechanisms and the genetic predisposition factors that may contribute to susceptibility to HCC development in the presence of thyroid disorders.
Acknowledgments
Supported by National Institutes of Health (NIH) grants RO3 FSl 1481 (to M.H.), CA106458-01 (to M.H.), and Texas Tobacco Settlement (to M. H.).
Abbreviations
- AOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence interval
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- SHBG
sex hormone–binding globulin
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35:266–269. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Preziati D, La Rosa L, Covini G, Marcelli R, Rescalli S, Persani L, et al. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol. 1995;132:587–593. doi: 10.1530/eje.0.1320587. [DOI] [PubMed] [Google Scholar]
- 7.Quaranta JF, Tran A, Regnier D, Letestu R, Beusnel C, Fuzibet JG, et al. High prevalence of antibodies to hepatitis C virus (HCV) in patients with anti-thyroid autoantibodies. J Hepatol. 1993;18:136–138. doi: 10.1016/s0168-8278(05)80022-4. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002) Thyroid. 2007;17:1211–1223. doi: 10.1089/thy.2006.0235. [DOI] [PubMed] [Google Scholar]
- 10.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S109–S112. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 11.Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for nonalcoholic steatohcpatitis? J Clin Gastroenterol. 2003;37:340–343. doi: 10.1097/00004836-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Krotkiewski M. Thyroid hormones and treatment of obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S116–S119. doi: 10.1038/sj.ijo.0801294. [DOI] [PubMed] [Google Scholar]
- 13.Dimitriadis G, Parry-Billings M, Bevan S, Leighton B, Krause U, Piva T, et al. The effects of insulin on transport and metabolism of glucose in skeletal muscle from hyperthyroid and hypothyroid rats. Eur J Clin Invest. 1997;27:475–483. doi: 10.1046/j.1365-2362.1997.1380688.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 15.Reddy A, Dash C, Leerapun A, Mettler TA, Stadheim LM, Lazaridis KN, et al. Hypothyroidism: a possible risk factor for liver cancer in patients with no known underlying cause of liver disease. Clin Gastroenterol Hepatol. 2007;5:118–123. doi: 10.1016/j.cgh.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am J Epidemiol. 1992;135:1042–1050. doi: 10.1093/oxfordjournals.aje.a116398. [DOI] [PubMed] [Google Scholar]
- 17.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol. 1992;135:1029–1041. doi: 10.1093/oxfordjournals.aje.a116397. [DOI] [PubMed] [Google Scholar]
- 18.Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I. Principles. Am J Epidemiol. 1992;135:1019–1028. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
- 19.Spitz MR, Fueger JJ, Newell GR. The development of a comprehensive, institution-based patient risk evaluation program: II. Validity and reliability of questionnaire data. Am J Prev Med. 1988;4:188–193. [PubMed] [Google Scholar]
- 20.Hassan MM, Spitz MR, Thomas MB, El-Deeb AS, Glover KY, Nguyen NT, et al. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: Case-control study. Int J Cancer. 2008;123:1883–1891. doi: 10.1002/ijc.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan MM, Spitz MR, Thomas MB, Curley SA, Patt YZ, Vauthey JN, et al. The association of family history of liver cancer with hepatocellular carcinoma: A case-control study in the United States. J Hepatol. 2009;50:334–341. doi: 10.1016/j.jhep.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 23.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 24.Aschengrau A, Seage GR. Essentials of Epidemiology in Public Health. 2. Sundbury, MA: Jones Bartlett; 2007. Effect measure modification; pp. 343–356. [Google Scholar]
- 25.Theise ND, Schwartz M, Miller C, Thung SN. Macroregenerative nodules and hepatocellular carcinoma in forty-four sequential adult liver explants with cirrhosis. Hepatology. 1992;16:949–955. doi: 10.1002/hep.1840160416. [DOI] [PubMed] [Google Scholar]
- 26.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Torres M, Rios-Bedoya CF, Ortiz-Lasanta G, Marxuach-Cuetara AM, Jimenez-Rivera J. Thyroid dysfunction (TD) among chronic hepatitis C patients with mild and severe hepatic fibrosis. Ann Hepatol. 2008;7:72–77. [PubMed] [Google Scholar]
- 28.Antonelli A, Ferri C, Fallahi P. Thyroid cancer in patients with hepatitis C infection. JAMA. 1999;281:1588. doi: 10.1001/jama.281.17.1588. [DOI] [PubMed] [Google Scholar]
- 29.Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med. 1995;12:622–627. doi: 10.1111/j.1464-5491.1995.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 30.Park JR, Jung TS, Jung JH, Lee GW, Kim MA, Park KJ, et al. A case of hypothytoidism and type 2 diabetes associated with type V hyperlipoproteinemia and eruptive xanthomas. J Korean Med Sci. 2005;20:502–505. doi: 10.3346/jkms.2005.20.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araujo J, Brandao LA, Guimaraes RL, Santos S, Falcao EA, Milanese M, et al. Prevalence of autoimmune thyroid disease and thyroid dysfunction in young brazilian patients with type 1 diabetes. Pediatr Diabetes. 2008;9:272–276. doi: 10.1111/j.1399-5448.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 32.Chubb SA, Davis WA, Inman Z, Davis TM. Prevalence and progression of subclinical hypothyroidism in women with type 2 diabetes: the Fremantle Diabetes Study. Clin Endocrinol (Oxf) 2005;62:480–486. doi: 10.1111/j.1365-2265.2005.02246.x. [DOI] [PubMed] [Google Scholar]
- 33.Chidakel A, Mentuccia D, Celi FS. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid. 2005;15:899–903. doi: 10.1089/thy.2005.15.899. [DOI] [PubMed] [Google Scholar]
- 34.Winger JM, Hornick T. Age-associated changes in the endocrine system. Nurs Clin North Am. 1996;31:827–844. [PubMed] [Google Scholar]
- 35.Jaworski MA, Slater JD, Severini A, Hennig KR, Mansour G, Mehta JG, et al. Unusual clustering of diseases in a Canadian Old Colony (Chortitza) Mennonite kindred and community. CMAJ. 1988;138:1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 36.Haffner SM. Sex hormones, obesity, fat distribution, type 2 diabetes and insulin resistance: epidemiological and clinical correlation. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S56–S58. doi: 10.1038/sj.ijo.0801279. [DOI] [PubMed] [Google Scholar]
- 37.Hautanen A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S64–S70. doi: 10.1038/sj.ijo.0801281. [DOI] [PubMed] [Google Scholar]
- 38.Hampl R, Kancheva R, Hill M, Bicikova M, Vondra K. Interpretation of sex hormone-binding globulin levels in thyroid disorders. Thyroid. 2003;13:755–760. doi: 10.1089/105072503768499644. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka K, Sakai H, Hashizume M, Hirohata T. Serum testosterone:estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res. 2000;60:5106–5110. [PubMed] [Google Scholar]
- 40.Garcia-Caballero T, Mertani HM, Lambert A, Gallego R, Fraga M, Pintos E, et al. Increased expression of growth hormone and prolactin receptors in hepatocellular carcinomas. Endocrine. 2000;12:265–271. doi: 10.1385/ENDO:12:3:265. [DOI] [PubMed] [Google Scholar]
- 41.Romero GS, Stephan DA, Sperling MA, Menon RK. Distinct sexual dimorphism in the effect of hypothyroidism on the expression of the growth hormone receptor and growth hormone-binding protein gene in rat liver. Horm Res. 1996;45:273–278. doi: 10.1159/000184805. [DOI] [PubMed] [Google Scholar]
- 42.Tellini U, Pellizzari L, Pravadelli B. Thyroid function in elderly with neoplasms, [in Italian] Minerva Med. 1999;90:111–121. [PubMed] [Google Scholar]


