Abstract
Purpose
To estimate the prevalence of and temporal trends in prenatal antipsychotic medication use within a cohort of pregnant women in the U.S.
Methods
We identified live born deliveries to women aged 15–45 years in 2001–2007 from 11 U.S. health plans participating in the Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP). We ascertained prenatal exposure to antipsychotics from health plan pharmacy dispensing files, gestational age from linked infant birth certificate files, and ICD-9-CM diagnosis codes from health plan claims files. We calculated the prevalence of prenatal use of atypical and typical antipsychotics according to year of delivery, trimester of pregnancy, and mental health diagnosis.
Results
Among 585,615 qualifying deliveries, 4,223 (0.72%) were to women who received an atypical antipsychotic and 548 (0.09%) were to women receiving a typical antipsychotic any time from 60 days before pregnancy through delivery. There was a 2.5-fold increase in atypical antipsychotic use during the study period, from 0.33% (95% confidence interval: 0.29%, 0.37%) in 2001 to 0.82% (0.76%, 0.88%) in 2007, while the use of typical antipsychotics remained stable. Depression was the most common mental health diagnosis among deliveries to women with atypical antipsychotic use (63%), followed by bipolar disorder (43%) and schizophrenia (13%).
Conclusions
The number and proportion of pregnancies exposed to atypical antipsychotics has increased dramatically in recent years. Studies are needed to examine the comparative safety and effectiveness of these medications relative to other therapeutic options in pregnancy.
Keywords: Antipsychotics, database, pregnancy, prevalence
Introduction
Atypical (second-generation) antipsychotics have replaced typical (first-generation) antipsychotics as the first-line treatment for schizophrenia and related psychotic disorders since their introduction in the 1990s (Lehman et al. 2004; Stanniland and Taylor 2000; Bagnall et al. 2003). In recent years, the indications for atypical antipsychotics have been expanded to bipolar disorder and depression. There is also evidence of increasing off-label use of these medications (Alexander et al. 2011).
For conditions that are the major indications for antipsychotic use – schizophrenia and related psychotic disorders, bipolar disorder, and depression – onset in women is usually before or during the childbearing years (Kessler et al. 2005; Leung and Chue 2000; Kennedy et al. 2005). Decisions about antipsychotic treatment during pregnancy must balance the risks of leaving these conditions untreated against potential medication-associated risks to the mother and the infant (ACOG Committee on Practice Bulletins--Obstetrics 2008; Altshuler et al. 1996; Viguera et al. 2002; Yonkers et al. 2004; Yonkers et al. 2009). Existing studies of birth outcomes in women treated with antipsychotics during pregnancy have produced contradictory results (Newham et al. 2008; Reis and Kallen 2008; Boden et al. 2012b; Johnson et al. 2012; McKenna et al. 2005; Babu et al. 2010; Ernst and Goldberg 2002; Gentile 2010; Grover et al. 2006; Newport et al. 2007; Trixler et al. 2005; Wichman 2009). For example, some studies (Newham et al. 2008; Reis and Kallen 2008; Boden et al. 2012b; Johnson et al. 2012) have observed an association between prenatal atypical antipsychotic exposure and congenital malformations, gestational diabetes, preterm delivery, macrosomia, or lower neuromotor performance, but others (McKenna et al. 2005; Coppola et al. 2007) have not.
It is unclear how many women are exposed to these medications during pregnancy as little is known about the prevalence of and temporal trends in the use of antipsychotics during the prenatal period. In this study, we examined the use of atypical and typical antipsychotics during pregnancy according to year of delivery, trimester of pregnancy, and mental health diagnosis within a large U.S. cohort of pregnant women.
Materials and Methods
Data source
This study used data from the Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP). MEPREP is a collaborative research program between the U.S. Food and Drug Administration and three contract sites: the HMO Research Network Center for Education and Research in Therapeutics, Kaiser Permanente of California, and Vanderbilt University School of Medicine/Tennessee State Medicaid (Andrade et al. 2012). Encompassed within these three contract sites are 11 health plan-affiliated research institutions, including Group Health Research Institute (Washington), Harvard Pilgrim Health Care Institute (Massachusetts), HealthPartners Research Foundation (Minnesota), Kaiser Permanente Colorado, Kaiser Permanente Georgia, Kaiser Permanente Northwest (Oregon), Meyers Primary Care Institute (Massachusetts), Lovelace Clinic Foundation (New Mexico), Kaiser Permanente Northern California, Kaiser Permanente Southern California, and Tennessee State Medicaid (through the auspices of Vanderbilt University School of Medicine). These health plans provide care to approximately 12 million current enrollees within nine states, covering geographically and ethnically diverse populations receiving care within a wide array of medical care delivery models.
To support multi-site studies of medication safety in pregnancy, the research institutions have extracted information on maternal and infant enrollment, demographics, outpatient pharmacy dispensings, and outpatient and inpatient health care encounters from their health plans’ administrative and claims databases. They have also linked health plan data to infants’ birth certificate files, which include information on sociodemographic, medical, and reproductive factors such as maternal race/ethnicity, parity and infants’ gestational age at birth. All data have been transformed into standardized datasets. The study was approved by the Institutional Review Board of each participating organization and the state departments of public health, where applicable.
Study population
The source population for the current study included all live born deliveries between January 1, 2001 and December 31, 2007. To be eligible, the mothers had to be between 15 and 45 years of age at the time of delivery, and to have been continuously enrolled in the health plan with pharmacy benefits from 180 days before pregnancy through delivery. We used the 180-day pre-pregnancy period to ascertain maternal characteristics, and capture antipsychotic use and medical diagnoses before pregnancy.
Antipsychotics of interest
The atypical antipsychotics of interest included aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone. The typical antipsychotics of interest were chlorpromazine, fluphenazine, haloperidol, loxapine, mesoridazine, molindone, perphenazine, pimozide, thioridazine, thiothixene, and trifluoperazine. In a secondary analysis, we further considered prochlorperazine and promethazine – two medications that are traditionally classified as typical antipsychotics but are more commonly used as anti-emetics in contemporary clinical practice.
We identified antipsychotic use from the outpatient pharmacy dispensing file using National Drug Codes and determined periods of drug exposure from the dispensing dates and days supplied. We incorporated a 14-day “grace period” after the calculated end of each prescription based on days supplied and considered women exposed during the grace period.
Definition and identification of trimesters of pregnancy
For deliveries for which the last menstrual period (LMP)-based gestational age was available in the birth certificate file (94% of the study population), we used the first day of the LMP as the beginning of the first trimester (“day zero”). We defined the first trimester as days 0–89, the second trimester as days 90–179, and the third trimester as day 180 through delivery. If the LMP was missing or had an improbable value (e.g., LMP-based gestational age <15 weeks), day zero was defined as delivery date minus the clinical or obstetric estimate-based gestational age. This method of assigning day zero is consistent with the approach used by the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention (Martin et al. 2010). The birth certificate LMP has been validated previously by one of the participating health plans, which found a concordance within two weeks between the birth certificate LMP and the hospital records in 94% of the records reviewed (Cooper et al. 2006).
For deliveries for which gestational age information was missing in the birth certificates (<1% of the study population), we applied a validated algorithm that used the delivery date and specific International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes recorded in the health plan claims data to estimate the trimesters (Li et al.). The algorithm assumed day zero as delivery date minus 270 days if there was no ICD-9-CM code for preterm birth; as delivery date minus 245 days if there was a code for preterm birth of unspecified gestational age; and as delivery date minus the upper limit of the gestational age range if there was a code for preterm birth with a specified range, e.g., delivery date minus 224 days for deliveries with an ICD-9-CM code 765.26 (“31 to 32 weeks of gestation”).
Identification of maternal characteristics
We obtained information on maternal age at delivery, calendar year of delivery, and diagnoses for potential indications for antipsychotic use from the health plan administrative and claims data. Information on maternal race/ethnicity, educational level, marital status, smoking, and alcohol consumption was extracted from the birth certificate file.
Statistical analysis
We identified deliveries exposed to either atypical or typical antipsychotics from any time from 60 days before pregnancy through delivery, and compared their maternal characteristics with those without any exposure to antipsychotic medication during the same period. We described the temporal trend in prenatal antipsychotic use by calendar year of delivery. We also estimated the prevalence during different pregnancy periods, including the 60-day period before pregnancy, any time during pregnancy, and each trimester of pregnancy; these time periods were not mutually exclusive, e.g., deliveries with atypical antispsychotic exposure during the first and second trimester would appear in both periods. Antipsychotic exposure status was also not mutually exclusive, e.g., deliveries with both atypical and typical antipsychotic exposure would be included in both groups.
We calculated the proportion of antispsychotic-exposed deliveries with a diagnosis of schizophrenia (ICD-9-CM codes 295.xx), bipolar disorder (296.0x, 296.1x, 296.4x–296.8x), or depression (296.2x, 296.3x, 300.4x, 311) any time from 180 days before pregnancy through delivery. Multiple diagnoses per delivery were recorded if they occurred. All analyses were performed separately for atypical and typical antipsychotics.
Results
The study population was composed of 585,615 deliveries. For 4,223 deliveries (0.7%) the mothers had filled at least one prescription for an atypical antipsychotic any time during the period from 60 days before pregnancy through delivery. Only 548 deliveries (0.09%) were to women exposed to typical antipsychotics during the same period. Compared with deliveries without any exposure to antipsychotics, deliveries with exposure to atypical or typical antipsychotics (not including prochlorperazine and promethazine) were more likely to be to women who were younger, non-Hispanic White or Black (rather than Hispanic or Asian), or had a lower education level (Table 1).
Table 1.
Maternal characteristics by prenatal antipsychotic use status, the Medication Exposure in Pregnancy Risk Evaluation Program, 2001–2007a
| Characteristics | Deliveries with atypical antipsychotic use |
Deliveries with typical antipsychotic use |
Deliveries with typical antipsychotic use including prochlorperazine and promethazine |
Deliveries with no Antipsychotic use |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
|
Total deliveries Maternal age at delivery (years) |
4,223 (100) | 548 (100) | 68,946 (100) | 514,782 (100) |
| <18 | 303 (7.2) | 10 (1.8) | 3,775 (5.5) | 15,772 (3.1) |
| 18–24 | 1,656 (39.2) | 192 (35.0) | 32,537 (47.2) | 117,838(22.9) |
| 25–34 | 1,756 (41.6) | 244 (44.5) | 27,051 (39.2) | 279,629 (54.3) |
| 35–45 | 508 (12.0) | 102 (18.6) | 5,583 (8.1) | 101,543 (19.7) |
| Calendar year of delivery | ||||
| 2001 | 264 (6.3) | 90 (16.4) | 8,432 (12.2) | 70,954 (13.8) |
| 2002 | 452 (10.7) | 72 (13.1) | 9,384 (13.6) | 74,064 (14.4) |
| 2003 | 555 (13.1) | 79 (14.4) | 9,345 (13.6) | 74,441 (14.5) |
| 2004 | 714 (16.9) | 75 (13.7) | 9,638 (14.0) | 73,106 (14.2) |
| 2005 | 794 (18.8) | 69 (12.6) | 10,335 (15.0) | 72,596 (14.1) |
| 2006 | 736 (17.4) | 83 (15.1) | 10,730 (15.6) | 74,676 (14.5) |
| 2007 | 708 (16.8) | 80 (14.6) | 11,082 (16.1) | 74,945(14.6) |
| Maternal race/ethnicity | ||||
| Non-Hispanic White | 2,967 (70.2) | 292 (53.3) | 39,991 (58.0) | 241,717 (47.0) |
| Black/African American | 911 (21.6) | 185 (33.8) | 18,536 (26.9) | 75,282 (14.6) |
| Asian American | 63 (1.5) | 16 (2.9) | 2,774 (4.0) | 61,104 (11.9) |
| Hispanic | 232 (5.5) | 47 (8.6) | 6,792 (9.9) | 122,944 (23.9) |
| Native American | 19 (0.4) | <5 (0.7) | 213 (0.3) | 1,532 (0.3) |
| Other | 14 (0.3) | <5 (0.4) | 187 (0.3) | 3,080 (0.6) |
| Unknown | 18 (0.4) | <5 (0.4) | 453 (0.7) | 9,123 (1.8) |
| Maternal education level (years) | ||||
| ≤12 | 3,108 (73.6) | 369 (67.3) | 46,139 (66.9) | 203,000 (39.4) |
| >12 | 1,036 (24.5) | 169 (30.8) | 21,301 (30.9) | 287,110 (55.7) |
| Unknown | 80 (1.9) | 10 (1.8) | 1,506 (2.2) | 24,672 (4.8) |
| Maternal marital status | ||||
| Married | 1,313 (31.1) | 164 (29.9) | 21,165 (30.7) | 137,718 (26.8) |
| Not married | 2,245 (53.1) | 251 (45.8) | 31,894 (46.3) | 78,091 (15.2) |
| Unknown | 666 (15.8) | 133 (24.3) | 15,887 (23.0) | 298,973 (58.0) |
| Smoked during pregnancy | ||||
| Yes | 1,533 (36.3) | 141 (25.7) | 14,630 (21.2) | 29,651 (5.8) |
| No | 1,313 (31.1) | 211 (38.5) | 28,947 (42.0) | 223,154 (43.3) |
| Unknown | 1,378 (32.6) | 196 (35.8) | 25,369 (36.8) | 261,977 (50.9) |
| Alcohol intake during pregnancy | ||||
| Yes | 46 (1.1) | 13 (2.4) | 386 (0.6) | 4,192 (0.8) |
| No | 1,108 (26.2) | 172 (31.4) | 22,123 (32.1) | 91,108 (17.7) |
| Unknown | 3,070 (72.7) | 363 (66.2) | 46,437 (67.4) | 419,482 (81.5) |
Antipsychotic exposure status was determined during the period from 60 days preceding pregnancy through delivery, it was not mutually exclusive (e.g., deliveries with both atypical and typical antipsychotic exposure would be included in both groups).
Atypical antipsychotic use
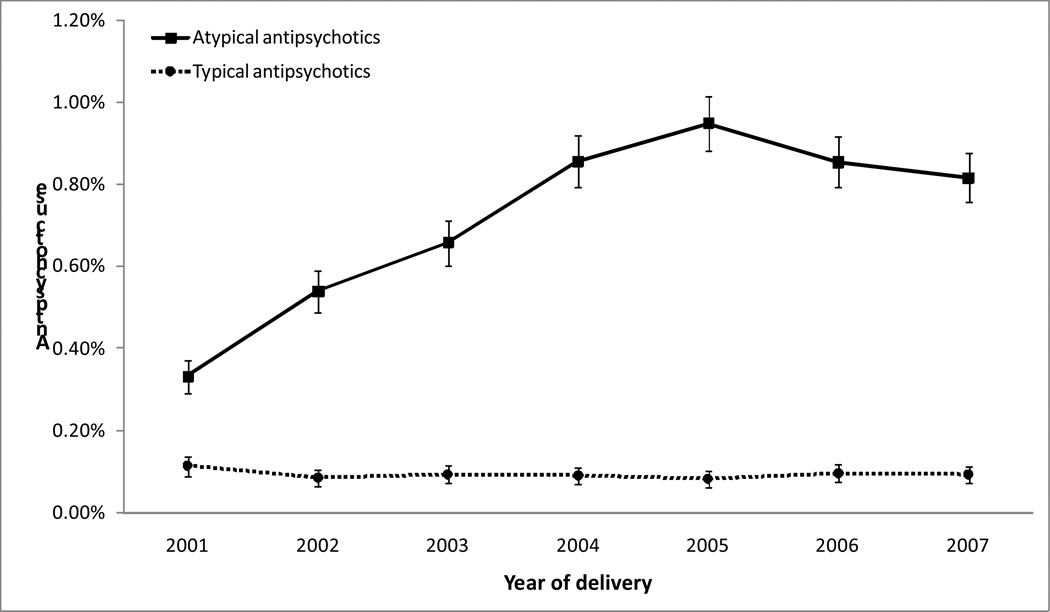
The prevalence of atypical antipsychotic exposure increased 2.5-fold during the study period, from 0.33% of deliveries (95% confidence interval: 0.29%, 0.37%) in 2001 to 0.82% (0.76%, 0.88%) in 2007 (Figure 1). The prevalence was highest at 0.5% during the first trimester, and decreased to 0.3% in the second trimester and 0.2% in the third trimester (Table 2). Quetiapine was the most commonly used atypical antipsychotics (42% of atypical antipsychotic use), followed by olanzapine (32%) and risperidone (23%).
Figure 1.
The prevalence of antipsychotic use from 60 days before pregnancy through delivery by year of delivery, the Medication Exposure in Pregnancy Risk Evaluation Program, 2001–2007 The proportion in a given year was calculated using the number of deliveries exposed to atypical (or typical) antipsychotics in that year as the numerator and the total number of deliveries in that year as the denominator. Bars represent the 95% confidence intervals.
Table 2.
Prenatal antipsychotic use by drug and gestational period, the Medication Exposure in Pregnancy Risk Evaluation Program, 2001–2007a
| Drugs | Any time from 60 days before pregnancy through delivery |
60-day pre pregnancy period |
Any time during pregnancy |
1st trimester | 2nd trimester | 3rd trimester | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | per 10,000 deliveries |
N | per 10,000 deliveries |
N | per 10,000 deliveries |
N | per 10,000 deliveries |
N | per 10,000 deliveries |
N | per 10,000 deliveries |
|
| Atypical antipsychotics | 4,223 | 72.0 | 3,169 | 54.0 | 3,476 | 59.3 | 3,030 | 51.7 | 1,565 | 26.7 | 1,144 | 19.5 |
| Quetiapine | 1,786 | 30.5 | 1,362 | 23.2 | 1,449 | 24.7 | 1,276 | 21.8 | 672 | 11.5 | 480 | 8.2 |
| Olanzapine | 1,342 | 22.9 | 884 | 15.1 | 1,077 | 18.4 | 867 | 14.8 | 465 | 7.9 | 358 | 6.1 |
| Risperidone | 970 | 16.5 | 676 | 11.5 | 750 | 12.8 | 628 | 10.7 | 303 | 5.2 | 211 | 3.6 |
| Aripiprazole | 443 | 7.6 | 331 | 5.6 | 351 | 6.0 | 310 | 5.3 | 140 | 2.4 | 87 | 1.5 |
| Ziprasidone | 314 | 5.4 | 229 | 3.9 | 249 | 4.2 | 223 | 3.8 | 92 | 1.6 | 60 | 1.0 |
| Clozapine | 12 | 0.2 | 8 | 0.1 | 12 | 0.2 | 10 | 0.2 | 5 | 0.1 | 7 | 0.1 |
| Paliperidone | <5 | 0.02 | <5 | 0.02 | <5 | 0.02 | <5 | 0.02 | <5 | 0.02 | <5 | 0.02 |
| Typical antipsychotics | 548 | 9.3 | 226 | 3.9 | 486 | 8.3 | 305 | 5.2 | 282 | 4.8 | 230 | 3.9 |
| Haloperidol | 225 | 3.8 | 76 | 1.3 | 206 | 3.5 | 112 | 1.9 | 126 | 2.1 | 119 | 2.0 |
| Chlorpromazine | 146 | 2.5 | 33 | 0.6 | 131 | 2.2 | 75 | 1.3 | 79 | 1.3 | 41 | 0.7 |
| Perphenazine | 95 | 1.6 | 59 | 1.0 | 81 | 1.4 | 64 | 1.1 | 44 | 0.8 | 32 | 0.5 |
| Thiothixene | 44 | 0.8 | 28 | 0.5 | 38 | 0.6 | 31 | 0.5 | 16 | 0.3 | 17 | 0.3 |
| Trifluoperazine | 27 | 0.5 | 10 | 0.2 | 24 | 0.4 | 13 | 0.2 | 14 | 0.2 | 11 | 0.2 |
| Thioridazine | 21 | 0.4 | 14 | 0.2 | 16 | 0.3 | 12 | 0.2 | 5 | 0.1 | 7 | 0.1 |
| Fluphenazine | 20 | 0.3 | 7 | 0.1 | 18 | 0.3 | 12 | 0.2 | 13 | 0.2 | 6 | 0.1 |
| Loxapine | 7 | 0.1 | 5 | 0.1 | 5 | 0.1 | 5 | 0.1 | <5 | 0.02 | <5 | 0.02 |
| Pimozide | <5 | 0.03 | <5 | 0.03 | <5 | 0.03 | <5 | 0.03 | <5 | 0.02 | <5 | 0.02 |
| Mesoridazine | <5 | 0.02 | <5 | 0.02 | <5 | 0.02 | <5 | 0.02 | 0 | 0 | 0 | 0 |
| Molindone | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Other typical antipsychotics | ||||||||||||
| Prochlorperazine | 6,266 | 106.8 | 841 | 14.3 | 5,659 | 96.5 | 3,643 | 62.1 | 2,856 | 48.7 | 946 | 16.1 |
| Promethazine | 64,037 | 1,091. 8 | 8,128 | 138.6 | 60,754 | 1,035.8 | 39,464 | 672.8 | 31,856 | 543.1 | 17,960 | 306.2 |
Antipsychotic exposure status was not mutually exclusive (e.g., deliveries with both atypical and typical antipsychotic exposure would be included in both groups). Exposure time periods were not mutually exclusive (e.g., deliveries with atypical antispsychotic exposure during the first and second trimester would appear in both periods). Some deliveries might be exposed to more than one drug of the same drug class, so the sum of deliveries exposed to individual drugs within each class might be greater than the overall number of exposed deliveries of that class.
About 81% of the deliveries to women with atypical antipsychotic exposure any time from 60 days before pregnancy through delivery had a recorded diagnosis of depression, bipolar disorder, or schizophrenia. Depression was the most common diagnosis among these deliveries – with 63% having such diagnosis – followed by bipolar disorder (43%) and schizophrenia (13%) (Table 3).
Table 3.
Mental health diagnoses among deliveries exposed to antipsychotics, by drug class and gestational period, the Medication Exposure in Pregnancy Risk Evaluation Program, 2001–2007a
| Drugs | Any time from 60 days before pregnancy through delivery |
60-day pre pregnancy period |
Any time during pregnancy |
1st trimester | 2nd trimester | 3rd trimester | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | %b | N | % | N | % | N | % | N | % | N | % | |
| Atypical antipsychotics | ||||||||||||
| Schizophrenia | 528 | 12.5 | 415 | 13.1 | 475 | 13.7 | 412 | 13.6 | 275 | 17.6 | 222 | 19.4 |
| Bipolar disorder | 1,801 | 42.6 | 1,366 | 43.1 | 1,560 | 44.9 | 1,354 | 44.7 | 769 | 49.1 | 598 | 52.3 |
| Depression | 2,644 | 62.6 | 1,997 | 63.0 | 2,200 | 63.3 | 1,928 | 63.6 | 993 | 63.5 | 723 | 63.2 |
| None of the above | 785 | 18.6 | 500 | 15.8 | 600 | 17.3 | 493 | 16.3 | 244 | 15.6 | 147 | 12.8 |
| Typical antipsychotics | ||||||||||||
| Schizophrenia | 145 | 26.5 | 70 | 31.0 | 135 | 27.8 | 87 | 28.5 | 86 | 30.5 | 86 | 37.4 |
| Bipolar disorder | 204 | 37.2 | 77 | 34.1 | 188 | 38.7 | 117 | 38.4 | 112 | 39.7 | 107 | 46.5 |
| Depression | 281 | 51.3 | 116 | 51.3 | 253 | 52.1 | 152 | 49.8 | 140 | 49.6 | 131 | 57.0 |
| None of the above | 149 | 27.2 | 49 | 21.7 | 126 | 25.9 | 77 | 25.2 | 69 | 24.5 | 34 | 14.8 |
Antipsychotic exposure status was not mutually exclusive (e.g., deliveries with both atypical and typical antipsychotic exposure would be included in both groups). Exposure time periods were not mutually exclusive (e.g., deliveries with atypical antispsychotic exposure during the first and second trimester would appear in both periods). Some deliveries might be exposed to more than one drug of the same drug class, so the sum of deliveries exposed to individual drugs within each class might be greater than the overall number of exposed deliveries of that class.
Within each drug class, proportions were calculated using the number of each cell as the numerator and the total number of each column as the denominator. Some deliveries might have more than one diagnosis so the sum of proportions might be greater than 100%.
During the study period, there appeared to be a modest shift in the diagnosis pattern among deliveries exposed to atypical antipsychotics, with more diagnosed with bipolar disorder and less with depression or schizophrenia over time (Table 4). In 2007, the proportion of deliveries with a diagnosis of bipolar disorder (53%) was similar to the proportion with a diagnosis of depression (58%), in contrast to 39% and 64%, respectively, in 2001.
Table 4.
Mental health diagnoses among deliveries exposed to antipsychotics, by drug class and year of delivery, the Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP), 2001–2007a
| Year of delivery | Schizophrenia | Bipolar disorder | Depression | None of the three | ||||
|---|---|---|---|---|---|---|---|---|
| N | %b | N | % | N | % | N | % | |
| Atypical antipsychotics | ||||||||
| 2001 (n=264) | 47 | 17.8 | 104 | 39.4 | 169 | 64.0 | 34 | 12.9 |
| 2002 (n=452) | 69 | 15.3 | 168 | 37.2 | 305 | 67.5 | 68 | 15.0 |
| 2003 (n=555) | 96 | 17.3 | 220 | 39.6 | 382 | 68.8 | 67 | 12.1 |
| 2004 (n=714) | 84 | 11.8 | 287 | 40.2 | 494 | 69.2 | 110 | 15.4 |
| 2005 (n=794) | 93 | 11.7 | 328 | 41.3 | 479 | 60.3 | 151 | 19.0 |
| 2006 (n=736) | 68 | 9.2 | 316 | 42.9 | 408 | 55.4 | 162 | 22.0 |
| 2007 (n=708) | 71 | 10.0 | 378 | 53.4 | 407 | 57.5 | 105 | 14.8 |
| Typical antipsychotics | ||||||||
| 2001 (n=90) | 24 | 26.7 | 25 | 27.8 | 43 | 47.8 | 24 | 26.7 |
| 2002 (n=72) | 26 | 36.1 | 19 | 26.4 | 39 | 54.2 | 18 | 25.0 |
| 2003 (n=79) | 20 | 25.3 | 30 | 38.0 | 40 | 50.6 | 20 | 25.3 |
| 2004 (n=75) | 20 | 26.7 | 38 | 50.7 | 43 | 57.3 | 20 | 26.7 |
| 2005 (n=69) | 16 | 23.2 | 27 | 39.1 | 32 | 46.4 | 21 | 30.4 |
| 2006 (n=83) | 19 | 22.9 | 34 | 41.0 | 40 | 48.2 | 23 | 27.7 |
| 2007 (n=80) | 20 | 25.0 | 31 | 38.8 | 44 | 55.0 | 23 | 28.8 |
Antipsychotic exposure status was not mutually exclusive (e.g., deliveries with both atypical and typical antipsychotic exposure would be included in both groups).
Within each drug class, proportions were calculated using the number of each cell as the numerator and the total number of each row as the denominator. Some deliveries might have more than one diagnosis so the sum of proportions might be greater than 100%.
Typical antipsychotics
The prevalence of typical antipsychotic use in each year was low and relatively stable at about 0.1% over the study period (Figure 1). Similar to what was observed for atypical antipsychotics, the prevalence was greatest during the first trimester, followed by a drop in the later two trimesters. Haloperidol and chlorpromazine had the highest use (Table 2). Approximately 73% of deliveries to women with typical antipsychotic exposure any time from 60 days before pregnancy through delivery had a diagnosis of bipolar disorder, schizophrenia, or depression (Table 3), with depression being the most common diagnosis (51%), followed by bipolar disorder (37%) and schizophrenia (27%). Compared with atypical antipsychotic-exposed deliveries, deliveries with typical antipsychotic use were more likely to have a diagnosis of schizophrenia (27% versus 13%).
Typical antipsychotics – Including prochlorperazine and promethazine
When we included prochlorperazine and promethazine, the number of deliveries with typical antipsychotic use became substantially higher (n=68,946, or 11.8% of all deliveries). Over the study years, prenatal use of typical antipsychotics including prochlorperazine and promethazine increased slightly from 10.6% in 2001 to 12.8% in 2007. The prevalence was highest at 7.3% during the first trimester, followed by 5.9% in the second trimester, 3.2% in the third trimester, and 1.5% in the 60-day pre-pregnancy period. Promethazine was prescribed far more commonly than prochlorperazine (10.9% vs. 1.1% of all deliveries). About 84% of deliveries were to women who were exposed to these two medications but did not have a diagnosis of schizophrenia, bipolar disorder, or depression. This, along with the low prevalence of use during the pre-pregnancy period, strongly suggested that these two medications were used as anti-emetics.
Discussion
In this study, we observed a 2.5-fold increase in the prevalence of prenatal use of atypical antipsychotics, from 0.3% (95% confidence interval: 0.3%, 0.4%) in 2001 to 0.8% (0.8%, 0.9%) in 2007. The prevalence of typical antipsychotics during pregnancy remained low and relatively stable. To our knowledge, this is the largest U.S. study to date to examine the prevalence of atypical and typical antipsychotic use during pregnancy.
The prevalence of prenatal antipsychotic use in our cohort was higher than previous estimates. A single-site U.S study found that 16 of the 30,092 women (0.05%) who delivered at the Mayo Clinic between 1993 and 2007 had used atypical antipsychotics during pregnancy (Wichman 2009). A study of 958,729 women in the Swedish Medical Birth Register from 1995 to 2005 found that 2,830 (0.3%) of them were exposed to antipsychotics while pregnant (Reis and Kallen 2008). However, 2,260 of these women used dixyrazine (not marketed in the U.S.) or prochlorperazine, two antipsychotics that are commonly used to treat nausea and vomiting during pregnancy and rarely used for psychiatric disorders. Only about 150 women (0.02%) used atypical antipsychotics. A separate study using the same national Swedish data from 2005 to 2009 found that the prevalence of atypical antipsychotic use during pregnancy was about 0.1% (Boden et al. 2012a). In a study conducted within the Medical Birth Registry of Norway, the prevalence of prenatal use of antipsychotic medications – identified by Anatomical Therapeutic Chemical (ATC) code N05A, which includes prochlorperazine and lithium – was 1.6% (1,736 out of 106,329 deliveries) during the 2004–2006 period (Engeland et al. 2008).
The higher prevalence in our study might be due to differences in the study population, clinical practice or medication use between countries, secular trends, or the methods used to identify medication exposure. Specifically, we included a large Medicaid population from the Tennessee Medicaid, which might be enriched with users of antipsychotic medications. A smaller study that used data from the Tennessee Medicaid observed an increase in atypical antipsychotic use during pregnancy, from 0.2% in 1985–2000 to 1.7% in 2005 (Epstein et al. 2012).
Findings from our study are consistent with an increase in the use of atypical antipsychotics and a shift in the diagnosis pattern from schizophrenia to bipolar disorder among atypical antipsychotics users identified from the general population over the same period (Verdoux et al. 2010; Alexander et al. 2011). The increase in use may in part be due to the expanded indications for use of atypical antipsychotics in bipolar disorder (of which the prevalence has been increasing in recent years (Blader and Carlson 2007)) and treatment-resistant depression (Nelson and Papakostas 2009), an increase in off-label use (Alexander et al. 2011), or a combination of these.
Decisions about the use of antipsychotic medications during pregnancy have to take into account the risks of not treating the underlying disorders, the risks associated with antipsychotic medications, the availability and safety of alternative therapies. Treating psychiatric conditions during pregnancy is generally recommended (ACOG Committee on Practice Bulletins--Obstetrics 2008; Altshuler et al. 1996; Viguera et al. 2002; Yonkers et al. 2004; Yonkers et al. 2009) but evidence to guide treatment choice is limited and conflicting (Trixler et al. 2005; Gentile 2010). It is unlikely that this critically needed information will come from randomized trials because of ethical concerns. Large, well-designed observational studies employing data from databases such as the MEPREP resources may help fill the knowledge gap about the comparative safety of antipsychotic medications in pregnancy.
Our study has several strengths. First, the large sample size and the demographic and geographic diversity of our population increase the generalizability of the study findings. Second, the use of electronic pharmacy dispensing data avoids the potential for recall bias that is common in studies that rely on patient self-report. Restricting the analysis to women with pharmacy benefits increases the likelihood that we captured most dispensings of the study medications. Third, linkage to birth certificate data allows us to define gestational age more accurately, reducing the potential for misclassification of prenatal exposure status (Toh et al. 2008; Li et al.).
On the other hand, our study is not without limitations. As with any study using pharmacy dispensing records to identify drug use, we could not ascertain whether antipsychotics dispensed were actually taken by the women. As we limited our study to live born deliveries, we were not able to assess the use of antipsychotics in pregnancies that did not result in a live birth. The prevalence is higher in the Medicaid population, but we did not stratify the analysis by insurance status. While not stratifying on insurance status would not affect the validity of the analysis, our overall findings may not be generalizable to other populations with different patient characteristics. We did not have data after 2007, so we could not examine the utilization pattern in recent years. The Tennessee Medicaid adopted a policy that capped the number of prescriptions per month at five for each patient in 2006. The cap may partially explain the apparent interruption in the temporal trend in atypical antipsychotic prevalence at the same time. However, we believe the impact of the cap was not likely to be major for two reasons: 1) the prescription limit did not apply to enrollees under the age of 21 years, and 2) antipsychotics are on the “Prescriber Attestation List”, meaning that a pregnant woman over 21 years of age can exceed the prescription limit and receive an antipsychotic medication so long as the prescriber preauthorizes the medicine. Finally, even though we were able to identify mental health diagnoses recorded during the prenatal period, we could not determine the accuracy of these diagnoses and whether the medications were actually being used for those indications. However, it is reassuring that the shift in the diagnosis pattern observed in our study was also seen in a large study of the U.S. general population (Alexander et al. 2011).
In conclusion, between 2001 and 2007, an increasing number and proportion of pregnancies were exposed to atypical antipsychotics. As the decision to initiate or continue antipsychotic medications during pregnancy must balance the risks of not treating the underlying psychiatric disorders against the risks associated with taking these medications, there is a need for large, well-designed studies to examine the comparative safety of antipsychotics and other treatment choices for each indication in pregnancy.
Acknowledgments
This study was supported through funding from contracts HHSF223200510012C, HHSF223200510009C, and HHSF223200510008C from the U.S. Food and Drug Administration (Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, Silver Spring, MD, USA). Dr. Dublin was supported by National Institute on Aging grant K23AG028954, Dr. Bobo was supported by National Institute of Mental Health grant K23MH087747. Dr. Andrade has received research funding from GlaxoSmithKline, a manufacturer of an antipsychotic medication, in the past 12 months for a project unrelated to antipsychotic medication use. The views expressed in this paper are those of the authors and are not intended to convey official U.S. Food and Drug Administration policy or guidance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Mental Health, or the National Institutes of Health.
References
- ACOG Committee on Practice Bulletins--Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001–1020. doi: 10.1097/AOG.0b013e31816fd910. [DOI] [PubMed] [Google Scholar]
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20:177–184. doi: 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153:592–606. doi: 10.1176/ajp.153.5.592. [DOI] [PubMed] [Google Scholar]
- Andrade SE, Davis RL, Cheetham TC, Cooper WO, Li DK, Amini T, Beaton SJ, Dublin S, Hammad TA, Pawloski PA, Raebel MA, Smith DH, Staffa JA, Toh S, Dashevsky I, Haffenreffer K, Lane K, Platt R, Scott PE. Medication Exposure in Pregnancy Risk Evaluation Program. Matern Child Health J. 2012;16:1349–1354. doi: 10.1007/s10995-011-0902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu GN, Desai G, Tippeswamy H, Chandra PS. Birth weight and use of olanzapine in pregnancy: a prospective comparative study. J Clin Psychopharmacol. 2010;30:331–332. doi: 10.1097/JCP.0b013e3181db8734. [DOI] [PubMed] [Google Scholar]
- Bagnall AM, Jones L, Ginnelly L, Lewis R, Glanville J, Gilbody S, Davies L, Torgerson D, Kleijnen J. A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess. 2003;7:1–193. doi: 10.3310/hta7130. [DOI] [PubMed] [Google Scholar]
- Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden R, Lundgren M, Brandt L, Reutfors J, Kieler H. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry. 2012a;69:715–721. doi: 10.1001/archgenpsychiatry.2011.1870. [DOI] [PubMed] [Google Scholar]
- Boden R, Lundgren M, Brandt L, Reutfors J, Kieler H. Antipsychotics During Pregnancy: Relation to Fetal and Maternal Metabolic EffectsAntipsychotics During Pregnancy. Arch Gen Psychiatry. 2012b;69:715–721. doi: 10.1001/archgenpsychiatry.2011.1870. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- Coppola D, Russo LJ, Kwarta RF, Jr, Varughese R, Schmider J. Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes. Drug Saf. 2007;30:247–264. doi: 10.2165/00002018-200730030-00006. [DOI] [PubMed] [Google Scholar]
- Engeland A, Bramness JG, Daltveit AK, Ronning M, Skurtveit S, Furu K. Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106,000 pregnancies in Norway 2004–2006. Br J Clin Pharmacol. 2008;65:653–660. doi: 10.1111/j.1365-2125.2008.03102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Bobo WV, Shelton RC, Arbogast PG, Morrow JA, Wang W, Chandrasekhar R, Cooper WO. Increasing use of atypical antipsychotics and anticonvulsants during pregnancy. Pharmacoepidemiol Drug Saf. 2012 doi: 10.1002/pds.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst CL, Goldberg JF. The reproductive safety profile of mood stabilizers, atypical antipsychotics, and broad-spectrum psychotropics. J Clin Psychiatry. 2002;4(63 Suppl):42–55. [PubMed] [Google Scholar]
- Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:518–544. doi: 10.1093/schbul/sbn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S, Avasthi A, Sharma Y. Psychotropics in pregnancy: weighing the risks. Indian J Med Res. 2006;123:497–512. [PubMed] [Google Scholar]
- Johnson KC, Laprairie JL, Brennan PA, Stowe ZN, Newport DJ. Prenatal Antipsychotic Exposure and Neuromotor Performance During Infancy. Arch Gen Psychiatry. 2012;69:787–794. doi: 10.1001/archgenpsychiatry.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N, Everitt B, Boydell J, Van Os J, Jones PB, Murray RM. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35:855–863. doi: 10.1017/s0033291704003307. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1–56. [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand. 2000;(401):3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Andrade SE, Cooper WO, Davis RL, Dublin S, Hammad TA, Pawloski PA, Pinheiro SP, Raebel MA, Scott PE, Smith DH, Dashevsky I, Haffenreffer K, Johnson KE, Toh S. Validation of an algorithm to estimate gestational age in electronic health plan databases. Pharmacoepidemiol Drug Saf. doi: 10.1002/pds.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, Osterman MJ. Births: final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- McKenna K, Koren G, Tetelbaum M, Wilton L, Shakir S, Diav-Citrin O, Levinson A, Zipursky RB, Einarson A. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66:444–449. doi: 10.4088/jcp.v66n0406. quiz 546. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166:980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- Newham JJ, Thomas SH, MacRitchie K, McElhatton PR, McAllister-Williams RH. Birth weight of infants after maternal exposure to typical and atypical antipsychotics: prospective comparison study. Br J Psychiatry. 2008;192:333–337. doi: 10.1192/bjp.bp.107.041541. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, Knight BT, Gibson BB, Viguera AC, Owens MJ, Nemeroff CB, Stowe ZN. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164:1214–1220. doi: 10.1176/appi.ajp.2007.06111886. [DOI] [PubMed] [Google Scholar]
- Reis M, Kallen B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28:279–288. doi: 10.1097/JCP.0b013e318172b8d5. [DOI] [PubMed] [Google Scholar]
- Stanniland C, Taylor D. Tolerability of atypical antipsychotics. Drug Saf. 2000;22:195–214. doi: 10.2165/00002018-200022030-00004. [DOI] [PubMed] [Google Scholar]
- Toh S, Mitchell AA, Werler MM, Hernández-Díaz S. Sensitivity and specificity of computerized algorithms to classify gestational periods in the absence of information on date of conception. Am J Epidemiol. 2008;167:633–640. doi: 10.1093/aje/kwm367. [DOI] [PubMed] [Google Scholar]
- Trixler M, Gati A, Fekete S, Tenyi T. Use of antipsychotics in the management of schizophrenia during pregnancy. Drugs. 2005;65:1193–1206. doi: 10.2165/00003495-200565090-00002. [DOI] [PubMed] [Google Scholar]
- Verdoux H, Tournier M, Begaud B. Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatr Scand. 2010;121:4–10. doi: 10.1111/j.1600-0447.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- Viguera AC, Cohen LS, Baldessarini RJ, Nonacs R. Managing bipolar disorder during pregnancy: weighing the risks and benefits. Can J Psychiatry. 2002;47:426–436. doi: 10.1177/070674370204700503. [DOI] [PubMed] [Google Scholar]
- Wichman CL. Atypical antipsychotic use in pregnancy: a retrospective review. Arch Womens Ment Health. 2009;12:53–57. doi: 10.1007/s00737-008-0044-3. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114:703–713. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stowe Z, Leibenluft E, Cohen L, Miller L, Manber R, Viguera A, Suppes T, Altshuler L. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry. 2004;161:608–620. doi: 10.1176/appi.ajp.161.4.608. [DOI] [PubMed] [Google Scholar]