Abstract
Cells are not passive bystanders in the process of hormonal signaling and instead can actively customize hormonal action. While diffusing from the plasma membrane to the nucleus, thyroid hormone is modified via the action of thioredoxin fold–containing selenoenzymes known as deiodinases. Whereas the type II deiodinase (D2) converts the prohormone thyroxine (T4) to the biologically active T3, the type III deiodinase (D3) converts it to reverse T3, an inactive metabolite. D3 also inactivates T3 to T2, terminating thyroid hormone action. Therefore, D2 provides cells with the ability to produce extra amounts of T3 and thus enhances thyroid hormone signaling. In contrast, expression of D3 results in the opposite action. In addition, the D2 protein is unique in that it can be switched off and on via an ubiquitin-regulated mechanism, triggered by catalysis of T4. Induction of D2 enhances local thyroid hormone signaling and energy expenditure during activation of brown adipose tissue by cold exposure or high fat diet. On the other hand, induction of D3 in myocardium and brain during ischemia and hypoxia decreases energy expenditure as part of a homeostatic mechanism to slow down cell metabolism in the face of limited O2 supply.
INTRODUCTION
Cells are not passive bystanders in the process of hormonal signaling and instead can customize the action of sexual hormones and other steroids as well as thyroid hormones. For example, 5α-reductase or P450 aromatase, respectively, transform testosterone into dihydrotestosterone or estradiol, locally changing testosterone's biological activity in opposite directions. A similar scenario exists in the case of the deiodinases, enzymes that can locally activate or inactivate thyroid hormone.
Membrane transporters are necessary for thyroid hormone access to the intracellular environment (1). Once inside the cells, thyroid hormone diffuses towards the nucleus and eventually binds to its receptors (TR), high affinity ligand-dependent transcription factors that modify gene expression (2). Once inside the cells, the prohormone T4 can be transformed to the biologically active T3 molecule via the type 2 deiodinase (D2) or it can be inactivated to form reverse T3 via the type 3 deiodinase (D3). Most importantly, T3 is also inactivated by D3, preventing or terminating thyroid hormone action (3).
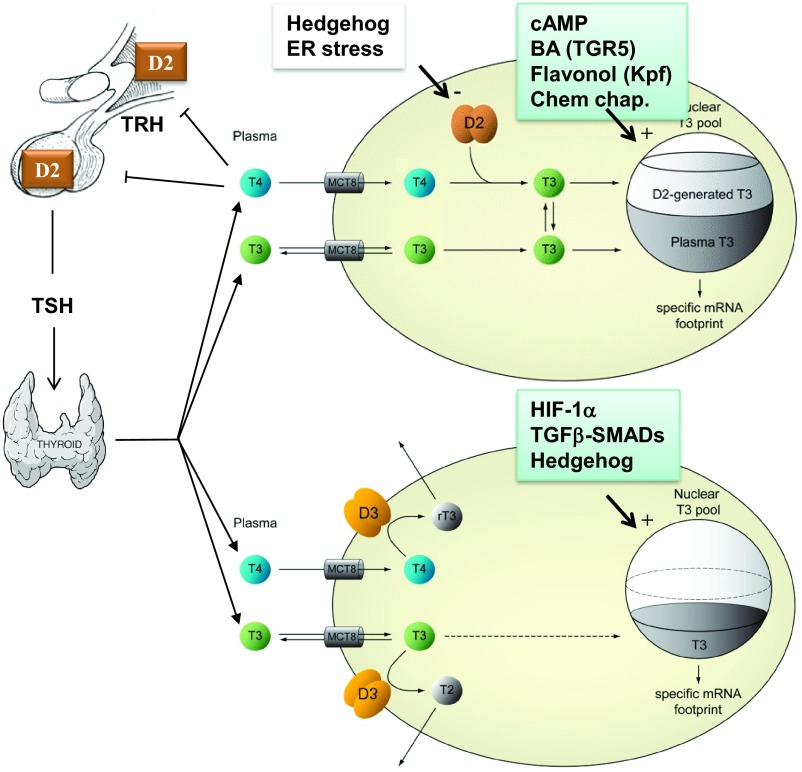
Deiodinases are dimeric integral-membrane thyroredoxin fold–containing selenoproteins of approximately 60 kDa (dimer) (4–9). Each dimer counterpart consists of a selenocystein-containing globular domain that is anchored to cellular membranes. D2 is retained in the endoplasmic reticulum (ER) and generates T3 in the proximity of the nuclear compartment (10). On the other hand, under normal circumstances D3 goes through the Golgi complex and reaches the plasma membrane where it undergoes endocytosis and recycles via the early endosomes (4). However, during ischemia (in the brain) or hypoxia D3 concentrates in the nucleus where it inactivates thyroid hormone and minimizes the metabolic footprint of T3 (11). Thus, D2 confers cells with the capacity to produce additional amounts of T3 and enhances thyroid hormone signaling. In contrast, expression of D3 results in the opposite action (Fig. 1). Furthermore, these events occur in the cell without relative changes to plasma thyroid hormone levels (12, 13).
Fig. 1.
Thyroid hormone signaling: T3-target cells are not passive bystanders. Deiodinases customize the action of thyroid hormone by augmenting or decreasing intracellular T3 levels.
The D2 protein is unique in that it can be switched off and on via an ubiquitin (Ub) regulated mechanism, triggered by catalysis of T4 (14–16). It is assumed that T4 deiodination exposes Lys-residues in D2's globular domain that are subsequently conjugated to Ub. Moreover, this results in inactivating D2 by disruption of the dimer formation (14). Ub-D2 is not immediately taken up by the proteasome and instead can be deubiquitinated and reactivated to produce another molecule of T3, repeating the cycle. While two Ub conjugases are involved in the process of D2 ubiquitination (17, 18), the limiting components of this pathway are two E3-ligase adaptors. These include the hedgehog-inducible SOCS-box containing WSB-1 (19), and TEB4 (20) a ligase involved in the ER-associated degradation (ERAD) program. In contrast, two Ub-specific proteases, USP20 and USP33, mediate deubiquitination and reactivation of Ub-D2 (15).
Thus, it is clear that thyroid hormone levels in the plasma do not faithfully reflect thyroid hormone signaling in cells; this action takes place inside the cell. A complex network of transcriptional and post-transcriptional mechanisms regulating deiodinase expression is at work in health and disease, mediating rapid customization of thyroid hormone signaling on a cell-specific basis.
DEIODINASES AND THE METABOLIC EFFECTS OF THYROID HORMONE
Thyroid hormone levels hardly fluctuate in the plasma of healthy individuals, as shown in a year-long study of serum levels of T4 and T3 (21). Thus, thyroid hormone–responsive metabolic processes are turned on and off by thyroid hormone via deiodination pathways that are taking place inside the target cells, seemingly invisible from the plasma viewpoint (12).
A glimpse into this world is available through the studies in which D2 and D3 expression reciprocally affect energy expenditure in a number of cell and animal models. For example, cAMP-dependent induction of D2 expression during activation of brown adipocytes by cold exposure or high fat diet enhances local thyroid hormone signaling and energy expenditure, the absence of which prevents normal BAT function (22–25). On the other hand, HIF-1α–dependent induction of D3 in myocardium and brain during ischemia and hypoxia decreases energy expenditure, supposedly as part of a homeostatic mechanism to slow down cell metabolism in the face of limited O2 supply (26, 27). In fact, D3 reactivation in disease states can be so powerful that it compromises systemic thyroid economy, leading to euthyroid sick syndrome (28). In rare instances, D3-mediated thyroid hormone inactivation is so dramatic that it exceeds the thyroidal synthetic capacity to sustain thyroid economy, leading to consumptive hypothyroidism (29).
D2 expression is the target of a rapidly growing number of molecules that accelerate energy expenditure and metabolic programs in cells and animal models. These include bile acids (30), flavonols (31), and chemical chaperones (32), which in turn confer protection against diet-induced obesity. Insulin and PPARγ agonists are also bona fide inducers of D2 in skeletal muscle (33). On the other hand, signaling through the D2 pathway is dampened by ER stress (34) and the LXR-RXR pathway (35), the metabolic consequence of which is currently under investigation.
During vertebrate embryogenesis, developmental signals control the expression interplay between D2 and D3 in metabolic relevant tissues such as BAT (36), pancreatic islets and skeletal muscle (37), explaining how “systemic” thyroid hormone can affect local control of tissue embryogenesis.
In the 3-day developmental snapshot during which BAT develops in mice (E16.5–18.5), D2 expression is upregulated approximately 5-fold and D3 expression decreases by 75%. This results in increased local net T3 availability, whilst serum T3 remains unchanged. This rapid enhancement in thyroid hormone signaling is critical for the expression of genes defining BAT identity, i.e., UCP1, PGC-1alpha, and Dio2 (36). Notably, these changes in gene expression are observed in utero, without a thermogenic challenge, which highlights the relevance of D2 and its ability to amplify thyroid hormone signaling in a developmental setting. The inactivation of the Dio2 gene as in the D2KO mouse results in a permanent BAT thermogenic defect, compromising thermoregulation and the ability to dissipate excessive calories from diet (22, 23).
The D2 pathway seems to also be critical for skeletal muscle development and function (37). Besides regulating insulin sensitivity in myocytes (33), D2-mediated T3 production is also required for the T3-dependent expression of myogenic factors, such as MyoD, which drive myocyte development. As in BAT, myocytes from D2KO mice have impaired development and function supposedly due to lower intracellular T3 generation. The control of the D2 pathway in myocytes is dependent on the transcription factor Fox03, which directly binds to the Dio2 promoter, upregulating D2 expression. The fact that Fox03KO myocytes also display impaired cellular development, easily reversed by the addition of exogenous T3 underscores the physiological relevance of the Fox03/D2 interplay.
The opposite scenario is observed during development of the pancreatic β-cells, with D3 expression keeping thyroid hormone signaling to a minimum, from late embryonic development throughout adulthood (38). The late emergence of D3 expression at E17.5 is restricted to insulin-positive cells, indicating a focused role in β- but not α-cell development. Alpha-cell development occurs at a much earlier phase of embryogenesis (by E9.5). As a result of untimely expression of thyroid hormone, D3KO animals exhibit a reduction in total islet area due to decreased β-cell area, insulin content, and lower expression of key islet genes involved in glucose sensing, insulin expression, and exocytosis. This is physiologically significant given that adult D3KO animals are glucose intolerant due to impaired glucose-stimulated insulin secretion, without changes in peripheral sensitivity to insulin.
CONCLUSIONS
From a broad perspective, deiodination supports a new paradigm in which hormones are activated or inactivated in a controlled fashion in specific thyroid hormone-target tissues. The role played by the deiodinases is analogous to 5α-reductase and P450 aromatase in sex steroid metabolism and to 11β-hydroxysteroid dehydrogenase in glucocorticoid metabolism. Compared to the field of steroid metabolizing enzymes, drug development for the control of deiodination is in its primordial. The therapeutic potential is obvious: if the D2 pathway can be harnessed pharmacologically, one should be able to control energy expenditure and perhaps contribute for the treatment of obesity, type 2 diabetes, and the metabolic syndrome. In turn, pharmacological control of the D3 pathway could help both metabolically and in the process recovery of illnesses.
ACKNOWLEDGEMENTS
Part of the work discussed here has been funded by NIDDK DK58538, DK65055, DK77148, and DK7856.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Hochberg, Baltimore: So, I am a rheumatologist. There are studies, particularly data from the Rotterdam Study, which have been confirmed in a couple of other European cohorts showing that a common variant in the Dio2 gene is associated with radiographic hip osteoarthritis, particularly the phenotype of decreased joint space width but not necessarily accompanied by osteophytosis. So, my question is: how do the common genetic variants in Dio2 affect the action of D2 at the cellular level in terms of inducing the hyperthyroid phenotype, which I presume would be related to an increase in catabolism of the chondrocyte, a catabolic phenotype of the chondrocyte?
Bianco, Miami: That's a great point. Thank you. We have looked very extensively at this polymorphism and could not find changes in D2 activity, even though the alteration in coding sequence it is located in the loop that we described as critical for the enzyme activity; however, enzyme kinetics are not changed. The hypothesis we tested was that if D2 activity would be decreased by virtue of having the polymorphism, then what would create a state of localized hypothyroidism? On the other hand, I suppose you could as well have increased catabolic efficiency of the D2 enzyme and created a state of localized hyperthyroidism. We just don't know because the studies so far, including from Rotterdam and our own laboratory, could not identify catalytic activity changes in D2 caused by the polymorphism. Alternatively, the association studies could reflect a gene linkage. It could be that the polymorphism is linked to another gene that has not been identified and this is an ongoing investigation in different labs.
Nestler, Richmond: Great presentation, but it actually raises a bit of a scary possibility. The symptoms of hypothyroidism are very vague. Endocrinologists are frequently getting patients coming saying, “I have these symptoms. I think I have hypothyroidism,” and we have, with great assurance said, “Your thyroid tests are normal. You don't have hypothyroidism,” but if these individuals were to have some reason or genetic reason for over-activation of D3, our studies would suggest they are hypothyroid and we can't detect it.
Bianco, Miami: Right.
Nestler, Richmond: So what do you suggest we do?
Bianco, Miami: Well again, this goes back to the polymorphism and I'll give you an example. About 85% of all hypothyroid patients that we commonly treat with levothyroxine are satisfied. However, a small fraction of these patients, about 10% to 15%, are never happy, never satisfied, even though serum TSH and free T4 levels are absolutely normal. It turns out that a recent study performed in the UK suggests that those unhappy hypothyroid patients are the ones that exhibit the Dio2 polymorphism. So, the underlying hypothesis is that when we treat those patients with levothyroxine alone, we are creating a state of brain hypothyroidism because D2 is so critical for the amount of T3 in the brain. By giving a combined therapy of both T4 and T3 to those patients you would actually fix that problem. That study was designed as a double-blind placebo-controlled study that enrolled about 600 patients. It is the largest study done so far and this is really promising in terms of treatment of hypothyroidism.
Reiser, Chicago: Thank you for a beautiful presentation. I was particularly intrigued by the cellular target customization of the signal. How widespread of a mechanism do you think that is? Is this applicable to other systemic molecules?
Bianco, Miami: Oh, I see. Well yes, if you look at hormones you would think that this is similar to what happens with testosterone, for example. You know testosterone serum levels hardly fluctuate but you do have activation of testosterone to dihydrotestosterone that it's a much more potent molecule and you can actually have testosterone being converted to estradiol in different tissues. So when you look at hormones, most molecules do fluctuate in the plasma. However, levels of thyroid hormones and steroid hormones are steady and they display this type of local control.
Gotto, New York: There are several thyroid agonists for the hepatic receptor in clinical trials for lowering cholesterol and LDL. Is it known what they do to the deiodinases?
Bianco, Miami: That's a great question. Thank you. So, by developing these thyroid hormone analogs that bind with higher affinity for TR-beta isoform of the thyroid hormone receptor one utilizes the same strategy I discussed with the deiodinases, that is to increase thyroid hormone action in a tissue-specific fashion. Even though we are talking about deiodinase and this is a completely different strategy, the goal is the same. However, the excitement with these analogs has decreased, even though they worked very well. The planning of clinical trials has been halted because of some serious side effects in the bone and the joints that were observed in dogs treated with the analogues. Now, to answer your question, by virtue of activating TR-beta, in fact, GC1 or other TR-beta analogs upregulate the type 1 deiodinase in the liver, explaining an increase in the serum T3/T4 ratio given the increased fractional conversion of T4 to T3.
Gotto, New York: I see. So this is not an off-target effect. This is mechanistically related.
Bianco, Miami: Right. As explained exactly.
Bishopric, Miami: Tony that was a beautiful talk.
Bianco, Miami: Thank you.
Bishopric, Miami: My favorite T3 target cell is the cardiac myocyte and I wondered if there is anything known about deiodinase expression during aging, for example, in that tissue because we have a lot of alterations in the expression of thyroid hormone toxicity in the heart with age.
Bianco, Miami: Right. No, unfortunately not. We would need to go and obtain the biopsies and it turns out that the mouse myocardium and the human myocardium are a little bit different in terms of the types of deiodinase they express. Human myocardium expresses type 2 deiodinase as opposed to the mouse in which you just don't find D2. However, that would be a very interesting topic for investigation. What we have shown is that the D3 knockout mouse loses the ability to undergo normal cardiac remodeling, developing cardiac-specific thyrotoxicosis and intense myocardial fibrosis. We've published this paper recently and it seems that it's actually the fibroblasts in the myocardium that have enhanced capacity to produce collagen, thus creating fibrosis, and we are actually looking further into that.
REFERENCES
- 1.Heuer H, Visser TJ. Minireview: pathophysiological importance of thyroid hormone transporters. Endocrinology. 2009;150:1078–83. doi: 10.1210/en.2008-1518. [DOI] [PubMed] [Google Scholar]
- 2.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianco AC, Larsen PR. Cellular and structural biology of the deiodinases. Thyroid. 2005;15:777–86. doi: 10.1089/thy.2005.15.777. [DOI] [PubMed] [Google Scholar]
- 4.Baqui M, Botero D, Gereben B, Curcio C, Harney JW, Salvatore D, et al. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem. 2003;278:1206–11. doi: 10.1074/jbc.M210266200. [DOI] [PubMed] [Google Scholar]
- 5.Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000;141:4309–12. doi: 10.1210/endo.141.11.7872. [DOI] [PubMed] [Google Scholar]
- 6.Curcio-Morelli C, Gereben B, Zavacki AM, Kim BW, Huang S, Harney JW, et al. In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology. 2003;144:3438–43. doi: 10.1210/en.2002-220960. [DOI] [PubMed] [Google Scholar]
- 7.Callebaut I, Curcio-Morelli C, Mornon JP, Gereben B, Buettner C, Huang S, et al. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J Biol Chem. 2003;278:36887–96. doi: 10.1074/jbc.M305725200. [DOI] [PubMed] [Google Scholar]
- 8.Berry MJ, Banu L, Larsen PR. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991;349:438–40. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- 9.Sagar GD, Gereben B, Callebaut I, Mornon JP, Zeold A, Curcio-Morelli C, et al. The thyroid hormone-inactivating deiodinase functions as a homodimer. Mol Endocrinol. 2008;22:1382–93. doi: 10.1210/me.2007-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeold A, Pormuller L, Dentice M, Harney JW, Curcio-Morelli C, Tente SM, et al. Metabolic instability of type 2 deiodinase is transferable to stable proteins independently of subcellular localization. J Biol Chem. 2006;281:31538–43. doi: 10.1074/jbc.M604728200. [DOI] [PubMed] [Google Scholar]
- 11.Jo S, Kallo I, Bardoczi Z, Arrojo EDR, Zeold A, Liposits Z, et al. Neuronal hypoxia induces hsp40-mediated nuclear import of type 3 deiodinase as an adaptive mechanism to reduce cellular metabolism. J Neurosci. 2012;32:8491–8500. doi: 10.1523/JNEUROSCI.6514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gereben B, Zeold A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci. 2008;65:570–90. doi: 10.1007/s00018-007-7396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagar GD, Gereben B, Callebaut I, Mornon JP, Zeold A, da Silva WS, et al. Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol. 2007;27:4774–83. doi: 10.1128/MCB.00283-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curcio-Morelli C, Zavacki AM, Christofollete M, Gereben B, de Freitas BC, Harney JW, et al. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest. 2003;112:189–96. doi: 10.1172/JCI18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gereben B, Goncalves C, Harney JW, Larsen PR, Bianco AC. Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol Endocrinol. 2000;14:1697–1708. doi: 10.1210/mend.14.11.0558. [DOI] [PubMed] [Google Scholar]
- 17.Botero D, Gereben B, Goncalves C, de Jesus LA, Harney JW, Bianco AC. Ubc6p and Ubc7p are required for normal and substrate-induced endoplasmic reticulum-associated degradation of the human selenoprotein type 2 iodothyronine monodeiodinase. Mol Endocrinol. 2002;16:1999–2007. doi: 10.1210/me.2002-0135. [DOI] [PubMed] [Google Scholar]
- 18.Kim BW, Zavacki AM, Curcio-Morelli C, Dentice M, Harney JW, Larsen PR, et al. Endoplasmic reticulum-associated degradation of the human type 2 iodothyronine deiodinase (D2) is mediated via an association between mammalian UBC7 and the carboxyl region of D2. Mol Endocrinol. 2003;17:2603–12. doi: 10.1210/me.2003-0082. [DOI] [PubMed] [Google Scholar]
- 19.Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005;7:698–705. doi: 10.1038/ncb1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavacki AM, Arrojo e Drigo R, Freitas BC, Chung M, Harney JW, Egri P, et al. The E3 ubiquitin ligase TEB4 mediates degradation of type 2 iodothyronine deiodinase. Mol Cell Biol. 2009;29:5339–47. doi: 10.1128/MCB.01498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069–78. doi: 10.1089/105072503770867237. [DOI] [PubMed] [Google Scholar]
- 22.Castillo M, Hall JA, Correa-Medina M, Ueta C, Won Kang H, Cohen DE, et al. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60:1082–9. doi: 10.2337/db10-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–85. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianco AC, Carvalho SD, Carvalho CR, Rabelo R, Moriscot AS. Thyroxine 5'-deiodination mediates norepinephrine-induced lipogenesis in dispersed brown adipocytes. Endocrinology. 1998;139:571–8. doi: 10.1210/endo.139.2.5737. [DOI] [PubMed] [Google Scholar]
- 25.Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest. 1987;79:295–300. doi: 10.1172/JCI112798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freitas BC, Gereben B, Castillo M, Kallo I, Zeold A, Egri P, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest. 2010;120:2206–17. doi: 10.1172/JCI41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest. 2008;118:975–83. doi: 10.1172/JCI32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab. 2008;4:148–55. doi: 10.1038/ncpendmet0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–9. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 31.da-Silva WS, Harney JW, Kim BW, Li J, Bianco SD, Crescenzi A, et al. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes. 2007;56:767–76. doi: 10.2337/db06-1488. [DOI] [PubMed] [Google Scholar]
- 32.da-Silva WS, Ribich S, Arrojo e Drigo R, Castillo M, Patti ME, Bianco AC. The chemical chaperones tauroursodeoxycholic and 4-phenylbutyric acid accelerate thyroid hormone activation and energy expenditure. FEBS Lett. 2011;585:539–44. doi: 10.1016/j.febslet.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grozovsky R, Ribich S, Rosene ML, Mulcahey MA, Huang SA, Patti ME, et al. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-gamma agonists in skeletal myocytes. Endocrinology. 2009;150:1976–83. doi: 10.1210/en.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arrojo E Drigo R, Fonseca TL, Castillo M, Salathe M, Simovic G, Mohácsik P, Gereben B, Bianco AC. Endoplasmic reticulum stress decreases intracellular thyroid hormone activation via an eIF2a-mediated decrease in type 2 deiodinase synthesis. Mol Endocrinol. 2011;25:2065–75. doi: 10.1210/me.2011-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christoffolete MA, Doleschall M, Egri P, Liposits Z, Zavacki AM, Bianco AC, et al. Regulation of thyroid hormone activation via the liver X-receptor/retinoid X-receptor pathway. J Endocrinol. 2010;205:179–86. doi: 10.1677/JOE-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall JA, Ribich S, Cristoffolete MA, Simovic G, Correa M, Patti ME, et al. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology. 2010;151:4573–82. doi: 10.1210/en.2010-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik JH, et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest. 2010;120:4021–30. doi: 10.1172/JCI43670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medina MC, Molina J, Gadea Y, Fachado A, Murillo M, Simovic G, et al. The thyroid hormone-inactivating type III deiodinase is expressed in mouse and human beta-cells and its targeted inactivation impairs insulin secretion. Endocrinology. 2011;152:3717–27. doi: 10.1210/en.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]