Abstract
Tissue injury may result as a consequence of a physical, chemical, or biological insult. Such injury recruits an adaptive response to restore homeostasis and protect against further injury. One of the most prompt protective and adaptive responses by all tissues is the robust activation of the highly inducible, anti-inflammatory, anti-oxidant, and anti-apoptotic protein, heme oxygenase-1 (HO-1). HO-1, a microsomal enzyme, catalyzes the breakdown of pro-oxidant heme, which is released from heme proteins to equimolar quantities of iron, carbon monoxide, and biliverdin. Biliverdin is converted to bilirubin by biliverdin reductase. The beneficial effects of HO-1 expression are not merely due to heme degradation but are also attributed to the cytoprotective properties of the byproducts of the reaction. Manipulation of this enzymatic system in a myriad of disease models has provided substantial evidence to support its role as a cytoprotective enzyme and is therefore an emerging therapeutic molecule.
INTRODUCTION
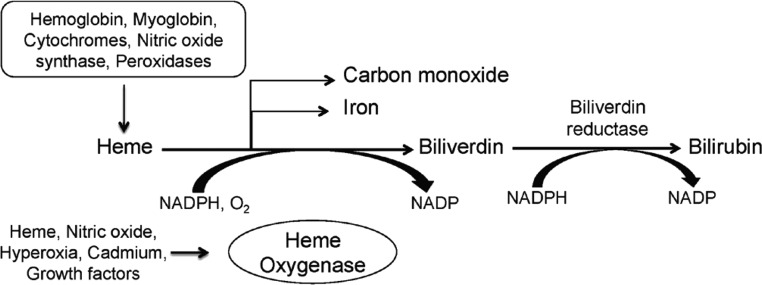
Heme oxygenase (HO) is the rate-limiting enzyme in the degradation of heme, which catalyzes the oxidative degradation of heme to equimolar quantities of carbon monoxide (CO), iron, and biliverdin (Figure 1). Biliverdin is consequently reduced to bilirubin by biliverdin reductase (1). Heme is derived from a number of heme proteins including hemoglobin, myoglobin, cytochromes, and enzymes such as nitric oxide synthase, myeloperoxidase, catalase and others that are present ubiquitously in all cells. Heme oxygenase primarily exists as two isoforms. HO-1, the inducible isoform is encoded on chromosome 22 (22q12) in the human genome and translates to a 32-KDa protein. On the other hand, HO-2 is the constitutive isoform encoded on chromosome 16 (16q12) and translates to a 36-kD protein. Although HO-1 and HO-2 share 40% homology, catalyze the same reaction, and have similar cofactor requirements (NADPH, O2), they substantially differ with respect to regulation and expression pattern. HO-2 is constitutively expressed in the testes, brain, and endothelium; however, HO-1 is expressed in all cells at low levels but is highly inducible by a wide variety of stimuli, including its own substrate heme (2). HO-2 is thought to regulate normal physiological functions, whereas HO-1 plays a protective role in modulating tissue responses to injury in several diseases including acute kidney injury, atherosclerosis, sepsis, organ transplant rejection, and others (Table 1).
Fig. 1.
Enzymatic reaction catalyzed by heme oxygenase-1.
TABLE 1.
Protective Role of HO-1 Expression in Diseases
| Organ | Disease | Reference |
|---|---|---|
| Brain | Cerebrovascular accident | (36, 37) |
| Eye | Corneal inflammation | (38) |
| Uveitis | (39) | |
| Ocular hypertension | (40) | |
| Ear | Noise-induced hearing loss | (41) |
| Drug-induced ototoxicity | (42) | |
| Lung | Hyperoxic injury | (43) |
| Pulmonary hypertension | (44) | |
| Pulmonary Fibrosis | (45) | |
| Heart | Cardiac transplant rejection | (46) |
| Ischemic heart disease | (47) | |
| Gastrointestinal | Inflammatory bowel disease | (48) |
| Liver | Ischemia/reperfusion injury | (49) |
| Drug-induced hepatotoxicity | (50) | |
| Transplantation | (51) | |
| Vasculature | Transplant arteriosclerosis | (52) |
| Atherosclerosis | (53) | |
| Hypertension | (54) | |
| Bone marrow | Transplantation | (55) |
| Kidney | Acute kidney injury | |
| Rhabdomyolysis | (4) | |
| Ischemia/reperfusion | (56, 57) | |
| Cisplatin nephrotoxicity | (58, 59) | |
| Contrast induced toxicity | (60) | |
| Mercuric chloride induced toxicity | (61) | |
| Glomerulonephritis | (62) | |
| Nephrotoxic nephritis | (63) | |
| Chronic renal allograft rejection | (64) | |
| Polycystic kidney disease | (65, 66) |
HO-1 Deficiency in Humans and Mice
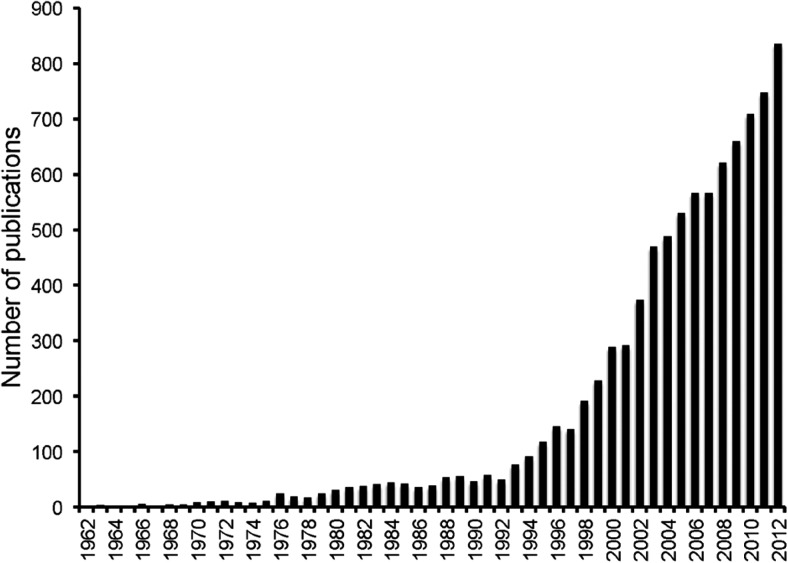
Heme oxygenase was first described by Tenhunen et al. in 1968 as an important regulator of heme turnover (3). They showed that this enzyme was capable of oxidative degradation of heme to bilirubin. After this observation, several researchers aimed at identifying the regulation and expression of this enzyme in different tissues. Seminal work by Nath et al. in 1992 provided the first demonstration that HO-1 was rapidly induced in the kidney in vivo during rhabdomyolysis and that this induction served as an important protective response against the inflicted heme-mediated injury (4). This study sparked an enormous interest in HO research that is reflected by an exponential increase in the number of articles published concerning this enzyme (Figure 2).
Fig. 2.
Number of publications from PubMed using the search term “heme oxygenase.”
Another breakthrough in the field was the generation of HO-1 knockout (HO-1-/-) mice by Poss and Tonegawa in 1997. These mice exhibited a decreased birth rate, growth retardation, microcytic hypochromic anemia, hematuria, proteinuria, kidney and liver iron deposition, along with a chronic inflammatory phenotype consisting of hepatosplenomegaly, lymphadenopathy, hepatic periportal inflammation, leukocytosis, and glomerulonephritis (5). The absence of HO-1 in a patient with phenotypic features similar to the HO-1–deficient mouse was described in 1999 by Yachie et al. (6). This patient, a 6-year-old boy, exhibited hematuria, proteinuria, hyperlipidemia, and hypobilirubinemia, and displayed progressive tubulointerstitial injury, iron deposition, inflammatory cell infiltrate, and vasculopathy in the kidney. The patient died at age 6 and was found to have high levels of oxidatively modified LDL in plasma and extensive fatty streaks and fibrous plaques in the aorta. A second patient with HO-1 deficiency was recently reported with a similar phenotype (7). The indisputable similarity between the mouse model and human HO-1 deficiency propelled research in this field to enable a comprehensive evaluation of the role of HO-1 in physiological and pathophysiological states.
Humanized HO-1 Transgenic Mice
Previous studies have shown major differences between the molecular regulation of the human HO-1 gene compared to the rodent HO-1 genes, although the function of the protein and enzymatic reaction is the same in both humans and rodents (8). To enable investigation of the human HO-1 gene in vivo, Kim et al. developed “humanized” HO-1 transgenic mice using an 87-kb bacterial artificial chromosome containing the entire HO-1 gene bred with HO-1 knockout mice (9). These humanized mice were able to rescue the phenotype of the HO-1–deficient mice described above and showed inducibility via transcriptional activation after injury. These mice offer a new tool to facilitate further translational studies to explore the therapeutic application of HO-1 in human diseases.
Modes of Protection
Although compelling evidence supports a role for HO-1 in cellular protection and survival, the mechanism(s) underlying such protection is attributable to multiple reasons. First, HO-1–mediated protection clearly relates to degradation of its substrate, heme, that has pro-oxidant effects (10, 11). Second, the by-products of the HO reaction also contribute to the protective response. CO, previously regarded as a toxic air pollutant and referred to as a “silent killer,” exerts cytoprotective effects through its potent anti-inflammatory, anti-apoptotic, and vasodilatory properties (12). CO also inhibits platelet aggregation and has bactericidal effects (13). Similarly, the bile pigments released from the HO reaction, biliverdin and bilirubin, are potent peroxy radical scavengers and can inhibit complement activation (14, 15). On the other hand, iron that is released from the reaction is capable of amplifying oxidative stress through reactive oxygen species generation. However, iron is safely sequestered by ferritin, an intracellular iron repository protein that is co-induced with HO-1 (16). Therefore, HO-1 not only removes toxic oxidant moities, but also protects the cells by providing anti-oxidant and anti-inflammatory molecules (17). Finally, the activation of HO-1 is also associated with upregulation of the cell cycle regulatory protein, p21, the latter mediating some of the cellular protective effects of HO-1 (18).
Clinical Implications
Given the significance of HO-1 expression during injury, research in the past decade has focused on targeting this enzymatic system as a therapeutic stratagem against diseases (reviewed in (19–24)). One approach that has gained considerable interest is the utilization of mesenchymal stem cells (MSC) to treat clinical conditions. These cells are multi-potent stem cells that have anti-inflammatory, angiogenic, and immunomodulatory properties that enable rapid repair and regeneration of damaged tissue (25). In fact, there are multiple ongoing clinical trials pertaining to various organ systems including the heart and kidney designed to evaluate the efficacy of MSC treatment during injury.
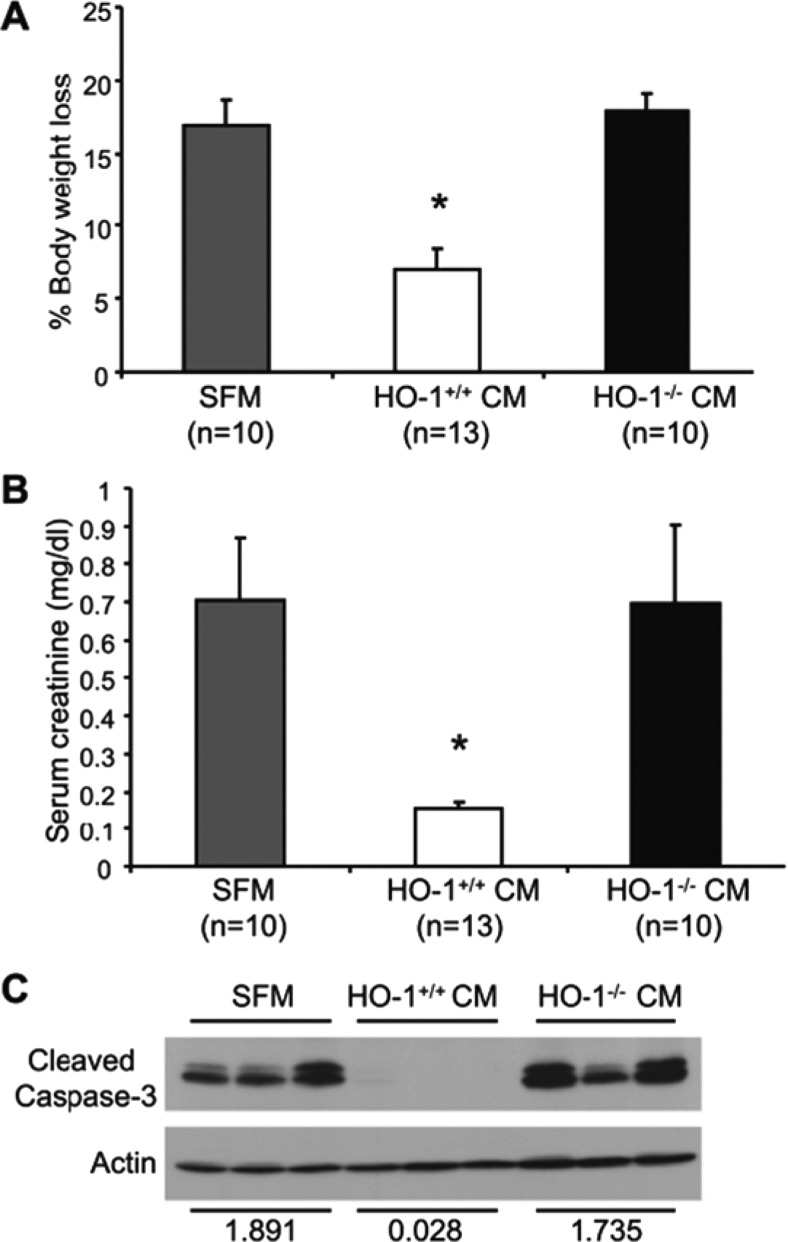
We have previously shown that MSCs lacking HO-1 expression have impaired paracrine effects and therapeutic potential (26). HO-1–deficient MSCs have reduced expression and secretion of important pro-angiogenic and growth factors such as stromal cell-derived factor-1, vascular endothelial growth factor–A, and hepatocyte growth factor. Furthermore, we showed that although conditioned media from HO-1+/+ MSCs provided significant structural and functional protection against cisplatin-induced acute kidney injury, conditioned medium from HO-1–deficient MSCs was not effective (Figure 3). These results indicate the important role of HO-1 in MSC-mediated protection and highlight the importance of screening MSC donors for HO-1 expression to increase their efficacy and therapeutic potential. This is particularly relevant because human HO-1 gene expression is regulated by the number of GT repeats in the proximal HO-1 promoter. Length polymorphisms in this GT repeat region correlate with levels of HO-1 expression and associates with several diseases (27). For example, shorter GT repeats (<25) account for higher HO-1 expression and are associated with better outcomes in clinical settings such as emphysema, vascular restenosis in coronary arteries, and hemodialysis-related arteriovenous fistulae, and in the setting of renal transplantation. In contrast, long GT repeats (>25) are associated with lower levels of HO-1 expression and worse outcomes in these disorders.
Fig. 3.
Conditioned media (CM) from HO-1+/+ mesenchymal stem cells (MSC) protects against cisplatin nephrotoxicity. (A) The amount of body weight loss following cisplatin administration was calculated and expressed as a percentage of the original weight. (B) Serum creatinine levels were measured and expressed as mg/dl. (C) Western blot analysis of cleaved caspase 3 expression in kidneys. Densitometric analysis of the bands on the caspase-3 and actin blots is indicated. Data are expressed as means ± SE and *P < 0.05. Reproduced with permission from Zarjou et al. (26).
Although these studies mainly focused on the induction of HO-1, recent work has directed attention to CO and CO-releasing molecules as a protective strategy against injury. Neto et al. showed in a model of transplant-induced renal ischemia reperfusion injury that low-dose CO inhalation led to increased protection and graft survival (28). Currently, there are several ongoing clinical trials that are aimed at CO-based therapies of clinical conditions such as pulmonary hypertension and fibrosis and renal transplantation.
Another area with important clinical implications stems from the observations that several protective agents that exert pleotropic effects such as neutrophil gelatinase-associated lipocalin (NGAL), statins, erythropoietin, α-melanocyte–stimulating hormone, and interleukin-10 — all require HO-1, at least in part, for their beneficial properties (29–34). For example, the protective effects of exogenous NGAL are lost in acute kidney injury when HO activity is inhibited (29). IL-10 is protective in sepsis and prevents transplant vascular rejection; however, these effects are lost when HO activity is blocked (35).
In summary, the biological implications of HO-1 expression are beyond just heme degradation and encompass regulation of cellular function through modulation of a myriad of pathways. Importantly, HO-1 expression is protective in tissue injury and is a common downstream mediator and therapeutic target for drugs and other small molecules. Furthermore, these protective effects are mediated via one or more of the byproducts of the HO reaction. Therefore, modulation of the HO-1 enzyme can determine the fate of cells during injury and targeting the heme oxygenase system is an emerging therapeutic intervention for diseases.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Sacher, Cincinnati: Thank you very much, very nice talk. I am intrigued about the Gilbert's relationship because, in fact, I think it's well-known that in Gilbert's syndrome individuals who, in fact, are starved, indirect bilirubin goes up and this is a protective functional response to stress.
Agarwal, Birmingham: I don't know if anyone has looked into that in particular but there have been large population studies since the initial work from the Czech Republic by Vitek and colleagues that unconjugated levels of bilirubin are protective, but when they've looked at obstructive jaundice, for example, it's mainly the conjugated fraction. That, in fact, is more toxic. For example, kids with neonatal hyperbilirubinemia from prematurity can get kernicterus. I don't think anybody has specifically studied the starvation response in terms of the higher level of bilirubin, but it is certainly worth exploring.
Quesenberry, Providence: Great talk, very exciting. There is some work we are collaborating with Giovanni Camussi in Torino who has looked at the mesenchymal stem cell healing of kidney injury from cisplatinum, glycerol, and ischemia reperfusion and his data offer an alternative potential and that's the transfer, probably, of microRNA in vesicles into damaged cells. Your comments?
Agarwal, Birmingham: That's a great question. There is elegant work from Camussi and others that, including endothelial progenitor cells or mesenchymal stem cells, when injected actually release microvesicles. These microvesicles are loaded with multiple factors including microRNA, DNA, and other particles that may be really getting transferred to the injured cell enhancing repair. We have preliminary data suggesting a defect in the HO-1–deficient mesenchymal stem cells in terms of secreting an adequate number of these microvesicles.
Quesenberry, Providence: I am just going to say that you are offering an alternative to his observations and actually since there's a common fund grant application coming out, that could be very helpful.
Agarwal, Birmingham: Thank you.
Lippman, Miami: I really enjoyed this talk though I'm not sure I completely follow. Perhaps you could say a bit more of the exact mechanism, what's known about how carbon monoxide actually has these beneficial effects, and whether or not in the knockout mouse you can ameliorate the effects by providing low doses of carbon monoxide.
Agarwal, Birmingham: We have not really done any of the CO experiments ourselves, but extensive work has been done by Augustine Choi, Leo Otterbein, Fritz Bach, and others where they showed that concentrations in the order of 250 parts per million (ppm) of CO afforded protection. A lot of skepticism was involved because for every molecule of heme that's degraded, equimolar amounts of CO are generated and you can rarely achieve 250 ppm concentrations of CO from endogenous breakdown of heme per se. Initially it was thought that CO was activating p38 MAP kinase and hence eliciting the protective effect, but now there is very nice data from several people where actually CO causes a preconditioning effect. It essentially poisons the mitochondrial cytochrome c-oxidase and stuns the cell and when the cell gets challenged with another insult it is ready to defend itself and this is, you know, a preconditioning phenomenon that has been well-known for over decades in different organ systems. It is believed that the heat shock proteins such as Hsp27 and Hsp90 are also involved with such a protective mechanism.
Lippman, Miami: So, would hypoxia be equivalent in that sort of suggestion to carbon monoxide?
Agarwal, Birmingham: Yes, so essentially CO is causing hypoxia to the cell. It's a hypoxic preconditioning and we know that CO binds to hemoglobin very avidly and, you know, that's how patients essentially die from CO toxicity. These results are just recently emerging that there are other alternate mechanisms.
Hochberg, Baltimore: I may have missed it if you mentioned it, but did you mention any agents which are able to induce the heme oxygenase enzyme?
Agarwal, Birmingham: There are multiple inducers of the heme oxygenase pathyway. In fact, we've all heard about a drug that was just withdrawn yesterday, bardoxolone methyl, which is a triterpenoid, very active inducer of heme oxygenase. Actually a proposed mechanism of action was through this pathway because it activates the transcription factor Nrf2. There are several other transcription factors involved. There are also drugs like statins, erythropoietin, IL-10, alpha-MSH, a lot of these agents have pleotropic effects, which are believed to work through the HO-1 pathway. For example, in HO-1–deficient animals or when HO activity is pharmacologically inhibited, these agents lose their protective effect. So, it could be a downstream mediator and several molecules that activate this enzyme system have been described.
REFERENCES
- 1.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 2.Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol. 2002;21:307–21. doi: 10.1089/104454902753759726. [DOI] [PubMed] [Google Scholar]
- 3.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–55. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath KA, Balla G, Vercellotti GM, Balla J, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–70. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919–24. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yachie A, Niida Y, Wada T, Igarashi N, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–35. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, et al. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol. 2011;33:74–8. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 8.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol. 2004;286:F425–41. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Zarjou A, Traylor AM, Bolisetty S, et al. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int. 2012;82:278–291. doi: 10.1038/ki.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–20. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 11.Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, et al. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64:648–55. [PubMed] [Google Scholar]
- 12.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–49. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 13.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–43. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 14.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 15.Nakagami T, Toyomura K, Kinoshita T, Morisawa S. A beneficial role of bile pigments as an endogenous tissue protector: anti-complement effects of biliverdin and conjugated bilirubin. Biochim Biophys Acta. 1993;1158:189–93. doi: 10.1016/0304-4165(93)90013-x. [DOI] [PubMed] [Google Scholar]
- 16.Balla G, Jacob HS, Balla J, Rosenberg M, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–53. [PubMed] [Google Scholar]
- 17.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–43. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 18.Inguaggiato P, Gonzalez-Michaca L, Croatt AJ, Haggard JJ, et al. Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int. 2001;60:2181–91. doi: 10.1046/j.1523-1755.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 19.Durante W. Targeting heme oxygenase-1 in vascular disease. Curr Drug Targets. 2010;11:1504–16. doi: 10.2174/1389450111009011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jazwa A, Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr Drug Targets. 2010;11:1517–31. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 21.Kim YM, Pae HO, Park JE, Lee YC, et al. Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;14:137–67. doi: 10.1089/ars.2010.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol. 2009;41:251–60. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham NG, Cao J, Sacerdoti D, Li X, et al. Heme oxygenase: the key to renal function regulation. Am J Physiol Renal Physiol. 2009;297:F1137–52. doi: 10.1152/ajprenal.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep. 2009;11:56–62. doi: 10.1007/s11906-009-0011-z. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–25. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 26.Zarjou A, Kim J, Traylor AM, Sanders PW, et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol. 2011;300:F254–62. doi: 10.1152/ajprenal.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, et al. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287:F979–89. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 29.Mori K, Lee HT, Rapoport D, Drexler IR, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 2005;19:1216–9. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- 31.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–54. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 32.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 33.Lam CW, Getting SJ, Perretti M. In vitro and in vivo induction of heme oxygenase 1 in mouse macrophages following melanocortin receptor activation. J Immunol. 2005;174:2297–304. doi: 10.4049/jimmunol.174.4.2297. [DOI] [PubMed] [Google Scholar]
- 34.Calo LA, Davis PA, Piccoli A, Pessina AC. A role for heme oxygenase-1 in the antioxidant and antiapoptotic effects of erythropoietin: the start of a good news/bad news story? Nephron Physiol. 2006;103:107–11. doi: 10.1159/000092213. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, et al. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci U S A. 2005;102:7251–6. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takizawa S, Hirabayashi H, Matsushima K, Tokuoka K, et al. Induction of heme oxygenase protein protects neurons in cortex and striatum, but not in hippocampus, against transient forebrain ischemia. J Cereb Blood Flow Metab. 1998;18:559–69. doi: 10.1097/00004647-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H, Kanamaru K, Tsunoda H, Inada H, et al. Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J Clin Invest. 1999;104:59–66. doi: 10.1172/JCI5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laniado-Schwartzman M, Abraham NG, Conners M, Dunn MW, et al. Heme oxygenase induction with attenuation of experimentally induced corneal inflammation. Biochem Pharmacol. 1997;53:1069–75. doi: 10.1016/s0006-2952(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 39.Ohta K, Kikuchi T, Arai S, Yoshida N, et al. Protective role of heme oxygenase-1 against endotoxin-induced uveitis in rats. Exp Eye Res. 2003;77:665–73. doi: 10.1016/j.exer.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Privitera MG, Potenza M, Bucolo C, Leggio GM, et al. Hemin, an inducer of heme oxygenase-1, lowers intraocular pressure in rabbits. J Ocul Pharmacol Ther. 2007;23:232–9. doi: 10.1089/jop.2006.101. [DOI] [PubMed] [Google Scholar]
- 41.Matsunobu T, Satoh Y, Ogawa K, Shiotani A. Heme oxygenase-1 expression in the guinea pig cochlea induced by intense noise stimulation. Acta Otolaryngol Suppl. 2009;562:18–23. doi: 10.1080/00016480902933056. [DOI] [PubMed] [Google Scholar]
- 42.Francis SP, Kramarenko II, Brandon CS, Lee FS, et al. Celastrol inhibits aminoglycoside-induced ototoxicity via heat shock protein 32. Cell Death Dis. 2011;2:e195. doi: 10.1038/cddis.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–94. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 44.Christou H, Morita T, Hsieh CM, Koike H, et al. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–29. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- 45.Tsuburai T, Suzuki M, Nagashima Y, Suzuki S, et al. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lung prevents bleomycin-induced pulmonary fibrosis via a Fas-Fas ligand-independent pathway. Hum Gene Ther. 2002;13:1945–60. doi: 10.1089/10430340260355356. [DOI] [PubMed] [Google Scholar]
- 46.Soares MP, Lin Y, Anrather J, Csizmadia E, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–7. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 47.Csonka C, Varga E, Kovacs P, Ferdinandy P, et al. Heme oxygenase and cardiac function in ischemic/reperfused rat hearts. Free Radic Biol Med. 1999;27:119–126. doi: 10.1016/s0891-5849(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 48.Wang WP, Guo X, Koo MW, Wong BC, et al. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G586–94. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- 49.Amersi F, Buelow R, Kato H, Ke B, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–9. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu H, Brittingham JA, Laskin DL. Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol Appl Pharmacol. 2002;181:106–15. doi: 10.1006/taap.2002.9409. [DOI] [PubMed] [Google Scholar]
- 51.Ke B, Buelow R, Shen XD, Melinek J, et al. Heme oxygenase 1 gene transfer prevents CD95/Fas ligand-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Hum Gene Ther. 2002;13:1189–99. doi: 10.1089/104303402320138970. [DOI] [PubMed] [Google Scholar]
- 52.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–6. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa K, Sugawara D, Wang X, Suzuki K, et al. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–12. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 54.Motterlini R, Gonzales A, Foresti R, Clark JE, et al. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–77. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 55.Gerbitz A, Ewing P, Wilke A, Schubert T, et al. Induction of heme oxygenase-1 before conditioning results in improved survival and reduced graft-versus-host disease after experimental allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2004;10:461–72. doi: 10.1016/j.bbmt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Maines MD, Raju VS, Panahian N. Spin trap (N-t-butyl-alpha-phenylnitrone)-mediated suprainduction of heme oxygenase-1 in kidney ischemia/reperfusion model: role of the oxygenase in protection against oxidative injury. J Pharmacol Exp Ther. 1999;291:911–9. [PubMed] [Google Scholar]
- 57.Shimizu H, Takahashi T, Suzuki T, Yamasaki A, et al. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28:809–17. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- 58.Agarwal A, Balla J, Alam J, Croatt AJ, et al. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48:1298–307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- 59.Shiraishi F, Curtis LM, Truong L, Poss K, et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;278:F726–36. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- 60.Goodman AI, Olszanecki R, Yang LM, Quan S, et al. Heme oxygenase-1 protects against radiocontrast-induced acute kidney injury by regulating anti-apoptotic proteins. Kidney Int. 2007;72:945–953. doi: 10.1038/sj.ki.5002447. [DOI] [PubMed] [Google Scholar]
- 61.Yoneya R, Ozasa H, Nagashima Y, Koike Y, et al. Hemin pretreatment ameliorates aspects of the nephropathy induced by mercuric chloride in the rat. Toxicol Lett. 2000;116:223–9. doi: 10.1016/s0378-4274(00)00222-8. [DOI] [PubMed] [Google Scholar]
- 62.Datta PK, Koukouritaki SB, Hopp KA, Lianos EA. Heme oxygenase-1 induction attenuates inducible nitric oxide synthase expression and proteinuria in glomerulonephritis. J Am Soc Nephrol. 1999;10:2540–50. doi: 10.1681/ASN.V10122540. [DOI] [PubMed] [Google Scholar]
- 63.Mosley K, Wembridge DE, Cattell V, Cook HT. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int. 1998;53:672–8. doi: 10.1046/j.1523-1755.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 64.Bedard EL, Jiang J, Parry N, Wang H, et al. Peritransplant treatment with cobalt protoporphyrin attenuates chronic renal allograft rejection. Transpl Int. 2005;18:341–9. doi: 10.1111/j.1432-2277.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 65.Maser RL, Vassmer D, Magenheimer BS, Calvet JP. Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J Am Soc Nephrol. 2002;13:991–9. doi: 10.1681/ASN.V134991. [DOI] [PubMed] [Google Scholar]
- 66.Zhou J, Ouyang X, Schoeb TR, Bolisetty S, et al. Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J Am Soc Nephrol. 2012;23:1161–71. doi: 10.1681/ASN.2011050442. [DOI] [PMC free article] [PubMed] [Google Scholar]