Abstract
The treatment of glycosphingolipid storage diseases by synthesis inhibition was first proposed 40 years ago as an alternative approach to enzyme replacement therapy. We have pursued this strategy through the rational design of potent and selective inhibitors of glucosylceramide synthase, the first step in glycosphingolipid synthesis. Eliglustat tartrate was the result of these efforts and is currently the focus of phase 3 trials for type 1 Gaucher disease. Phase 2 studies showed a reduction in splenomegaly and hepatomegaly and improvements of anemia and thrombocytopenia at levels equivalent to or exceeding the historic response to imiglucerase. Structural analogues of eliglustat have also been designed that lack pgp-1 recognition and cross the blood brain barrier. These may have utility for central nervous system– based sphingolipidoses. Because glycosphingolipids are important regulators of receptor tyrosine kinases, glucosylceramide synthase inhibitors may also be beneficial for disorders such as type 2 diabetes mellitus and polycystic kidney disease.
INTRODUCTION
“Necessity is the mother of invention” is an adage that is often invoked to explain scientific and technological progress in many fields of endeavor, including medicine. The origin of this aphorism is appears to be the moral of Aesop's fable of The Crow and the Pitcher. In this story, a thirsty crow cannot reach the water at the bottom of the vessel and with great ingenuity adds pebbles to the pitcher to raise the water level. The history of medicine is replete with many examples in which clinical science has discovered solutions to seemingly intractable problems.
It took more than 2500 years for the relationship between necessity and invention to be inverted by the social critic and economist Thorstein Veblen. In his book The Theory of the Leisure Class, Veblen opined that invention is just as often the mother of necessity (1). Inventions that are created for one purpose are rapidly adapted for other uses, often leading to great financial gain. The phonograph and internet are among the most commonly cited examples of this phenomenon. Indeed, modern medicine is characterized by the adoption of new medicines and technologies justified by the improvement of medical care and the quality of life for patients.
With the development and approval of each new therapeutic, investigators ask whether such drugs may have a place beyond their initially intended and approved use. In particular, drugs developed for orphan diseases targeting very small numbers of affected individuals may find applications for more common disorders. Imatinib, erythropoietin, and botulinum toxin developed for chronic myelogenous leukemia, anemia in renal failure, and hemifacial spasm, respectively, are noteworthy examples of new therapeutics resulting in previously unappreciated clinical necessities. In this review I discuss a novel, first-in-class small molecule, a glycolipid synthesis inhibitor, developed out of work emanating from our laboratory for the treatment of type I Gaucher disease, and the possibility that it might find use in the treatment of more common clinical disorders.
STRATEGIES FOR THE TREATMENT OF LYSOSOMAL STORAGE DISEASES
Of the 6000 to 7000 rare diseases, approximately 50 are lysosomal storage diseases (2). Lysosomal storage diseases commonly arise as the result of monogenic disorders leading to an absent or defective gene product in the form of a lysosomal hydrolase or membrane transporter. Gaucher disease is the most common and intensively studied lysosomal storage disease. In this autosomal recessive disorder, a deficiency or absence of the enzyme β-glucocerebrosidase results in the lysosomal accumulation of the simplest glycosphingolipid, glucosylceramide. The clinical spectrum of Gaucher disease is extensive. At one end of the spectrum, patients are asymptomatic and may be incidentally diagnosed after evaluation for anemia or an enlarged spleen. At the other end of the clinical spectrum, affected individuals have significant central nervous system involvement and skin abnormalities (3).
There is a strong association between the severity of the clinical phenotype and the residual activity of the β-glucocerebrosidase. Type 1 Gaucher disease is the most common subgroup. Type 1 patients typically develop enlarged spleens and livers, significant anemia, and thrombocytopenia. Among the most debilitating complications of type 1 Gaucher are deforming bone disease and pulmonary involvement that may eventually result in pulmonary fibrosis. Several biomarkers have been identified, most notably elevated chitotriosidase levels. Gaucher type 3 disease is associated with the type 1 phenotype in addition to central nervous system involvement. These may include Parkinsonian symptoms, cognitive developmental delay, and eye movement disorders. Finally, type 2 disease is associated with neonatal onset with severe neurological involvement including seizure disorders and neurological degeneration. Additionally, dermatological manifestations in the form of ichthyosis or hydrops fetalis can be observed.
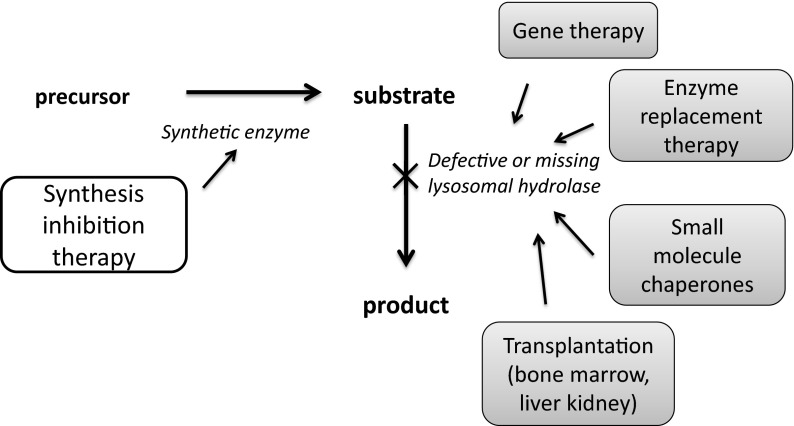
Historically, treatment strategies for Gaucher disease have focused on the restoration of β-glucocerebrosidase activity (Fig. 1) (4). In principle, this can be accomplished in several ways that include transplantation, gene therapy, and most recently the use of chemical chaperones. However, enzyme replacement therapy, most recently using recombinant β-glucocerebrosidase, has emerged as the standard of care for type 1 Gaucher disease (5). The success of this strategy is based on the ability of macrophages to bind, endocytose, and traffic to the lysosome the mannose-terminated enzyme. Enzyme replacement therapy, although effective in reducing spleen and liver size and in improving anemia and thrombocytopenia, has several limitations. These limitations include the need for intravenous administration, the development of immune responses limiting the efficacy of the enzyme, and high expense. The cost of enzyme replacement for Gaucher disease typically exceeds $200,000 annually for life. The most significant pharmacological limitation of enzyme replacement is the limited distribution of enzyme to compartments where it is required to reverse or prevent disease. The poor distribution of infused enzyme to bone limits its effectiveness in preventing osteonecrosis, osteopenia, and bone pain; the poor distribution to lung limits its utility for preventing pulmonary hypertension and fibrosis. Most importantly, the inability of the enzyme to cross the blood brain barrier has meant that this strategy is ineffective for the neurological complications of the type 2 and 3 forms of Gaucher disease.
Fig. 1.
Alternative strategies for the treatment of Gaucher disease and other glycosphingolipidoses such as Fabry disease. Most strategies have focused on the restoration of defective missing glycosidase. For type 1 Gaucher disease, enzyme replacement with imiglucerase is the current standard of care. Synthesis inhibition therapy targeting glucosylceramide synthase is an alternative approach.
An alternative strategy for treating sphingolipidoses was proposed in 1972 by Norman Radin at the University of Michigan (6). Radin speculated that targeting the anabolic pathways of sphingolipid metabolism would provide an additional and perhaps superior approach to these diseases. In glycosphingolipidoses such as type I Gaucher disease, some residual catabolic activity of the glycosidase is present. Thus, he reasoned that changing the equilibrium between synthesis and degradation might be sufficient to have a therapeutic benefit (Fig. 2).
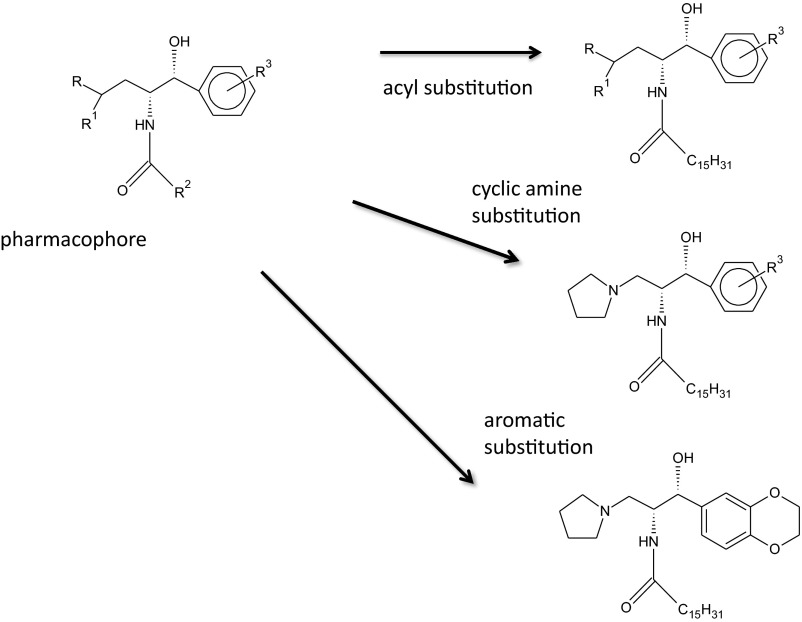
Fig. 2.
Summary of the medicinal chemistry strategies used in the design and identification of eliglustat. Preliminary work resulted in the identification of a pharmacophore, the key molecular structure required for glucosylceramide synthase inhibition. Systematic substitutions of the fatty acyl chain in amide linkage, the amine, and the aromatic group led to the identification of a lead compound with nanomolar activity in inhibiting the glycolipid synthase.
THE SEARCH FOR SMALL MOLECULE INHIBITORS OF GLUCOSYLCERAMIDE SYNTHASE
To test this hypothesis, we targeted the first step in glycolipid synthesis, the enzyme glucosylceramide synthase. This enzyme, localized to the cytosolic side of the Golgi membrane, catalyzes the formation of glucosylceramide from ceramide and UDP-glucose. Earlier work by Radin had identified a lead compound that inhibited glucosylceramide synthase at micromolar concentrations and with limited specificity toward the enzyme (7). This compound, PDMP, contains three primary functional groups including a cyclic amine, aromatic group, and fatty acid in amide linkage. A pharmacophore, a core chemical structure that minimally defines the activity of the inhibitor, was identified. We subsequently undertook the systematic substitution of these groups to identify potential glycolipid synthesis inhibitors with higher activity and greater specificity.
Empirical substitutions were first made for the amine (morpholino group) and fatty acyl group (8, 9). We determined that cyclic amines were required for activity and that a five-membered ring (pyrrolidine group) was optimal. Similarly, increasing the carbon chain length resulted in measurable improvements in the IC50 (the concentration at which the drug achieves 50% of enzyme inhibition) of the inhibitor. Subsequently, a form of rational drug design, Hansch analysis, was used. This strategy, long used by medicinal chemists, predicted that the substitution of the aromatic phenyl group with an aromatic 1, 2-ethylenedioxyphenyl group would improve activity. Indeed, a greater than 1000-fold increase in inhibitory activity against the glycolipid synthase was observed (Fig. 3) (10).
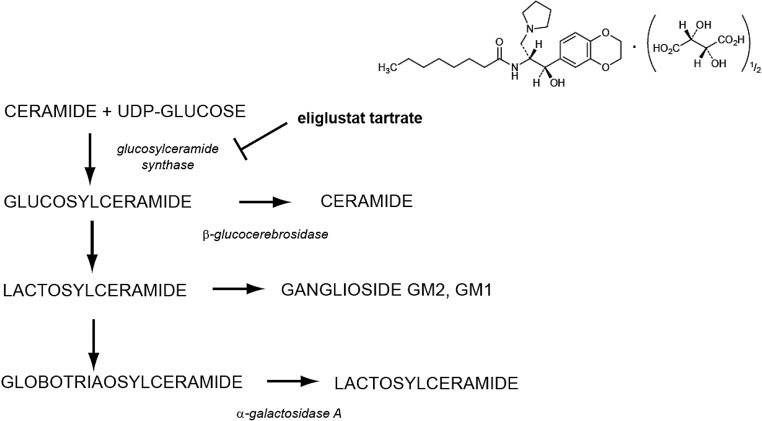
Fig. 3.
Structure and site of action of eliglustat tartrate. Eliglustat binds to glucosylceramide synthase inhibiting the formation of glucosylceramide, the glycolipid that accumulates in Gaucher disease due to low or absent activity of β-glucocerebrosidase. Eliglustat also inhibits the formation of globotriaosylceramide the glycolipid that accumulates in Fabry disease in addition to several gangliosides.
The identification of a potent glucosylceramide synthase inhibitor with an IC50 in the low nanomolar range rapidly led to proof of principle studies in models of glycosphingolipidoses. At this time, the most suitable mouse model of a glycolipid storage disorder without central nervous system involvement was the α-galactosidase knockout, a model of Fabry disease. Both in vitro and in vivo studies confirmed that the D-threo-1,2 ethylenedioxyphenyl-2-palmitoylamino-3-pyrrolidino analogue marked lowered glucosylceramide and globotriaosylceramide levels (11, 12). This was in marked contrast to another glucosylceramide synthase inhibitor, miglustat. This imino sugar showed no efficacy in decreasing globotriaosylceramide levels and only modestly inhibited glucosylceramide synthase at mid-micromolar concentrations. Subsequently, studies in more recently developed mouse models of type 1 Gaucher disease confirmed the potential utility of this molecule in blocking glucosylceramide accumulation and Gaucher cell formation (13).
Based on the promising results from the Fabry mouse studies, Genzyme Corporation licensed these newer PDMP based compounds for clinical development. A collaborative series of enabling studies were pursued between the Genzyme and Michigan groups. This work has been detailed in a recent review article (14). The decanoyl substituted compound was initially studied. Although many favorable properties were noted for this lead, it was observed that the pharmacokinetics of the longer fatty acyl chain substituted homologues were unfavorable. Specifically, their half-lives were markedly prolonged and the volumes of distribution of these drugs were very high. This undoubtedly was a reflection of the higher lipophilicity of these homologues. A simple decrease in the fatty acyl chain length to 8 carbons resulted in a drug that had a half-life of 5 hours with only a modestly higher IC50 against the glucosylceramide synthase. Additional work identified the tartrate salt as most favorable for bioavailability and stability. Another important finding was that eliglustat is a cytochrome P450 2D6 inhibitor. An investigational new drug application for this compound, now known as eliglustat tartrate, was filed and clinical trials were initiated.
CLINICAL TRIALS WITH ELIGLUSTAT TARTRATE
Eliglustat tartrate has been the subject of seven clinical trials (Table 1; clinicaltrials.gov website shows further details regarding the dates, inclusion, and exclusion criteria for these studies). Phase 1 trials assessed safety, tolerability, and pharmacokinetics in escalating single and multiple doses (15). Single doses of less than 20 mg/kg and multiple dosing at less than 200 mg twice daily were well tolerated. A dose of 50 mg twice daily resulted in therapeutic plasma concentrations. The terminal half-life was approximately 6 hours. Single doses at greater than10 mg/kg produced mild increases in the QT/QTc interval. Based on the assessment that eliglustat was safe, a phase 2 trial was initiated.
TABLE 1.
Registered Clinical Trials Using Eliglustat Tartrate
| Trial | Phase | Subject No. | Design | Status |
|---|---|---|---|---|
| NCT01452542 | 1 | 22 | Pharmacokinetics of eliglustat tartrate in healthy subjects | Completed |
| NCT01357811 | 1 | 28 | Effects of eliglustat on the pharmacokinetics of digoxin | Completed |
| NCT01659944 | 1 | 14 | Effects of eliglustat on the pharmacokinetics of metoprolol | Completed |
| NCT00358150 | 2 | 22 | Open label, type 1 Gaucher patients naïve to treatment | Completed fifth year of extension trial |
| ENGAGE NCT00891202 | 3 | 40 | Randomized control, double -blind, treatment -naïve type 1 Gaucher patients | Primary treatment period completed |
| ENCORE NCT00943111 | 3 | 160 | Randomized, type 1 patients stabilized on ERT, imiglucerase versus eliglustat | Primary treatment period completed |
| EDGE NCT01074944 | 3 | 171 | Single- versus twice-daily dosing of eliglustat | Ongoing |
The primary goals of this open-label, single-arm study were to evaluate the efficacy, safety, and pharmacokinetics in type I Gaucher patients. The entry criteria included patients aged 18 to 65 years with documented β-glucocerebrosidase deficiency and the absence of treatment with enzyme or miglustat for 12 months before treatment. The primary endpoints for efficacy were a reduction in spleen size, improvement in anemia, and thrombocytopenia. Secondary endpoints included a reduction in liver volume; improvement in plasma biomarkers including chitotriosidase, CCL18, ACE, and TRAP; reduction in plasma glucosylceramide and ganglioside GM3 levels; and improvement in skeletal changes. The results of the initial 12-month treatment period and 2-year extension trial have been published (16, 17). At 1 year, 77% of the subjects had achieved the primary endpoints by an intention-to-treat analysis and 91% of treatment completers (20 of 26 subjects) had done so. Four of 26 subjects withdrew early from the study for non-sustained ventricular tachycardia (thought to be unrelated to the drug) and pregnancy.
Recently, the results from the 4-year treatment of the extension trial of the phase 2 study were reported in an unpublished poster. Nineteen of the original 20 completers elected to remain on eliglustat tartrate. The mean hemoglobin increased by 2.3 ± 1.5 g/dL and platelet counts by 95%. The mean spleen and liver volumes decreased by 63% and 28%, respectively. Chitotriosidase and CCL18 levels both decreased by 82%. Importantly, significant improvements were observed in bone health including significant improvements in bone mineral density, reduction in dark marrow, and the absence of bone crises. No serious adverse effects have been observed during the last 2 years. Collectively, the improvements in spleen size, hemoglobin, and platelet counts exceed the 95% confidence limits observed historically in type I patients treated with imiglucerase. However, clinical data comparing directly imiglucerase infusions with eliglustat tartrate have not been reported. The overall responses of the phase 2 subjects to eliglustat tartrate at 12 and 24 months of treatment are shown in Table 2.
TABLE 2.
The Primary Endpoint Response to Eliglustat Tartrate in Type 1 Gaucher Patients Participating in the Phase 2 Trial
| 12 Months | 48 Months | |
|---|---|---|
| Hemoglobin | +1.62 g/dL | +2.3 g/dL |
| Platelet count | +40.3% | +95% |
| Spleen volume | −38.5% | −63% |
| Liver volume | −27.0% | −28% |
Nineteen patients completed 4 years of treatment with eliglustat. The overall change in hemoglobin was from 11.3 ± 1.5 to 13.6 ± 1.2 g/dL and in platelet count from 68,700 ± 21,200 to 125,400 ± 51,100/mm3. Spleen and liver volumes were measured as multiples of normal (MN). The spleen volumes decreased from 17.3 ± 9.5 to 6.1 ± 3.4 MN and the liver volumes decreased from 1.7 ± 0.4 to 1.2 ± 0.3 MN.
At the time this review is being written, the primary treatment periods for the two pivotal phase 3 trials have been completed but the data have not yet been reported in a scientific forum. The ENGAGE trial is a randomized, double-blind, placebo-controlled trial designed to establish the efficacy and safety of eliglustat in type 1 Gaucher disease patients. The ENCORE study is a randomized, multicenter, open-label comparator trial designed to evaluate the safety and efficacy of eliglustat in patients already stabilized on imiglucerase. The results of these trials will be reported in 2013.
One can conclude from the reported data to date that eliglustat tartrate has promising efficacy and safety. The primary outcomes of organ size reduction and improvements in hematological parameters are comparable to or exceed those observed with imiglucerase. Additionally, the limited clinical data suggest encouraging efficacy with regard to stabilization and improvement in bone health, a complication that has been largely refractory to enzyme replacement therapy. Finally, the data on efficacy and safety of eliglustat tartrate would appear to be markedly superior to that associated with miglustat, the latter agent showing much less significant responses to clinical outcomes and a significantly less favorable profile of untoward effects. Independent of whether eliglustat tartrate itself will ultimately be approved for clinical use, it can now be argued that synthesis inhibition therapy is a clinically viable strategy for the treatment of type I Gaucher disease.
POTENTIAL EXTENDED USE APPLICATIONS OF GLUCOSYLCERAMIDE SYNTHASE INHIBITORS
Can synthesis inhibition be applied to other glycosphingolipid storage disorders? The most obvious additional clinical use for eliglustat would be for Fabry disease (Fig. 3). This disorder arises from an X-linked deficiency in the lysosomal enzyme α-galactosidase A. The enzyme degrades terminal galactosyl residues on glycolipids, most notably globotriaosylceramide (Gb3). The significant clinical phenotype of Fabry disease reflects accumulation of Gb3 in the vasculature of affected patients and includes renal failure, strokes, and myocardial disease. Other important symptoms include pain, ocular keratopathy, and angiokeratomas (18).
As noted above, the original proof of principle studies for the ethylenedioxyphenyl-substituted analogues of PDMP were in a mouse model of Fabry disease (12). Recently, the potential efficacy of eliglustat tartrate in the Fabry mouse model was evaluated. In this study eliglustat was compared to recombinant α-galactosidase A in the reduction of Gb3 in visceral tissues (19). Synthesis inhibition with eliglustat was more effective than enzyme replacement in reducing renal Gb3 levels. The converse was observed in liver and cardiac Gb3 reductions. In combination, both therapies were superior to either treatment alone in Gb3 responses. The functional responses of polyuria and nociceptive latency also improved with all treatments. These data suggest that either alone or in combination with α-galactosidase A, eliglustat should be studied as a therapeutic option for Fabry disease.
One set of glycosphingolipidoses in which eliglustat clearly will not show efficacy is those associated with central nervous system involvement. These would include types 2 and 3 Gaucher disease, Tay-Sachs and Sandhoff diseases, and GM1 gangliosidosis. Each of these disorders is associated with the accumulation of either glucosylceramide or a glucosylceramide-based glycosphingolipid such as ganglioside GM2. Experimental studies in which knockout mice for the GM2 gangliosidosis Sandhoff disease were crossed with mice deficient in the synthetic enzyme N-acetylgalactosylaminyl transferase showed that both neurological symptoms and survival could be obviated and prolonged if synthesis was blocked (20).
Eliglustat, however, does not cross the blood brain barrier due to recognition by the multidrug transporter pgp-1. Thus, there is no significant distribution of the drug into the brain. Recently, we have addressed this problem by the property-based design of an eliglustat homologue that is not recognized by the multidrug resistance transport system (21). Preliminary data show that this compound, an indanyl substituted homologue of eliglustat, can reduce the GM2 levels in the Sandhoff mouse. Significant proof of principle on drug design work remains before one might consider the use of this or related compounds for any of these disorders, but the ability to design a compound that crosses the blood brain barrier and lowers glucosylceramide based lipids is a promising advance.
The potential use of eliglustat or of CNS permeant homologues for other glycosphingolipidoses would appear to be an obvious extension of the synthesis inhibition hypothesis. However, a large body of research has been conducted that would indicate a role for glycosphingolipids in fundamental physiological and pathophysiological responses. More specifically, as first discovered by Bremer and Hakomori, glycolipids have been strongly associated with the function of receptor tyrosine kinases and associated signaling pathways (22). Notable examples for which a role for glucosylceramide-based glycolipids has been shown are in the regulation of the insulin and EGF receptor–mediated pathways.
A potential role for glucosylceramide synthesis inhibition was raised in earlier work showing that hyperglycemia was associated with the de novo synthesis of glucosylceramide and ganglioside GM3 in streptozotocin-induced diabetes mellitus (23). In this study, the increased synthesis of glucosylceramide-based glycolipids was the consequence of higher UDP-glucose and reducing equivalents in the form of NADPH, an important substrate and cofactor for glycolipid formation, respectively. Subsequently, an in vitro model showed a potentially beneficial effect of glucosylceramide synthesis inhibition by PDMP in an in vitro model of diabetes (24). Further support for a direct role in blocking ganglioside GM3 synthesis was reported by the Proia group who observed that GM3 synthase knockout mice exhibited a marked increase in insulin sensitivity (25).
Two groups, using different classes of glucosylceramide synthase inhibitors, subsequently reported the reversal of the insulin resistant phenotype in mouse models of diabetes (26, 27). The metabolic syndrome phenotype appears to be sensitive to glycosphingolipid synthesis inhibition as well (28).
The pathophysiological basis of another clinical disorder, polycystic kidney disease, is based in part on dysregulation of EGF receptor signaling. An early article on the cpk mouse, a model of autosomal recessive polycystic kidney disease, reported increased glucosylceramide and ganglioside GM3 levels in the kidneys of these mice (29). The Genzyme group subsequently tested whether glucosylceramide synthase inhibition with a close analogue of eliglustat, Genz-123346, could prevent cyst development (30). Three mouse models of cystic kidney disease were studied. These included the Pkd1 conditional mouse, an orthologous model of autosomal dominant polycystic kidney disease, and jck and pcy mice, models of nephronopthesis. In all three models, regardless of the genetic basis of the cystogenesis, glucosylceramide synthesis inhibition prevented or mitigated cyst growth.
CONCLUSION
The discovery and clinical development of eliglustat tartrate as a first in class glucosylceramide synthase inhibitor is the result of a 40-year endeavor, beginning with a novel hypothesis formulated by Norman Radin. If approved, this drug will not only provide a new therapeutic option for type 1 Gaucher patients, but will also carry with it significant cost savings for the Gaucher community at large.
As investigators continue to explore the pathophysiology of glycosphingolipids in clinical disorders beyond classic lysosomal storage diseases, new possibilities for targeting synthetic enzymes in sphingolipid pathways should emerge. With a renewed focus on diabetes, cystic kidney disease, and many as yet unforeseen opportunities, the next 40 years should be comparably exciting.
ACKNOWLEDGMENTS
This work would not have been possible without the active collaboration and efforts of many investigators at the University of Michigan and Genzyme Corporation. I would especially acknowledge the efforts of Akira Abe, a longtime colleague, and Norm Radin, a great mentor. More recently, Scott Larsen and Richard Keep have been key collaborators on the development of CNS permeant compounds. Important colleagues at Genzyme include Craig Siegel, Diane Copeland, Carol Nelson, Seng Cheng, and Judy Peterschmitt, representing their respective groups.
Footnotes
Potential conflicts of interest: James A. Shayman is an inventor on patents covering the composition of matter, synthesis, and uses of eliglustat tartrate and related compounds. These patents are held by the University of Michigan were licensed to Genzyme/Sanofi Corporation. As an employee of the University of Michigan, the author has recused himself from participation in the clinical trials on eliglustat tartrate to avoid the potential for a conflict of interest. This work is currently supported by NIH grants RO1 DK055823, 5RO1AR056991, and 1R43FD004052.
DISCUSSION
Gotto, New York: The FDA in the last 2 days had an advisory committee recommend two new agents for homozygous familial hypercholesterolemia. I just want to raise a general question of the cost and can we afford not to treat these patients but with a price ranging from $200,000 I think for the Olexin agent, which inhibits terminal part of complement. It is over $400,000 a year, so how do you see a balance between the cost and the benefit?
Shayman, Ann Arbor: The cost of drugs for orphan diseases is clearly a major social challenge. In this particular case, one of our motivators was to find a less expensive therapy compared to traditional enzyme replacement. In point of fact, if eliglustat is approved by the FDA, I anticipate that the cost of treating type 1 Gaucher patients will fall by about 50%, so there is potential social benefit there. Moreover, with many of orphan drugs, if these agents can be repurposed or extended use applications can be found beyond the initial intended use, this can drive down the cost as well. The final point I would raise is that there are many more patients with Gaucher disease than originally believed. Firstly, Gaucher disease is panethnic. It is not simply a disease of the Ashkenazi Jewish population. There are many people that suffer from this disease around the world for whom their governments are unwilling or unable to pay the currently high prices for enzyme replacement. Secondly, if we say as a community that we have the challenge of finding effective therapeutics for 5000 or more different rare diseases, then how do we find ways to lower the cost of drug development? I think that's an academic challenge and that involves many secondary questions. How do we make the drug discovery process more efficient? How do we design clinical trials for very small numbers of patients? How do we create registries that define the natural history of rare diseases? How can we identify biomarkers so we can design the best clinical trials possible? I think that finding answers to these questions is a real challenge and a particular goal for academic medicine.
Mitch, Houston: Thank you. I know you've been at this a very long time and you've made a lot of progress. I was just curious in designing the drugs. So, you take the drug to find a specific area in the enzyme that it can inhibit or augment the activity. What lessons have you learned about the pharmacokinetics, because if you find that action, then of course you want to maximize it by reducing excretion or reducing metabolism, et cetera?
Shayman, Ann Arbor: Well one surprise to me was that the actual endpoint in developing a drug might be in finding a compound that is not the absolute most effective one. The compound we initially licensed to Genzyme had an IC50 of about 10 nanomolar. It was also very lipophilic having an extremely long half-life. The pharmacokinetics and the activity were such that it really was not an ideal drug because when given to an animal, it would take many days to washout. You can imagine that if you were giving this particular compound to a patient and if there were untoward effects, it would take many, many days for those effects to be reversed. Second of all, the lead drug was so active that using standard mass spec techniques, it was very hard to follow plasma levels of the compound in order to perform the pharmacokinetic studies. So we actually “dumbed down” the drug, made it less active, shortening the half-life and thus made it more “drug-like.” That was a real lesson for me. The second observation was that the drug discovery phase is just the first step. The drug development phase is equally challenging. So again, another epiphany, if you will, was the fact that when you go to the FDA when you are doing your phase I trials, the FDA actually is not looking for drugs that are completely devoid of side effects. In fact, they expect and require in your phase I trials that you find adverse effects. So, when Genzyme went to the FDA, they proposed an escalating dose of eliglustat at seven different concentrations of drug. However, there were no adverse effects suggesting eliglustat had a very wide therapeutic index. The investigators had to use another six concentrations before some grade I effects were seen. In one respect, that was very promising as well. The last point I would raise is that a real challenge for finding therapeutics for rare diseases is the patient numbers. Patient numbers are a challenge not just for clinical trial design, but to result in enough confidence that anything that you are going to offer to patients is not, at some point, going to have some unknown and significant toxicity. In this case, the eliglustat studies have comprised the largest collection of clinical trials on Gaucher patients ever done. However many of the diseases we would like to target may have 50 identifiable patients in total, representing another challenge.
REFERENCES
- 1.Veblen T. The Theory of the Leisure Class : An Economic Study in the Evolution of Institutions. New York: Macmillan; 1899. [Google Scholar]
- 2.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–65. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 3.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E. Gaucher disease: multiple lessons from a single gene disorder. Acta Paediatr. 2006;95:103–9. doi: 10.1111/j.1651-2227.2006.tb02398.x. [DOI] [PubMed] [Google Scholar]
- 5.Mistry PK, Weinreb NJ, Brady RO, Grabowski GA. Gaucher disease: resetting the clinical and scientific agenda. Am J Hematol. 2009;84:205–7. doi: 10.1002/ajh.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radin NS, Arora RC, Ullman MD, et al. A possible therapeutic approach to Krabbe's globoid leukodystrophy and the status of cerebroside synthesis in the disorder. Res Commun Chem Pathol Pharmacol. 1972;3:637–44. [PubMed] [Google Scholar]
- 7.Vunnam RR, Radin NS. Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chem Phys Lipids. 1980;26:265–78. doi: 10.1016/0009-3084(80)90057-2. [DOI] [PubMed] [Google Scholar]
- 8.Carson KG, Ganem B, Radin NS, et al. Studies on morpholinosphingolipids—potent inhibitors of glucosylceramide synthase. Tetrahedron Lett. 1994;35:2659–62. [Google Scholar]
- 9.Abe A, Radin NS, Shayman JA, et al. Structural and stereochemical studies of potent inhibitors of glucosylceramide synthase and tumor cell growth. J Lipid Res. 1995;36:611–21. [PubMed] [Google Scholar]
- 10.Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J Biol Chem. 1999;274:14662–9. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- 11.Abe A, Arend LJ, Lee L, et al. Glycosphingolipid depletion in Fabry disease lymphoblasts with potent inhibitors of glucosylceramide synthase. Kidney Int. 2000;57:446–54. doi: 10.1046/j.1523-1755.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 12.Abe A, Gregory S, Lee L, et al. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest. 2000;105:1563–71. doi: 10.1172/JCI9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEachern KA, Fung J, Komarnitsky S, et al. A specific and potent inhibitor of glucosylceramide synthase for substrate inhibition therapy of Gaucher disease. Mol Genet Metab. 2007;91:259–67. doi: 10.1016/j.ymgme.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Shayman JA. Eliglustat tartrate: glucosylceramide synthase inhibitor treatment of type 1 Gaucher disease. Drugs Future. 2010;35:613–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Peterschmitt MJ, Burke A, Blankstein L, et al. Safety, tolerability, and pharmacokinetics of eliglustat tartrate (Genz-112638) after single doses, multiple doses, and food in healthy volunteers. J Clin Pharmacol. 2011;51:695–705. doi: 10.1177/0091270010372387. [DOI] [PubMed] [Google Scholar]
- 16.Lukina E, Watman N, Arreguin EA, et al. A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1. Blood. 2010;116:893–9. doi: 10.1182/blood-2010-03-273151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukina E, Watman N, Arreguin EA, et al. Improvement in hematological, visceral, and skeletal manifestations of Gaucher disease type 1 with oral eliglustat tartrate (Genz-112638) treatment: 2-year results of a phase 2 study. Blood. 2010;116:4095–8. doi: 10.1182/blood-2010-06-293902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shayman JA, Killen PD. Fabry Disease. In: Mount DB, Pollak MR, editors. Molecular and Genetic Basis of Renal Disease. Philadelphia: Saunders Elsevier; 2008. pp. 195–9. [Google Scholar]
- 19.Marshall J, Ashe KM, Bangari D, et al. Substrate reduction augments the efficacy of enzyme therapy in a mouse model of Fabry disease. PloS one. 2010;5:e15033. doi: 10.1371/journal.pone.0015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Wada R, Kawai H, et al. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J Clin Invest. 1999;103:497–505. doi: 10.1172/JCI5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen SD, Wilson MW, Abe A, et al. Property-based design of a glucosylceramide synthase inhibitor that reduces glucosylceramide in the brain. J Lipid Res. 2012;53:282–91. doi: 10.1194/jlr.M021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bremer EG, Hakomori S, Bowen-Pope DF, et al. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984;259:6818–25. [PubMed] [Google Scholar]
- 23.Zador IZ, Deshmukh GD, Kunkel R, et al. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest. 1993;91:797–803. doi: 10.1172/JCI116299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagami S, Inokuchi Ji J, Kabayama K, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–92. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita T, Hashiramoto A, Haluzik M, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A. 2003;100:3445–9. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aerts JM, Ottenhoff R, Powlson AS, et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–9. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Przybylska M, Wu IH, et al. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–8. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 28.Yew NS, Zhao H, Hong EG, et al. Increased hepatic insulin action in diet-induced obese mice following inhibition of glucosylceramide synthase. PLoS One. 2010;5:e11239. doi: 10.1371/journal.pone.0011239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshmukh GD, Radin NS, Gattone VH, 2nd, Shayman JA. Abnormalities of glycosphingolipid, sulfatide, and ceramide in the polycystic (cpk/cpk) mouse. J Lipid Res. 1994;35:1611–8. [PubMed] [Google Scholar]
- 30.Natoli TA, Smith LA, Rogers KA, et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16:788–92. doi: 10.1038/nm.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]