Abstract
Inflammation contributes to all phases of the atherothrombotic process, patients with elevated inflammatory biomarkers such as high-sensitivity C-reactive protein (hsCRP) have increased cardiovascular risk, and recent work directly implicates the interleukin-1 (IL-1) and interleukin-6 (IL-6) pathways in atherogenesis. Yet, it remains unknown whether targeted inhibition of inflammation will reduce cardiovascular event rates. To address directly this fundamental hypothesis, our research group has initiated two large-scale, randomized, placebo-controlled trials using targeted anti-inflammatory agents for the secondary prevention of myocardial infarction. The first trial, the Cardiovascular Inflammation Reduction Trial (CIRT), has been funded by the NHLBI and will evaluate whether low-dose methotrexate (target dose, 20 mg/wk) as compared to placebo will reduce major vascular events among a group of post-myocardial infarction patients with either diabetes or metabolic syndrome, groups known to have high risk on the basis of a persistent pro-inflammatory response. CIRT is based, in part, on observational evidence of reduced vascular event rates among those treated with methotrexate in the setting of rheumatoid arthritis or psoriatic arthritis and on the ability of methotrexate to reduce TNF, IL-6, and CRP levels. The second trial, the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS), will evaluate whether interleukin-1β (IL-1β) inhibition as compared to placebo can reduce rates of recurrent myocardial infarction, stroke, and cardiovascular death among stable coronary artery disease patients who remain at high vascular risk due to persistent elevations of hsCRP (_2 mg/L) despite contemporary secondary prevention strategies. Canakinumab is a human monoclonal antibody that selectively neutralizes IL-1β, a pro-inflammatory cytokine that plays multiple roles in the atherothrombotic process and that undergoes activation by the NLRP3 inflammasome, a process promoted by cholesterol crystals that in turn leads directly to increased production of IL-1 and IL-6. Together, CIRT and CANTOS will enroll more than 25,000 patients worldwide and provide a fundamental test of the inflammatory hypothesis of atherothrombosis.
THE INFLAMMATORY HYPOTHESIS OF ATHEROTHROMBOSIS: CLINICAL EVIDENCE
Inflammation is recognized as a pathologic hallmark in all stages of atherogenesis from early endothelial dysfunction through the process of acute plaque (1). Components of both the innate and adaptive immune systems contribute to this process. With regard to innate immunity and the immediate protection it provides as a non-specific first line of host defense, multiple pattern recognition receptors including disease associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) result in leukocyte activation, cytokine generation, and trafficking of mast cells, eosinophils, neutrophils, and macrophages, all with effects on foam cell accumulation and plaque generation. With regard to adaptive immunity, recent work has similarly implicated this highly specific form of host defense based on antigen presentation and lymphocyte production of antibodies in multiple facets of cell adhesion, lesion propagation, matrix and collagen degradation, smooth muscle proliferation, platelet reactivity, and acute thrombotic occlusion.
From a clinical perspective, translation of the inflammatory hypothesis of atherosclerosis has been based largely on epidemiologic evidence linking inflammatory biomarkers such as IL-6 (2), soluble intercellular adhesion molecule-1 (sICAM-1) (3), fibrinogen (4), and C-reactive protein, particularly when measured with high-sensitivity assays (hsCRP) (5). Of these, hsCRP has emerged as the most clinically useful in part due to its ease of measurement and temporal stability (6, 7). hsCRP has proven capable of identifying patient populations at high vascular risk on the basis of a pro-inflammatory response, even when other traditional risk factors are absent (8, 9). As reviewed in a comprehensive meta-analysis conducted by the Emerging Risk Factor Collaboration (6), the magnitude of cardiovascular risk associated with a one standard deviation increase in hsCRP is at least as large as that associated with a one standard deviation increase in either hyperlipidemia or blood pressure (7). Further, as shown in the Reynolds Risk Scores for men and women (10), the addition of hsCRP, along with family history, significantly improves global risk prediction. Indeed, in direct comparisons performed in multiple cohorts including the Framingham Heart study itself (11), predictive risk scores inclusive of hsCRP consistently improve model fit and clinical efficacy over risk scores based on traditional factors alone (12, 13).
Despite the consistency of data describing hsCRP as a clinically useful biomarker of inflammation, whether CRP itself plays any causal role in atherogenesis is uncertain. On the one hand, CRP could be causal as it plays direct roles in complement activation and serving as a pattern recognition molecule, CRP is a highly conserved component of innate immunity. On the other hand, no data to date have implicated CRP reduction per se in vascular risk reduction and genetic studies using the concepts of Mendelian randomization have not provided positive evidence of linkage between genes known to influence CRP and long-term clinical outcomes (14, 15). I have argued for some time that CRP is most likely a biomarker for a more causal pro-inflammatory response triggered by the IL-1 and IL-6 cascade. In support of this position, recent genetic data has linked specific polymorphisms in the IL-6 receptor gene, IL6R, to both reduced levels of CRP and fibrinogen, as well as reduced risks of vascular disease (16, 17). In these recent reports, genetic effects were similar to that associated with the clinical use of tocilizumab, a monoclonal antibody that specifically targets the IL-6 receptor.
To date, the ability of hsCRP to serve as a method to improve targeting of drug therapy has primarily been in the context of statin prescription. As we first described in the Cholesterol and Recurrent Events (CARE) trial of pravastatin in the secondary prevention of myocardial infarction, the clinical benefit of statin therapy is greater in the presence of elevated hsCRP levels (18). Further, as we then showed in both the AFCAPS/TexCAPS trial of lovastatin (19) and in the large-scale JUPITER trial of rosuvastatin (20), statins are highly effective for reducing vascular event rates among apparently healthy individuals with low levels of LDL cholesterol (who otherwise would not qualify for treatment) but who are selected for increased risk on the basis of high levels of hsCRP. In JUPITER, absolute vascular risk and the magnitude of absolute risk reduction associated with statin therapy increased with increasing levels of baseline hsCRP. Further, in that trial, on-treatment hsCRP (but not on-treatment HDL-C) was a powerful predictor of residual risk. These data are consistent with reports from the PROVE IT, A to Z, REVERSAL, CORONA, and ASCOT trials, all of which reported that achieving low levels of hsCRP contributes to event reduction in a manner analogous to achieving low levels of LDL cholesterol (21–25).
Although hypothesis generating, analyses of statin trials cannot evaluate whether decreasing inflammation decreases vascular risk because statins potently reduce LDL cholesterol as well as inflammation. Nonetheless, the clinical, laboratory, genetic, and pathologic data described above all contribute to the core scientific basis for proceeding with a series of “cardiovascular inflammation reduction trials” designed to test directly whether known anti-inflammatory agents without confounding effects on cholesterol or platelet function can reduce cardiovascular events (26). In the past year, our research group has initiated two such trials, each using a novel strategy to target inflammation without altering other concomitant pathways for vascular disease. Those two trials, the Cardiovascular Inflammation Reduction Trial (CIRT) (26) of low-dose methotrexate and the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) (27) evaluating interleukin-1β (IL-1β) inhibition, are described below along with summaries of the pathophysiologic and clinical data supporting them.
LOW-DOSE METHOTREXATE AS AN AGENT TO TARGET IL-6 AND CRP AND POTENTIALLY REDUCE CARDIOVASCULAR EVENT RATES AND NEW ONSET DIABETES: THE CIRT TRIAL
Testing the inflammatory hypothesis of atherothrombosis requires a specific intervention that (a) inhibits inflammation without having substantive impact on other pathways of the atherothrombotic process, and (b) has a safety profile allowing evaluation in randomized trial settings (26). Low-dose methotrexate (LDM) has multiple attributes that make it an appropriate agent to test directly the inflammatory hypothesis of atherothrombosis.
First, LDM (range, 10 to 20 mg/wk) is widely used and has an enviable safety profile among patients with rheumatoid arthritis and psoriasis, patient groups known to have increased risks for cardiovascular disease. Further, comprehensive guidelines from the American College of Rheumatology exist regarding dosing regimens, drug monitoring, and the identification of high-risk patient subgroups (28). This experience greatly reduces the potential for unanticipated off-target toxicity.
Second, LDM reduces several inflammatory biomarkers including CRP and IL-6 in patients with rheumatoid arthritis (RA) and psoriasis. By contrast, LDM does not have substantive effects on lipid levels, hemostasis, or platelet function. Thus, LDM provides a mechanism to test the inflammatory hypothesis of atherothrombosis without confounding effects on other important vascular pathways.
Third, among both RA and psoriasis patients assessed in seven cohort and case-control settings (29–35), available observational epidemiologic data suggest that exposure to LDM is associated with reductions in cardiovascular morbidity and mortality, even though those receiving LDM have worse vascular risk factor profiles, data mitigating against indication bias. These data have been verified in a recent systematic overview (36). Of interest, the cardiovascular benefit of LDM was observed despite the fact that patients initiating treatment (mean dose, 13 mg/wk) had worse prognostic factors for mortality when compared to patients not being treated with LDM. Other observational studies of RA patients taking LDM have shown improvement in heart failure and reduction in carotid intima media thickness (37). Excess vascular risk unexplained by traditional risk factors among those with RA or psoriasis also supports the conceptual basis for a trial of anti-inflammatory therapy among those with a persistent enhancement of the innate immune response.
Fourth, mechanistic studies suggest that atheroprotective effects of methotrexate may accrue from enhanced release of adenosine which in turn leads to facilitation of cholesterol efflux and reverse cholesterol transport from arterial wall foam cells via upregulated expression of cholesterol 27-hydroxylase (HY27) and the ATP-binding cassette transporter (ABCA1) (38, 39). Recent data indicating enhanced gene expression of HY27 and ABCA1 with clinical use of LDM also supports this emerging hypothesis. Other work suggests that methotrexate has direct effects on apoptosis and on the suppression of adhesion molecule function, both of which play relevant roles in atherothrombosis (40–42).
Finally, LDM is a generic therapy given orally as a once-weekly agent allowing for the efficient and safe conduct of a large simple trial. This simplicity has been incorporated into the CIRT trial in such a way that ongoing safety evaluations can use a centralized methodology that improves participant safety, maintains the study blind while allowing for in-trial dose adjustments, and provides an efficient method to address issues of compliance and follow-up on a cost-effective centralized basis.
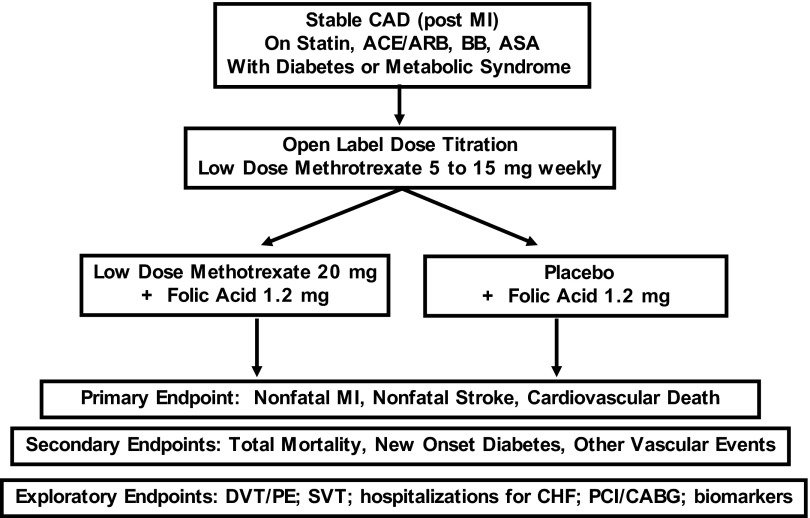
The primary aim of the CIRT trial is to directly test the inflammatory hypothesis of atherothrombosis by evaluating whether or not LDM will reduce rates of recurrent myocardial infarction, stroke, and cardiovascular death among stable post-myocardial infarction patients with type 2 diabetes or metabolic syndrome, conditions associated with an enhanced pro-inflammatory response. Funded by the National Heart Lung and Blood Institute, the CIRT trial is a randomized, double-blind, placebo-controlled, multi-center, event-driven trial that will enroll 7000 men and women from the United States and Canada. After a 4-week open-label run-in, eligible participants who have suffered documented myocardial infarction in the past 5 years will be randomly allocated to usual care plus placebo or usual care plus LDM (initial target dose, 15 mg orally per week with a safety-based titration to 20 mg orally per week after 4 months based on evidence of safety and tolerability). All study participants will additionally receive 1 mg daily oral folate. LDM complications will be minimized through education programs for all investigators and coordinators through enhanced communication with study participants by limiting enrollment to those with no evidence of malignancy, hepatitis, renal dysfunction, chronic infection, or other methotrexate risk factors; by conducting an initial 4-week active-therapy run-in designed to eliminate individuals intolerant to treatment before randomization; and through regular monitoring of liver function and hematologic indices using a centralized methodology designed to ensure participant safety, allow for dose reductions while maintaining the study blind, and provide an efficient method to address issues of compliance and follow-up on a cost-effective centralized basis.
The primary trial endpoint of CIRT is the rate of recurrent myocardial infarction, stroke, or cardiovascular death. Secondary endpoints include all-cause mortality, incident diabetes among those with metabolic syndrome at study entry, hemoglobin A1c (HbA1c) control among those with diabetes at study entry, incident venous thrombosis, and incident atrial fibrillation. The trial is event driven such that in the absence of extreme effects, the trial will conclude after accrual of at least 530 primary endpoints, an effect estimated to provide 90% power to detect a 25% relative risk reduction (Fig. 1).
Fig. 1.
Design of the Cardiovascular Inflammation Reduction Trial (CIRT).
CANAKINUMAB AS AN AGENT TO TARGET INTERLEUKIN-1β AND POTENTIALLY REDUCE CARDIOVASCULAR EVENT RATES AND NEW ONSET DIABETES: THE CANAKINUMAB ANTI-INFLAMMATORY THROMBOSIS OUTCOMES STUDY (CANTOS)
Of inflammatory cytokines implicated in atherothrombosis, IL-1 plays a particularly substantive role (42). In simplified form, the IL-1 signaling system involves two agonists, IL-1α and IL-1β (which exert pro-inflammatory effects through binding to the IL-1 type 1 receptor), and an endogenous antagonist for the IL-1 receptor (IL-1Ra, which blocks binding of IL-1 α and β to the IL-1 type I receptor) (44). Cells that produce IL-1β have an additional regulatory step in that the initial protein product, pro-IL-1β, is inactive and requires proteolytic cleavage by caspase-1 to attain biological activity. This latter cleavage process is controlled by an important complex of intra-cellular proteins known as the NLRP3 inflammasome that can be activated by several exogenous “danger signals” including crystalline compounds (45). As examples, silica, asbestos, hydroxyapetite, and uric acid crystals are all known to enhance IL-1β production through inflammasome interactions. Specific amino acid mutations altering the NLRP3 inflammasome can severely increase secretion of IL-1β and result in a group of rare auto-inflammatory disorders (such as the Muckle-Wells syndrome) that respond to treatment with monoclonal antibodies targeted against IL-1β or with exogenous IL-1R antagonists (46–48). Moderate imbalances in the IL-1/IL-1Ra system are believed to contribute to chronic inflammatory conditions including type 2 diabetes, inflammatory arthritis, psoriasis, gout, and inflammatory bowel disease (49–52).
Recent observations directly implicate the NLRP3 inflammasome and its resulting production of IL-1β in the atherosclerotic process. First, genome-wide association studies have found the NLRP3 loci to be among those determining plasma CRP levels (53). Second, the NLRP3 inflammasome can be activated by cholesterol crystals and minimally modified LDL cholesterol (54, 55). This latter observation implicates crystalline cholesterol as a trigger for IL-1β activation, and thus provides a critical linkage between hyperlipidemia and inflammation. Third, IL-1β–induced inflammation in pancreatic islets of patients with type 2 diabetes has been reported to be mediated through the NLRP3 inflammasome (56). Last, as IL-1β drives the acute phase response, these inflammasome activation data provide a unifying causal pathway explaining why systemic biomarkers of inflammation such as hsCRP, IL-6, fibrinogen, and sICAM-1 levels are elevated many years in advance of acute coronary occlusion.
Although the NLRP3 inflammasome has only recently been described, experimental data has long suggested a role for IL-1 in atherothrombosis (57). As examples, IL-1 is known to induce pro-coagulant activity as well as monocyte and leukocyte adhesion in human vascular endothelial cells; endotoxin and tumor necrosis factor can induce IL-1 gene expression in human vascular endothelial as well as smooth muscle cell; and atherosclerotic lesions contain both IL-1β and IL-1Ra (58–60). Further, reduced lesion formation has been reported in atherosclerosis-prone mice deficient in either IL-1 or the type I IL-1 receptor, whereas IL-1Ra–deficient mice have increased atherogenesis (61–64). In a parallel manner, studies performed on pig coronary arteries show increased neointimal formation with periadventitial administration of IL-1 and reduced neointima formation in the presence of IL-1Ra (65, 66). Human studies supporting these concepts include the observation that atherosclerotic as compared to normal coronary arteries have increased IL-1β levels; that IL-1Ra concentrations are higher among those with acute coronary syndromes as compared to asymptomatic patients or those with chronic stable coronary disease; and that polymorphism in IL-1Ra associates with the burden of coronary lesions found on angiography, with rates of stent re-stenosis, and with atherosclerotic progression (67–70).
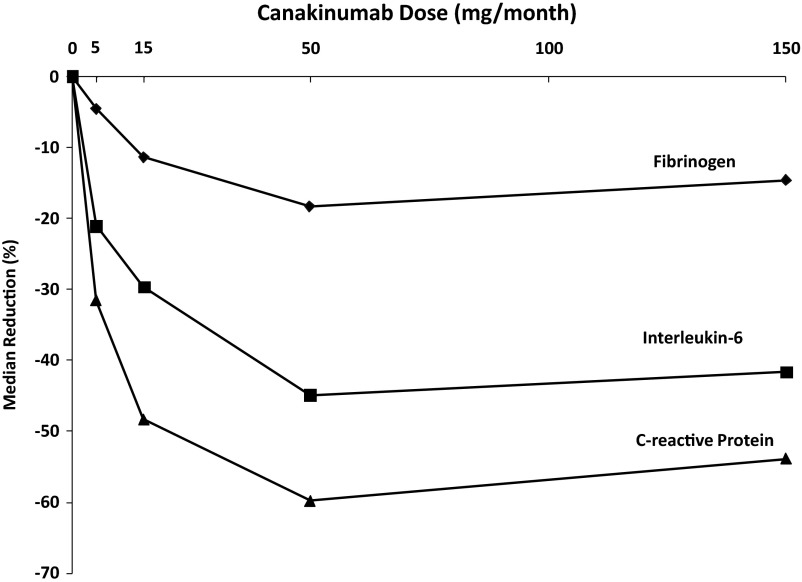
Given the multiple roles played by IL-1β in atherothrombosis, we have hypothesized that IL-1β inhibition with canakinumab, a human monoclonal anti-human IL-1β antibody, might serve as a novel treatment to reduce cardiovascular event rates (27). Canakinumab directly binds human IL-1β and thus blocks the interaction of this cytokine with its type I and type II receptors. In patients with type 2 diabetes, IL-1β antagonism with canakinumab produces a rapid and dose-dependent inhibition of the acute phase response resulting in sustained reductions in fibrinogen, IL-6, and CRP without impacting on lipid levels (Fig. 2) (71). Thus, similar to LDM, canakinumab provides a specific targeted method directly test the inflammatory hypothesis of atherothrombosis.
Fig. 2.
Effects of monthly subcutaneous canakinumab on fibrinogen, IL-6, and hsCRP. [Data from Ridker et al (71)].
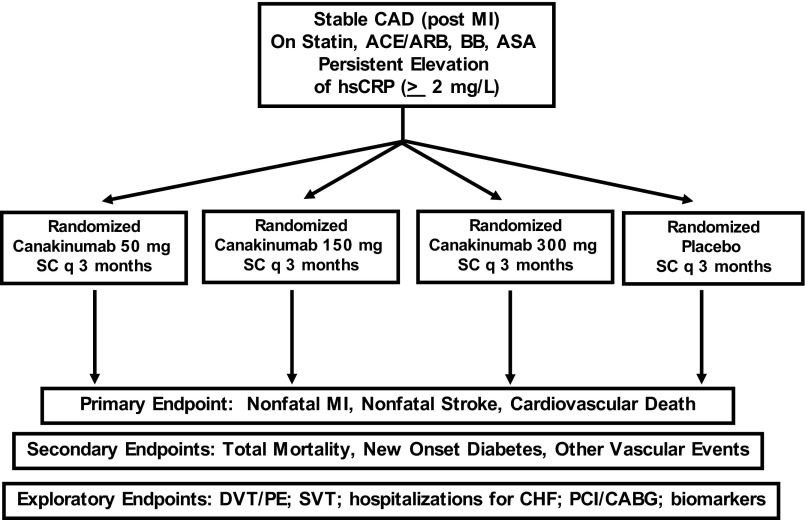
The primary aim of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) is to evaluate whether long-term treatment with canakinumab as compared to placebo will reduce rates of recurrent cardiovascular events among stable post-myocardial infarction patients who remain at increased vascular risk due to persistently elevated levels of hsCRP (2 mg/L) despite usual care, including statin therapy (27). In contrast to the CIRT trial for which there is ample prior data to select a single dose for methotrexate (and thus a simple two-arm trial can be conducted), CANTOS will evaluate three active doses of canakinumab (50 mg, 150 mg, or 300 mg subcutaneously every 3 months) in comparison to placebo (and thus requires a more complex four-arm trial structure) (Fig. 3). The trial primary endpoint will be recurrent major cardiovascular events, defined as non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death. Secondary objectives of CANTOS include determination of the safety and efficacy of long-term canakinumab therapy among post-myocardial infarction patients on total mortality and on other vascular events including hospitalization for unstable angina requiring revascularization. Further, among those with normal or impaired fasting glucose at the time of randomization, CANTOS will also address whether canakinumab will reduce the incidence of new onset diabetes. In exploratory pre-specified analyses, CANTOS will also address the impact of IL-1β inhibition with canakinumab on the incidence of several clinical conditions known to associate with chronic inflammation including venous thromboembolism, atrial fibrillation, stent thrombosis, and hospitalization for congestive heart failure.
Fig. 3.
Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS).
CANTOS began enrollment in late 2011 and ultimately will include 17,200 adult men and women who have suffered a documented acute myocardial infarction at least 30 days before randomization, have completed any planned revascularization procedures associated with their initial infarction, and have evidence of systemic inflammation on the basis of an hsCRP 2 mg/L despite the stable use of standard secondary prevention therapies. As canakinumab is an inhibitor of innate immunity, patients with a suspected or known immunocompromised state, those being administered another biologic agent that targets the immune system (TNF blockers, anakinra, rituximab, abatacept, tocilizumab), and those already receiving methotrexate at a dose exceeding 15 mg weekly will not be eligible. Specific details of CANTOS have been presented elsewhere (27).
Similar to the CIRT trial, CANTOS is designed as an event-driven trial with all primary analyses conducted on an intention-to-treat basis. Trial completion is anticipated to occur after the accrual of 1400 primary endpoints during an estimated 4.5-year period. This number of primary endpoints should provide approximately 90% power to detect the superiority of at least one dose of canakinumab compared to placebo, assuming a hazard reduction of 20%. The protocol also pre-specifies analyses to detect the superiority of the combined canakinumab arms compared to placebo.
Although side effects of canakinumab when given as a treatment for gout, arthritis, or diabetes are few, as in the CIRT trial, all participants in CANTOS will undergo specific monitoring for infection and incident cancer. In both trials, those with a history of or at high risk for either tuberculosis- or HIV-related disease will not be eligible, nor will any individuals with chronic infections or the need for other systemic anti-inflammatory therapies.
WHY PERFORM THE CIRT AND CANTOS TRIALS?
CIRT and CANTOS will be the first randomized trials to formally address the inflammatory hypothesis of atherothrombosis (26, 27); together, these trials will enroll more than 25,000 patients worldwide. Each trial uses an event-driven protocol to address whether LDM (CIRT) or IL-1β inhibition with canakinumab (CANTOS) as compared to placebo can reduce rates of recurrent myocardial infarction, stroke, and cardiovascular death among stable coronary artery disease patients who are selected for a high likelihood of a persistent pro-inflammatory response (in CIRT using the additional entry criteria of the presence of either diabetes or metabolic syndrome and in CANTOS using the additional entry criteria of hsCRP 2 mg/L). Most importantly, both trials seek to expand our understanding of how the balance of innate immunity contributes to cardiovascular health and to diabetes prevention, and both will provide critical safety and efficacy data on long-term inhibition of immunity.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Mushlin, New York: Paul that was a beautiful summary of a spectrum of work that is important for all of us to hear about and understand more. My question is really pretty simple and that is, I noticed that in both CIRT and in CANTOS, all-cause mortality is not an endpoint. Is that, in your mind, just a power and sample size issue or is there a conceptual reason why you're not looking at all-cause mortality?
Ridker, Boston: The primary endpoint of CIRT as well as CANTOS is recurrent non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death. In this regard, both trials are conceived of as proof of concept, and thus relate primarily to cardiovascular events and cardiovascular deaths. That being said, in both CIRT and CANTOS, all-cause mortality is a very highly ranked secondary endpoint.
Bray, Penn Valley: Paul that was great, really clear and it's exciting to see that these trials will hopefully answer this question. So, can you tell us if you're having trouble recruiting because of the same things Dr Weinblatt had to overcome?
Ridker, Boston: When Mike Weinblatt and his colleagues began studying methotrexate for rheumatoid arthritis, they ran into considerable skepticism from those who only thought of the drug in terms of cancer treatment. We still run into that problem, but unlike Mike, I have the advantage of his work demonstrating that at low doses, methotrexate is an effective anti-inflammatory with a fully acceptable and well-described safety profile. And, of course, there are now several million older Americans who take low-dose methotrexate as first-line treatment for arthritis, so I think we can overcome any reluctance to randomize into CIRT in the cardiovascular setting. It interests me from a social perspective that our CANTOS trial using canakinumab is recruiting well already, suggesting that it sometimes is easier to study a drug physicians know little about compared to a drug like methotrexate where the risks are well established.
Gotto, New York: Paul thanks for this lecture and really opening up a new area of research and preventing cardiovascular disease. I want to ask you a question about HDL which also has anti-inflammatory action. In the JUPITER trial, you reported that that if we get the LDL low enough, the HDL didn't seem to matter. Other trials reported different results and if you take the HDL from a patient who has had a recent acute coronary syndrome, that HDL has been reported to lose its anti-inflammatory properties for at least up to a year. So, are you planning to look at HDL or any of the other associated factors with HDL functionality in these studies?
Ridker, Boston: Dr Gotto, let me begin by thanking you for helping to bring me into this organization and thank you again for your willingness several years ago to share data from your AFCAPS/TexCAPS trial that ultimately led to JUPITER. Yes, we are planning to measure HDL functionality in both CIRT and CANTOS, and we are working with Dan Rader looking at their HDL efflux assay for this exact reason. I agree with you that there is something important about inflammatory HDL and we need to go well beyond simple chemical measures of HDL cholesterol to understand its role in atherogenesis.
Shayman, Ann Arbor: That was a very provocative talk. Thank you. You seem to imply that any form of inflammation in this setting would be something to impact on but have your other groups looked at ways to try to tease out what the specific inflammatory pathways would be in terms of looking at, let's say, tyrosine modifications to sort out myeloperoxidase activation or eNOS (endothelial nitric oxide synthase) uncoupling leading to ROS (reactive oxygen species), for example.
Ridker, Boston: When one looks simply at biomarkers of this process, we get similar epidemiologic relationships for myeloperoxidase and Lp-pLA2 as we do for CRP or IL-6. But you are correct in that we do not yet know the specific underlying source for the inflammation being detected. Thus, the trick in figuring out how to get trials done was to pick anti-inflammatory agents that we believe will impact on atherosclerosis and fit a profile that allows for adequate safety exposure. Methotrexate is being used as a broad-term “upstream” anti-inflammatory while canakinumab will provide a much more focused approach, thus giving us two swings at the bat. But the point you are making is very relevant; both CIRT and CANTOS are designed as proof of concept studies. As in any good trial, if either is positive, we will have raised as many questions as we have answered.
Hochberg, Baltimore: I will do a short question since one of my Chairs is the Chair of the session. TNF inhibitors may reduce cardiovascular events in patients with rheumatoid arthritis, but as you showed on your observational slide, they raise LDL levels. So do you think the effect is more important in terms of the reduction in inflammation as the LDL levels are going up in that situation and then can you make a brief comment on the trial which the FDA required Genetech to perform regarding tocilizumab, the IL-6 monoclonal antibody versus etanercept?
Ridker, Boston: As a cardiologist, I would never suggest that inflammation inhibition is more important than aggressive lowering of LDL cholesterol; in both CIRT and CANTOS, the expectation is that all participants will already be on high-dose statin therapy. The question, however, is whether we can additionally reduce inflammation as another method to reduce event rates. We chose methotrexate and canakinumab because neither adversely impacts on lipid levels. As you correctly point out, the commercial TNF inhibitors and IL-6 inhibitors increase LDL, and thus the manufacturers of these agents need to address cardiovascular safety.
Palmer, New York: With the cholesterol that's related to crystals, presumably there is more free cholesterol. Is there a relationship between the atheromatous individuals who have elevated CRP and the amount of free cholesterol in the lesions?
Ridker, Boston: That's a superb question because there is little to no relationship between the level of CRP in plasma and the volume of atherosclerosis measured systemically, yet there is a relationship between the earliest deposition of cholesterol crystals and the initiation of inflammation, likely through the NLRP3 inflammasome.
Palmer, New York: Thank you.
Bishopric, Miami: I would like to know whether you have thoughts about why all the anti-inflammatory agents that have gotten into trouble for increasing rates of atherosclerotic and adverse cardiovascular events have been so contradictory to this theory.
Ridker, Boston: Earlier in this session we heard an elegant discussion about COX-1 and COX-2 inhibitors, and it is largely these anti-inflammatory agents that have been associated with small but real adverse cardiovascular effects. However, those effects have really been based on platelet aggregation effects and represent a very different kind of inflammation reduction than we are talking about in the new trials.
REFERENCES
- 1.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 6.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55:305–12. doi: 10.1373/clinchem.2008.120642. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, Pencina M, Jacques P, Selhub J, D'Agostino R, Sr, O'Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–7. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–9. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 13.Cook NR, Paynter NP, Eaton CB, Manson JE, Martin LW, Robinson JG, et al. Comparison of the Framingham and Reynolds Risk Scores for global cardiovascular risk prediction in the multiethnic Women's Health Initiative. Circulation. 2012;125:1748–56. doi: 10.1161/CIRCULATIONAHA.111.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 15.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–44. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–8. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 22.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 23.McMurray JJ, Kjekshus J, Gullestad L, Dunselman P, Hjalmarson A, Wedel H, et al. Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): a retrospective analysis. Circulation. 2009;120:2188–96. doi: 10.1161/CIRCULATIONAHA.109.849117. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 25.Braunwald E. Creating controversy where none exists: the important role of C-reactive protein in the CARE, AFCAPS/TexCAPS, PROVE IT, REVERSAL, A to Z, JUPITER, HEART PROTECTION, and ASCOT trials. Eur Heart J. 2012;33:430–2. doi: 10.1093/eurheartj/ehr310. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(suppl 1):332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 29.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 30.van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8(5):R151. doi: 10.1186/ar2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–7. doi: 10.1016/j.jaad.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH, Avorn J, Katz JN, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(12):3790–8. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 33.Suissa S, Bernatsky S, Hudson M. Antirheumatic drug use and the risk of acute myocardial infarction. Arthritis Rheum. 2006;55(4):531–6. doi: 10.1002/art.22094. [DOI] [PubMed] [Google Scholar]
- 34.Naranjo A, Sokka T, Descalzo MA, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10(2):R30. doi: 10.1186/ar2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochberg MC, Johnston SS, John AK. The incidence and prevalence of extra-articular and systemic manifestations in a cohort of newly-diagnosed patients with rheumatoid arthritis between 1999 and 2006. Curr Med Res Opin. 2008;24(2):469–80. doi: 10.1185/030079908x261177. [DOI] [PubMed] [Google Scholar]
- 36.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 49(2):295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 37.Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford) 2005;44(5):677–80. doi: 10.1093/rheumatology/keh610. [DOI] [PubMed] [Google Scholar]
- 38.Reiss AB, Carsons SE, Anwar K, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008;58(12):3675–3683. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coomes E, Chan ES, Reiss AB. Methotrexate in atherogenesis and cholesterol metabolism. Cholesterol. 2011;2011:503028. doi: 10.1155/2011/503028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen DY, Chih HM, Lan JL, Chang HY, Chen WW, Chiang EP. Blood lipid profiles and peripheral blood mononuclear cell cholesterol metabolism gene expression in patients with and without methotrexate treatment. BMC Med. 2011;9:4. doi: 10.1186/1741-7015-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 2005;114(2):154–63. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology (Oxford) 2003;42(10):1189–96. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 43.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–4. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 44.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 45.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–11. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinarello CA. Interleukin-1beta and the autoinflammatory diseases. N Engl J Med. 2009;360:2467–70. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416–25. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 49.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 50.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 51.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 52.Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. a novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–40. [PubMed] [Google Scholar]
- 53.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in 80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2012;123:731–8. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajama K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donath MY, Schoelson SE. Type 2 diabetes as an inflammatory disease. Nature Rev Immunology. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 57.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–9. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 58.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–23. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–11. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986;124:179–85. [PMC free article] [PubMed] [Google Scholar]
- 61.Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, et al. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 63.Isoda K, Shiigai M, Ishigami N, Matsuki T, Horai R, Nishikawa K, et al. Deficiency of interleukin-1 receptor antagonist promotes neointimal formation after injury. Circulation. 2003;108:516–8. doi: 10.1161/01.CIR.0000085567.18648.21. [DOI] [PubMed] [Google Scholar]
- 64.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, et al. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–73. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 65.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, et al. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97:769–76. doi: 10.1172/JCI118476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morton AC, Arnold ND, Gunn J, Varcoe R, Francis SE, Dower SK, et al. Interleukin-1 receptor antagonist alters the response to vessel wall injury in a porcine coronary artery model. Cardiovasc Res. 2005;68:493–501. doi: 10.1016/j.cardiores.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–6. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 68.Kastrati A, Koch W, Berger PB, Mehilli J, Stephenson K, Neumann FJ, et al. Protective role against restenosis from an interleukin-1 receptor antagonist gene polymorphism in patients treated with coronary stenting. J Am Coll Cardiol. 2000;36:2168–73. doi: 10.1016/s0735-1097(00)01014-7. [DOI] [PubMed] [Google Scholar]
- 69.Francis SE, Camp NJ, Dewberry RM, Gunn J, Syrris P, Carter ND, et al. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation. 1999;99:861–6. doi: 10.1161/01.cir.99.7.861. [DOI] [PubMed] [Google Scholar]
- 70.Olofsson PS, Sheikine Y, Jatta K, Ghaderi M, Samnegard A, Eriksson P, et al. A functional interleukin-1 receptor antagonist polymorphism influences atherosclerosis development. The interleukin-1beta:interleukin-1 receptor antagonist balance in atherosclerosis. Circ J. 2009;73:1531–6. doi: 10.1253/circj.cj-08-1150. [DOI] [PubMed] [Google Scholar]
- 71.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]