Abstract
The development of human embryonic stem cell (hESC) lines in 1998 offered the prospect of a new era of regenerative medicine in which cell therapy might cure intractable diseases such as type 1 diabetes, Parkinson's disease, and spinal cord injury. The Bush Administration decision in 2001 to restrict federal funding of hESC research touched off a controversy that continues to the present. One response to the Bush policy was establishment of state stem cell research funding programs, notably the California Institute of Regenerative Medicine (CIRM). As Director of the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) and Vice Chair of the National Institutes of Health (NIH) Stem Cell Task Force, and now as a member of the Empire State Stem Cell Funding Board and member of an Institute of Medicine (IOM) committee charged with evaluation of the CIRM, I have had the opportunity to gain a first-hand perspective of the field. Here I present my impressions of the legal and science policy debates and selectively summarize research progress toward the hoped-for cures.
INTRODUCTION
On August 2, 2001, I found myself in the Oval Office of the White House along with two colleagues from the NIH in a meeting on hESC research with President George W. Bush, his senior political advisor, Karl Rove, and his Chief of Staff, Andrew Card. This was one in a series of meetings (Fig. 1) held by the President between July 9th and August 2nd of that year, prior to his address on August 9th setting out his policy for federal funding of hESC research. Lana Skirboll, head of the NIH Office of Science Policy, had been communicating with White House staff concerning the number of established hESC lines available for research, and had arranged the Oval Office meeting with them. Ron McKay, a neuroscientist in the NIH intramural program, participated because of his expertise in stem cell research and studies he had done on treatment of Parkinson's disease in mouse models using embryonic stem cells. I was included because type 1 diabetes was considered a prime candidate for cell therapies derived from hESCs. NIDDK, which I directed from 1999–2006, was the lead Institute at NIH for support of diabetes research.
Fig. 1.
A listing released by the White House of meetings on embryonic stem cell research held by President George W. Bush in July and August 2001 before his August 9, 2001, address defining his policy for federal funding of embryonic stem cell research (reprinted from The New York Times, August 11, 2001).
In this article, I provide: 1) a brief background on stem cell biology and how this field of research generated a major controversy over the issue of federal funding for hESC research; 2) a chronologic summary of the major legal and political developments in this controversy; and 3) a perspective on current and future prospects for stem cell research leading to “cures” of serious diseases.
STEM CELL BIOLOGY
Mario Capecchi, Martin Evans, and Oliver Smithies received the 2007 Nobel Prize in Physiology or Medicine (1) for work going back to 1981 on development of mouse embryonic stem cell technology that allowed any gene to be “knocked out” in mice. This enabled creation of mouse models of human disease that have proved to be powerful tools for understanding pathophysiology and for testing possible therapies. Creation of mouse embryonic stem cells involved dissociating mouse blastocysts, and culturing cells derived from the inner cell mass to establish cell lines capable of differentiating into any adult cell type. Indeed, these cells were pluripotent (Fig. 2) and could give rise to teratomas, as well as contribute to creation of a new mouse (chimerism) when injected into an unrelated mouse blastocyst. None of this was considered controversial because the work involved destruction of mouse (as opposed to human) embryos, and Federal funding for this line of research in the United States was robust.
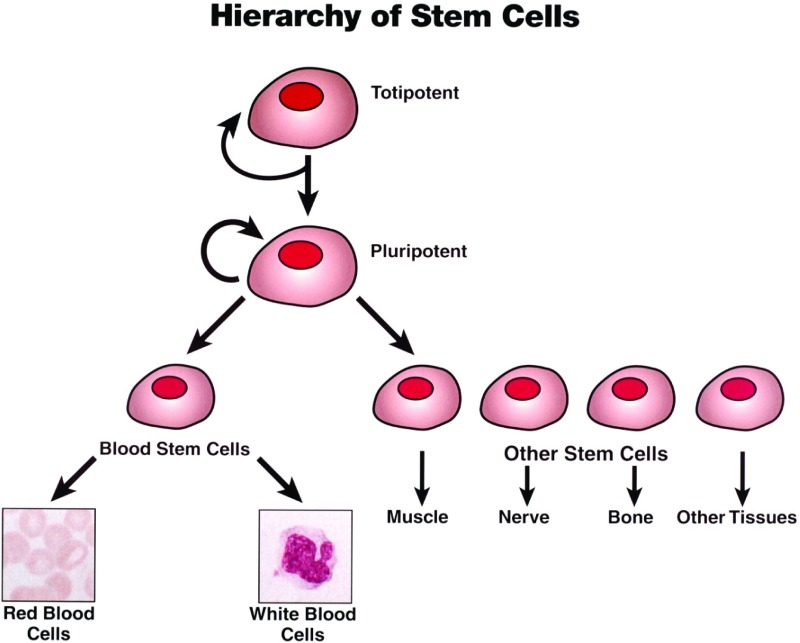
Fig. 2.
A schematic diagram showing the hierarchy of stem cells from totipotent (able to give rise to entire organism) to pluripotent (able to give rise to any cell type but not entire organism) to so-called adult stem cells, with more limited ability to generate various cell types. The circular arrows shown for totipotent and pluripotent stem cells signify their ability to undergo asymmetric cell division, giving rise to both a daughter stem cell of identical potential and a daughter progenitor cell that is committed to differentiate along a particular cell lineage.
But in 1998, Jamie Thomson et al. at the University of Wisconsin, adapting the mouse techniques to human blastocysts, showed for the first time that hESC lines could be established (2). This triggered the controversy that will be described in the next section. Here, we first review basic aspects of stem cell biology to understand the significance of Thomson's breakthrough.
A stem cell is defined as a cell that is capable of asymmetric division, giving rise to a daughter cell that retains “stemness” and to another daughter cell that is a progenitor for a particular cell lineage. In 1961, Ernest A. McCulloch and James E. Till discovered that a single bone marrow precursor cell capable of forming a colony in an irradiated recipient mouse could give rise to multiple lineages of hematopoietic cells (3). This discovery gave rise to the concept of hematopoietic stem cells, one example of an “adult” stem cell, i.e., a cell capable of giving rise to some but not all cell lineages. Skin stem cells and intestinal lining stem cells are other examples of adult stem cells (Fig. 2). In contrast, embryonic stem cells derived from the inner cell mass of the blastocyst are pluripotent. They can give rise to any cell type but not to the entire organism. Only totipotent cells such as the zygote have that capability (Fig. 3).
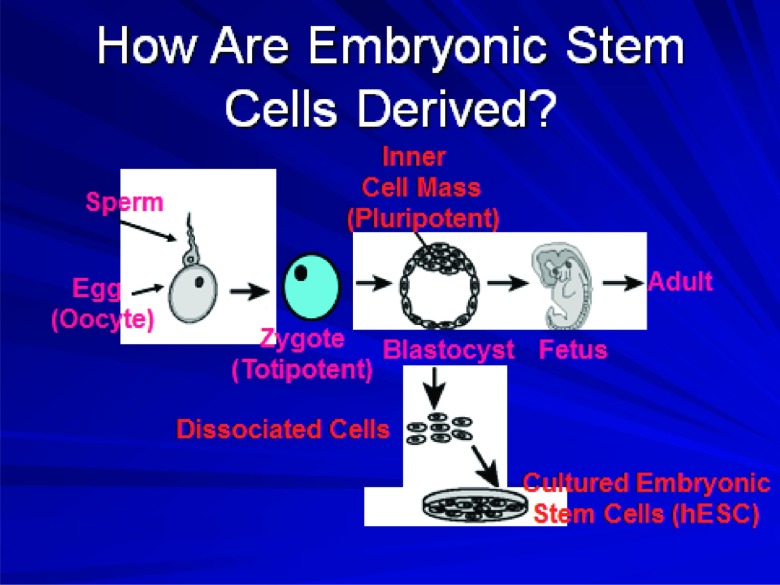
Fig. 3.
A schematic diagram showing the steps from fertilization and creation of the zygote to development of the blastocyst. When the latter is implanted within the uterus, normal fetal development may proceed. In the process of in vitro fertilization, the embryo develops to the blastocyst stage and may then be placed in the uterus for implantation to occur. Derivation of embryonic stem cell lines involves dissociation of the blastocyst and culture of the cells from the inner cell mass under special conditions.
hESCs derived from the inner cell mass of the blastocyst must be cultured under specialized conditions in order for them to maintain their pluripotent nature and their ability to replicate indefinitely. Initially, this involved growing hESCs on mouse “feeder” cell layers which secreted factor(s) that inhibited hESC differentiation. Without the feeder layer, hESCs spontaneously differentiated into multiple cell types including beating cardiomyocytes and neurons. Contamination of the earliest hESC lines by mouse feeder cell-derived products, thus rendering them unsuitable for cell therapy in humans, became one argument for liberalizing federal funding for hESC research (see next section). Later, cell-free media were developed to allow growth of hESC lines without spontaneous differentiation.
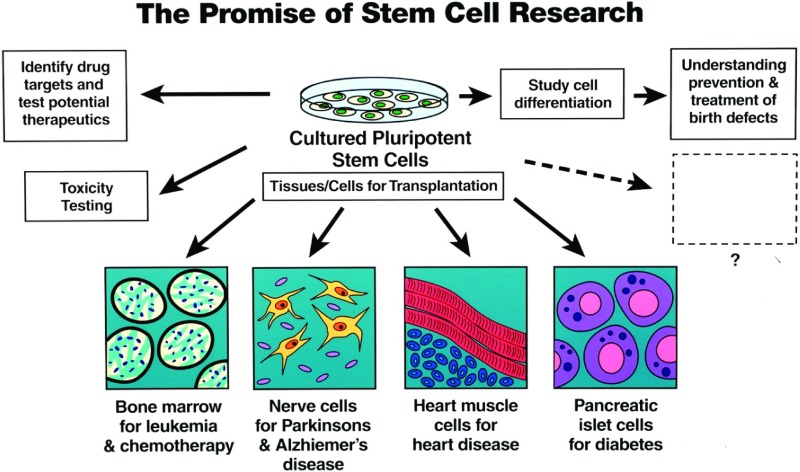
The ability of hESCs to differentiate into any cell type spurred research on defining optimal conditions for their differentiation into specific cell types that could be used in cell therapy of disease (Fig. 4). This ability to differentiate into any cell type, coupled with their ability to replicate indefinitely and generate a virtually unlimited supply of cells, generated huge enthusiasm for their therapeutic potential. In addition to their potential utility for cell therapy, hESC offered great promise in understanding the mechanisms of normal cell differentiation and organ development; could be used for testing novel drugs and other therapeutic agents; and could allow toxicity testing “in a dish” (Fig. 4).
Fig. 4.
A schematic diagram showing potential uses of pluripotent stem cells such as embryonic stem cells. These include differentiation to specific cell types for cell therapy of various diseases, use in vitro for testing of drug treatments and of possible drug toxicity, and studies of normal cellular differentiation and development.
A distinctive feature of the controversy over hESC research was the intrusion of ideology into the science. Opponents of hESC research argued that it was unnecessary because adult stem cells were much more “plastic” than had been assumed, and could differentiate into any cell type under appropriate conditions. Many of the reports of such adult stem cell plasticity were later shown to involve cell fusion or other artifacts rather than true pluripotency (4). Then, in 2007, Yamanaka et al. in Japan reported on a method to create pluripotent ES-like cells from adult human fibroblasts (5). These so-called induced pluripotent stem cells (iPSC) were created by the addition of four genes that “reprogrammed” an adult, differentiated cell back to a state of pluripotency. The creation of iPSCs was quickly seized upon by opponents of hESC research as rendering the latter unnecessary. But careful studies have shown that iPSCs may differ in important respects from hESC lines because of incomplete reprogramming. However, iPSCs do provide a powerful tool for modeling human disease. Skin fibroblasts or other adult cells taken from patients with specific diseases can be reprogrammed to iPSCs, and then differentiated to the cell type of interest for the disease, e.g., cardiomyocytes in patients with long QT syndrome. This allows both mechanistic studies and testing of potential treatments.
Yamanaka, for his discovery of iPSCs, shared the 2012 Nobel Prize in Physiology or Medicine with John Gurdon who had shown decades before that adult somatic cell nuclei transplanted into enucleated frog oocytes could be reprogrammed and give rise to new frogs (6). In 1997, Wilmut et al. showed that fundamentally the same procedure could be used to “clone” a mammal, Dolly the sheep (7). Both Gurdon's and Wilmut's achievements involved a process termed somatic cell nuclear transfer (SCNT). Evidently, a factor (or factors) in oocyte cytoplasm was capable of reprogramming the genome present within the transplanted somatic cell nucleus back to a state of pluripotency. Every adult nucleated cell possesses an entire copy of the genome, but epigenetic modifications occur with cell differentiation that regulate gene expression in ways characteristic of each specific cell type. We now know that reprogramming involves erasure of these epigenetic marks. Yamanka's breakthrough was to identify a finite set of specific genes which when expressed in an adult somatic cell mimicked the reprogramming effected by oocyte cytoplasm.
The conjunction of Dolly the sheep, as well as the cloning of numerous other mammalian species which soon followed, and Thomson's creation of hESC lines raised the prospect of performing SCNT with nuclei derived from human somatic cells and human oocytes. In this way, an embryo could be created with the genome of the somatic cell donor. Allowing the embryo to develop to the blastocyst stage in vitro, and then deriving a new hESC line from the inner cell mass could, in theory, allow generation of autologous cells differentiated from the custom hESC line. Thus, autologous neurons could be generated to be used for cell therapy of a subject who had suffered acute spinal cord injury. This application of SCNT was termed “therapeutic cloning,” to distinguish it from a procedure in which the blastocyst created via SCNT would be implanted in the uterus of a surrogate mother to give birth to a new organism, i.e., “reproductive cloning” as in the creation of Dolly. The use of the term “cloning” and the fact that obtaining oocytes from donor women was rate-limiting for therapeutic cloning added fuel to the already raging fire enveloping hESC research.
THE CONTROVERSY OVER hESC RESEARCH
The controversy over hESC research had its origins in the controversy over abortion policy and by extension federal funding of fetal tissue research. Scientists in the United States have performed research on human fetal tissues for decades (8). Human fetal kidney cells were used in development of the polio vaccine. Transplantation of human fetal adrenal tissue into the brains of patients with Parkinson's disease to restore neuronal dopamine secretion showed some promise, although a 2001 clinical trial was halted because of adverse neurologic effects. In 1988, President Reagan imposed a moratorium on federal funding for human fetal tissue transplantation research (but not for other types of human fetal tissue research). The NIH Revitalization Act of 1993 (Public Law 103-43) formalized President Clinton's lifting of the 1988 ban (8).
After the 1994 election in which Republicans took control of both houses of Congress, policy shifted on federal funding. Although the ban on funding any type of human fetal tissue research was not re-imposed, an amendment to the authorization bill for the department of Health and Human Services was passed in 1996. The Dickey-Wicker amendment, named for its authors, Jay Dickey (R–Arkansas) and Roger Wicker (R–Mississippi), banned federal funding for research in which a human embryo is destroyed, discarded, or knowingly subjected to risk of injury or death (9). This amendment has been attached to the corresponding House appropriations bill each year since.
Thomson's derivation of hESC lines, which clearly involved destruction of human embryos, was not supported by NIH or other federal funds because that would have violated the Dickey-Wicker amendment. As the potential for hESC research to revolutionize treatments for a number of serious diseases became apparent, several members of Congress, most notably the late Senator Arlen Specter (at that time R–Pennsylvania) and Senator Tom Harken (D–Iowa) began holding hearings on this subject. Specter and Harken, who alternated as Chairs of the Senate Appropriations subcommittee with jurisdiction over NIH, were both champions for NIH funding in general and for NIH support of hESC research in particular. Harold Varmus, then NIH Director, testified at hearings before Specter and Harken on December 2, 1998, and January 26, 1999 (10). After these hearings, the Clinton administration issued guidelines through the NIH in 2000 for funding grants using hESC lines (11). The guidelines permitted funding for research on hESC lines, but not for derivation of new hESC lines. However, no NIH grants for hESC research were issued before the end of the Clinton administration.
My personal involvement with this issue began shortly after I had been appointed Director of NIDDK by Harold Varmus in November 1999. At my first appearance, along with the NIH Director and other Institute Directors, before the Senate Appropriations subcommittee for the annual hearing on the NIH budget in April 2000, I was questioned by Senator Harken about the “Edmonton Protocol” and its implications for possible cure of type 1 diabetes. Although pancreatic islet transplantation had been tried for more than 2 decades as a treatment for type 1 diabetes, it had been largely unsuccessful. A new immunosuppressive regimen lacking glucocorticoids, newer methods for islet harvest from cadaveric human pancreases, and a larger number of islets infused via the portal vein allowed investigators in Edmonton, Alberta, to render several patients with longstanding insulin-dependent type 1 diabetes insulin independent (12). The implication was that islet transplantation could become a definitive or at least temporary cure for type 1 diabetes, but this immediately begged the question of where the supply of islets for transplantation of even a portion of the estimated 1 million people, adults and children, with type 1 diabetes in the United States would come from. The approximately 9000 cadaver pancreases that became available for transplantation, either as whole organs or for islet harvest, each year in the United States would never suffice. That was exactly the question posed to me, as Director of NIDDK, by Senator Harken. The question had been planted by a patient advocacy organization (as was customary practice), in this case the Juvenile Diabetes Research Foundation (JDRF), whose leadership had become enthusiastic about islet transplantation as a cure and the potential of hESC research to solve the dilemma of inadequate islet supply.
My responses to his questions evidently satisfied both Senator Harken and the JDRF sufficiently that I was invited back for some “encore” performances, including hearings exclusively focused on hESC research on April 26, 2000, and September 7, 2000. For these, I was joined by my colleague, Gerald Fischbach, then Director of the National Institute of Neurologic Disorders and Stroke (NINDS). The rationale for involving the NINDS Director was that Parkinson's disease (for which human fetal tissue transplantation had already been attempted; see above) and spinal cord injury were considered prime candidates for cell therapy using neurons differentiated from hESC lines. These hearings were remarkable media events, not obviously because of my and Gerry Fischbach's appearance, but because the entire controversy had seized the public's imagination, with extensive coverage in print and broadcast media. To further capture attention, celebrity “witnesses” were invited to the hearings. I will never forget the poignant sight of Christopher Reeve, quadriplegic following his accident, testifying at the April 2000 hearing. But the Country's polarization on the issue was mirrored in the witnesses who appeared at these hearings. Supporters of hESC research included distinguished scientists, celebrities, biotechnology company leaders, and political figures such as Senator Gordon Smith (R–Oregon). Opponents of hESC research included scientists such as David Prentice, a biologist at Indiana State University, Catholic and other theologians, and Senators such as Sam Brownback (R–Kansas).
The controversy centered on the status of the human embryo. Opponents argued that destruction of any human embryo was destruction of human life, and that federal support for hESC research encouraged further destruction of embryos even if the Dickey-Wicker amendment forbade support for hESC derivation itself. Proponents argued that IVF clinics in the United States were already destroying thousands of embryos each year and that the number of frozen embryos, estimated at 100,000, was vastly more than would ever be used for implantation for reproductive purposes. As long as strict ethical guidelines were followed, they argued, it was actually immoral to deny patients suffering from incurable diseases the possibility of new forms of therapy that might be developed from research on hESC lines. Furthermore, supporters pointed out that a ban on federal funding for hESC research would leave it to the private sector to pursue such work, arguably under less carefully regulated standards than under NIH auspices.
Two further dimensions of the controversy have already been alluded to in the preceding section. One was the contention by opponents of hESC research that all the potential for treatments promised by hESC work could be obtained from adult stem cells that carried no ethical “baggage.” In reality, the plasticity potential of adult stem cells could only be defined through careful scientific research comparing adult and hESCs, not through ideology-driven positions. But that did not prevent definitive pronouncements often supported by no or questionable data. The other issue related to SCNT and therapeutic cloning. The appeal of the latter, with Christopher Reeve as the “poster child,” was that the potential for immune rejection of transplanted cells could theoretically be obviated by transplanting autologous cells derived via SCNT. But this had several problematic features, not least, the need for women to serve as oocyte donors. Although this was routine and remunerated practice for reproductive purposes in IVF clinics, donation of oocytes for research purposes was viewed as a different matter, conceptually different from donation of embryos as a “byproduct” of couples already seeking help at IVF clinics. SCNT was further stigmatized by opponents as potentially leading to reproductive cloning; this despite the fact that no one to date, notwithstanding the work of Hwang in South Korea which was found to be fraudulent, had succeeded in generating a viable human diploid blastocyst via SCNT. The development of iPSCs by Yamanaka largely displaced interest in SCNT. Not only could iPSC lines derived on a customized basis theoretically address the immune rejection advantage offered by SCNT, but iPSC technology could and has been fully exploited to model various diseases, arguably one of the stronger scientific rationales for use of SCNT.
With the election of George W. Bush as President in November 2000, responsibility for setting policy on NIH funding for hESC research passed to his administration. It was in this context that the series of Oval Office meetings in the summer of 2001 described at the start of this article occurred (Fig. 1). These culminated in the President's speech at 9PM on August 9, 2001, from his ranch in Crawford, Texas, setting out a policy permitting NIH funding for research on hESC lines established before the exact time of his speech, but not for any lines derived after that time. This “Solomonic” (or perhaps more likely Karl “Rovian”) decision upset both opponents and supporters of hESC research. The former group's reaction was intuitively obvious, but why were supporters unhappy? In part because any intrusion of what was viewed as “abortion politics” into science policy was viewed as problematic. NIH funding had heretofore enjoyed vigorous, bipartisan support. Indeed, the doubling of the NIH budget, started during the Clinton administration, continued under that of Bush. But first, the Dickey-Wicker amendment and now the Bush policy were placing restrictions on NIH funding of what was believed to be a critical field of research. Patients and patient advocates were concerned about anything that might limit hESC research conducted under appropriate, ethically approved guidelines. They were concerned that such restrictions might limit the entry of both new and established scientists into this nascent field. However, the most immediate and concrete concern was related to the adequacy of the number of hESC lines sanctioned by the Bush policy for NIH funding. At the outset, the number of approved lines was estimated at ∼70, a number attested to by the NIH Office of Science Policy under Lana Skirboll. Indeed, at the news conference in Crawford, Texas, the day after the President's speech, when asked by a reporter whether the number of lines approved was adequate, the President responded to the effect that the “NIH came into the Oval Office and looked me in the eye” and assured him that the number of lines was adequate. Unfortunately, subsequent events would prove that the actual number of lines was much lower, ∼20. Questions about their genetic diversity being adequate for treatment of the US population, about their contamination by mouse feeder cell-derived factors, and about their genomic integrity following multiple passages led to mounting pressure to reverse the Bush policy and allow NIH funding for newly derived lines untainted by the preceding problems.
One consequence of the President's restrictive policy was a move by several states to initiate stem cell funding programs of their own. The most ambitious was that of California, which, with the $3 billion bond approved by the State's voters on proposition 71, allowed creation of the California Institute for Regenerative Medicine (CIRM). New York State created NYSTEM, an initiative funded through annual appropriations that were eventually to total $600 million [the Institute of Medicine (11) reviews these and other state stem cell funding initiatives]. Another consequence was that Congress attempted to pass new legislation enabling NIH to fund hESC research on newly derived lines. Rep. Michael Castle introduced the Stem Cell Research Enhancement Act in 2005 which passed both houses of Congress, but was vetoed by President Bush. Congress failed on that occasion and subsequently to override the veto. Only with the election of President Obama was the policy changed by Executive Order in March 2009. The NIH issued new guidelines for approving hESC lines eligible for NIH funding and as of December 2012, a total of 198 lines are listed on a specially created NIH registry (13).
President Obama's reelection in November 2012 assured continuation of his policy on funding hESC research, a policy candidate Romney had promised to reverse. But that policy also came under legal challenge by plaintiffs arguing that it violated the Dickey-Wicker amendment [Table 1; Cohen et al. (9) reviews the various court proceedings]. An initial ruling led to a temporary injunction against NIH funding of hESC research, in essence suspending the Obama Executive Order. That ruling and the ban were subsequently reversed, and a decision of the US Court of Appeals for the District of Columbia Circuit on August 24, 2012, ruled that the policy did not violate the amendment. Now the matter may be considered by the Supreme Court, so that the possibility that NIH funding will once again be halted remains.1
TABLE 1.
Timeline of Human Embryonic Stem Cell (hESC) Controversy
|
STEM CELL RESEARCH: PROGRESS TOWARD THE CLINIC
Translating basic research discoveries into safe and effective treatments for human disease is always an enormous challenge. One need only consider monoclonal antibodies which took approximately 2 decades to move from discovery to powerful research tool to diagnostics for human disease and finally to the current status in which a number of monoclonal antibodies is approved for treatment of cancer, autoimmune, and other diseases. Gene therapy, in contrast, has yet to achieve meaningful success after several decades of research and trials, with several high-profile setbacks during that time only recently being overcome with promising results in a few cases. Against this background, it should be no surprise that 14 years after Thomson derived the first hESC lines, there is as yet no approved disease treatment derived from hESC lines.
Added to the usual translational challenges, the field of hESC research had the additional burden of the controversy over federal funding. One unfortunate consequence of the controversy was the tendency of some supporters of hESC research to unrealistically raise expectations for the cures sure to come. A pernicious consequence of these unrealistically raised expectations has been the proliferation of “charlatan” stem cell clinics promising treatments for a multitude of diseases, but in fact, using unproven and in many cases unsafe methods. Educating the public about the dangers of such fraudulent practitioners has been undertaken by organizations such as the International Society for Stem Cell Research. Translation of hESC research into the clinic poses additional challenges related to the nature of cell therapy. Unlike treatment with orally available, small molecule drugs or even injected biologics, the business model for cell therapy is not well defined, hence dampening industry interest. One of the few currently approved forms of cell therapy, bone marrow transplantation, is practiced in specialized academic medical centers without industry providing the cell product. Unique problems that must be addressed if hESC-derived cells are to be administered therapeutically include the need for standardization of cell product, evidence that teratomas and other neoplastic processes will not ensue after cell transplantation, and methods to track the fate and efficacy of administered cells (14).
These challenges should not obscure the fact that hESC research has already led to major advances in basic developmental biology because the processes regulating cell differentiation have been intensively studied. Our understanding of the epigenetic processes that underlie both cell differentiation and reprogramming has increased dramatically. Models of human disease using iPSC- and hESC-derived cells have led to important advances in understanding etiology, and directly to testing potential therapeutics. The latter is an area of intense industry interest, as is toxicity testing using hESC- and iPSC-derived cells. Work on adult stem cells such as hematopoietic stem cells, mesenchymal stem cells, and cardiac stem cells has progressed significantly, including late-stage clinical trials in some cases. Close collaboration between clinicians and industry will be required if cell therapy is to become successful (15).
Given the magnitude of funding through CIRM, the critical mass of academic medical centers, research institutes and biotech companies in California, and the mandate to turn hESC research discoveries into cures, it should not be surprising that CIRM is sponsoring some of the leading efforts in cell therapy (16). A number of “disease teams” has been funded to move toward clinical trials for indications ranging from HIV to cancer. Even here though, the challenges cited above are apparent. A trial of hESC-derived neurons for repair of spinal cord injury that was supported by CIRM was halted after the enrollment of four patients, not because of apparent adverse effects, but because the company performing the trial, Geron, made the business decision that their limited funds were better spent on another therapeutic area. Another company funded by CIRM, ViaCyte, is working on hESC-derived cells that produce insulin in a glucose-responsive manner. Animal studies of such cells have proven effective in keeping diabetes under control, but extrapolating from these results to success in human trials is far from certain. Perhaps the most promising results to date involve transplant of a sheet of retinal pigment epithelial cells derived from hESCs for treatment of macular degeneration. The small number of cells required, the immune privileged site of the eye, and the straightforward endpoint for successful treatment, namely the ability to read an eye chart, all make macular degeneration the likeliest candidate for the first successful application of hESC research to human disease. The late Senator Specter used to ask me and other NIH directors during his hearings for a prediction of how many years it would take to develop a cure for a particular disease. Then, as now, I'm reluctant to try to make a specific prediction, but I do feel it's likely that we will see hESC-derived treatments for at least some serious diseases in the future.
Footnotes
Potential Conflicts of Interest: None disclosed.
On 1/7/13 the Supreme Court decided not to take up this case, effectively allowing the US Court of Appeals decision to reverse the ban stand.
DISCUSSION
Mackowiak, Baltimore: Allen, do you believe that President Bush understood what you were telling him in your meeting with him?
Spiegel, Bronx: They say that memory is selective and that you also remember well things which are surrounded by increased beta receptor activation; both are the case here. First of all, the primer which I showed you, his comment was, “This is terrific because even a C student like me can understand it.” That was point one. Point two; I was literally taken aback at one moment in this 45-minute meeting. I am explaining about islets and the Edmonton protocol and why this is important for type 1 diabetes, and the president says, “Now I can see why these JDRF [Juvenile Diabetes Research Foundation] folks are so cranked up about this,” but then, a voice over my right shoulder, Karl Rove, says, “What about porcine islets?” and what he was talking about was xenotransplantation. Why do you need embryonic stem cells? Before I could talk about porcine endogenous retroviruses, et cetera, the President laughs and chuckles and says, “Porcine. If you'd have been back in Texas you'd have said pig.” He knew what porcine meant.
Alpert, Tucson: The last two talks are just terrific and the topic that I've been talking about for the last 2 years with a very close colleague of mine, who is what I call a molecular cardiologist, a genetic and molecular biologist. A couple of comments. First of all, I think to tie what you said to what Andy Feinberg said, Dolly the sheep, of course, had premature aging and then the theory is that there were, of course, epigenetic factors that didn't come over with the nucleus so there is a lot more to go. As we have been talking about this and we have actually written an editorial together for the “Green Journal” (The American Journal of Medicine) following a series of articles in Science and other places like the ones that Andy showed from The New York Times that said, “Has the genomic revolution failed?”, I think that what we were all taught in medical school was that the sickle cell anemia model, in which one gene is abnormal, and one amino acid is abnormal, and therefore you have the disease. This is opposed by the beautiful description from Andy and also that relates very nicely to your descriptions. The analogy that we have used (and I think it's very apt) is that we were taught in medical school was similar to going to the piano and hitting middle C repeatedly, in other words, a single note; whereas what's really going on would be musically similar to what might occur if a meeting room like this held five orchestras and five chorales performing five different Bach cantatas all at the same time! And you're trying to figure out where the melody is! So, it's just a lot more complicated, but the kinds of things that we've heard in the last two talks, I think, are going to put us in the right direction.
Spiegel, Bronx: Well thank you for those comments; if I can respond just briefly on two points you made. When speaking to lay audiences, I try to use a musical analogy, so I view the genome, per se, as the score for an entire symphony, and every cell has the entire score. A pluripotent cell has the score unmarked. By the time you get to a liver cell, it's maybe only the woodwind section, a neuron is the strings and brass, and reprogramming these epigenetic changes involves erasing those marks so that you get back to the entire score, but it doesn't happen perfectly. These iPS [induced pluripotent stem] cells, and even human embryonic stem cells, will vary in the degree of erasure, so that's an important point. I also would, if I may be permitted, like to harken back to Dr Feinberg's remarkable talk. There are recognitions of the fact that even monogenic Mendelian disorders, leave aside epigenetic changes, have modifier genes, and you mentioned sickle cell anemia. My medical school classmate, Stu Orkin, made the brilliant discovery that there is a transcription factor which regulates the amount of fetal hemoglobin and that's known to be a crucial variable in terms of the sickle crises and the degree of expression of the disease. So, while epigenetics is a lot, it's not everything. There are still modifiers and complexity and that may have therapeutic potential if we can just modify, not by hydroxyurea but that transcription factor, the regulation of hemoglobin F, we may be able to ameliorate sickle cell disease.
Hoffman, New York: That was a wonderful summary of a dramatic series of events. One of the issues with the use of these cells that's always troubled me is their lack of transplantability because if you are going to use them as a therapeutic, you would hope that they would be able to sustain their effect for a long period of time. The other problem is one of the readouts for each of these cell types is the formation of teratomas, which is a form of cancer. So how would you resolve those two issues really to make this a reality?
Spiegel, Bronx: The real target is exactly the two issues that you just described and this is what I was hinting at with the regulatory challenges. Geron, this biotech company, provided reams and reams of data to the FDA to get regulatory approval for taking their human embryonic stem cell line and turning it into neurons for treatment of spinal cord injury. The fact that four patients enrolled in their clinical trial have not, as yet, suffered any consequences is not sufficient assurance. They ethically need to be followed for years, but I pointed out the eye is really a very, very special case. I am not trivializing the complexity there but it turns out that just 200,000 retinal pigment epithelial cells, differentiated either from iPS or human embryonic stem cells, placed on an extracellular matrix, which is what Bruch's membrane is in the retina, can restore function to the macula and reverse macular degeneration. The readout, by the way, is an eye chart. Nothing could be simpler and more dramatic. Pfizer has invested in this because, in part as you know, Avastin (bevacizumab) and Lucentis (ranibizumab) are so expensive and require constant injections to the eye, so they see a whole new business model; yet what if there is a teratoma? Well the glib response will be, “Well you'll enucleate like we do for retinoblastoma.” That wouldn't be a good outcome but again the eye offers, I think, a tremendous arena for these kinds of studies and eventually we will have to know whether standardized cell lines, which are really GMP-approved and tested will be feasible. It is a huge challenge. I don't mean to trivialize it. Thank you.
REFERENCES
- 1.Mak TW. Gene targeting in embryonic stem cells scores a knockout in Stockholm. Cell. 2007;131:1027–31. doi: 10.1016/j.cell.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor SS, Shapiro MA, et al. Embryonic stem cells derived from human blastocysts. Science. 1998;282:1145–47. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–22. [PubMed] [Google Scholar]
- 4.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–48. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe M, Ohnuki M, et al. Induction of pluripotent stem cells from adult human skin fibrobalsts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–5. doi: 10.1038/182064a0. [DOI] [PubMed] [Google Scholar]
- 7.Wilmut I, Sullivan G, Taylor J. A decade of progress since the birth of Dolly. Reprod Fertil Dev. 2009;21:95–100. doi: 10.1071/rd08216. [DOI] [PubMed] [Google Scholar]
- 8. [accessed December 22, 2012]. http://www.ascb.org/newsfiles/fetaltissue.pdf.
- 9.Cohen IG, Feigenbaum J, Adashi EY. Sherley v Sebelius and the future of stem cell research. JAMA. 2012;308:2087–8. doi: 10.1001/jama.2012.36633. [DOI] [PubMed] [Google Scholar]
- 10. [accessed December 7, 2012]. http://www.aaas.org/spp/cstc/briefs/stemcells/stemhearings.shtml.
- 11.IOM (Institute of Medicine) The California Institute for Regenerative Medicine: Science, Governance and the Pursuit of Cures. Washington D.C.: The National Academies Press; 2013. [Google Scholar]
- 12.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticosteroid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 13. [accessed December 21, 2012]. http://stemcells.nih.gov/research/registry.
- 14.Rao MS, Collins FS. Steering a new course for stem cell research: NIH's Intramural Center for Regenerative Medicine. Stem Cells Trans Med. 2012;1:15–7. doi: 10.5966/sctm.2011-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley L, Whitaker M. Cell therapies: the route to widespread adoption. Stem Cells Trans Med. 2012;1:438–47. doi: 10.5966/sctm.2011-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trounson A. California Institute for Regenerative Medicine: accelerating stem cell therapies in California and beyond. Stem Cells Trans Med. 2012;1:6–8. doi: 10.5966/sctm.2011-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]