Abstract
Flaviviruses are small enveloped virions that enter target cells in a pH-dependent fashion. Virus attachment, entry, and membrane fusion are orchestrated by the envelope (E) and pre-membrane (prM) proteins, the two structural proteins displayed on the surface of virions. Flaviviruses assemble as an immature non-infectious form onto which prM and E form trimeric spikes. During egress from infected cells, flaviviruses undergo dramatic structural changes characterized by the formation of a herringbone arrangement of E proteins that lay flat against the surface of the virion and cleavage of the prM protein by the cellular protease furin. The result is a relatively smooth, infectious mature virion. This dynamic process is now understood in structural detail at the atomic level. However, recent studies indicate that many of the virions released from cells share structural features of both immature and mature virus particles. These mosaic and partially mature virions are infectious and interact uniquely with target cells and the host immune response. Here, we will discuss recent advances in our understanding of the biology and significance of partially mature flaviviruses.
Flaviviruses are a group of enveloped positive-stranded RNA viruses responsible for considerable morbidity and mortality throughout the world. Members of this genus with a significant impact on public health include dengue virus (DENV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), tick-borne encephalitis virus (TBEV) and West Nile virus (WNV). These viruses are typically transmitted to humans through the bite of mosquitoes or ticks, and cause a spectrum of severe illnesses that includes encephalitis and hemorrhagic disease. While vaccines have been effective at reducing the burden of several flaviviruses when available (YFV, JEV, and TBEV) [1-3], an urgent need exists for additional vaccines and therapeutics against this genus of viruses. Antiviral antibodies contribute significantly to protection against flavivirus infection [4,5], and have proven to be a good correlate of protection for existing flavivirus vaccines [2,6]. An understanding of the structural and immunological basis for antibody-mediated protection against flavivirus infection has evolved rapidly [5]. However, recent insights into the composition, structure, and dynamics of flavivirus virions identify previously unappreciated complexities that may impact the potency of anti-flavivirus antibodies and, in the case of DENV, their potential to exacerbate disease [7,8]. This review will discuss new insights into the structural heterogeneity of flaviviruses, and how this advances our current understanding of the biology of the virus particle and its interaction with the humoral immune response.
The envelope proteins
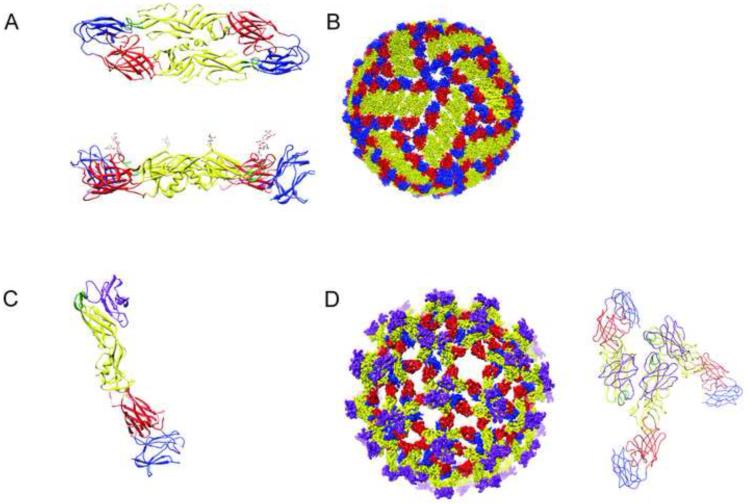
The ~11kb positive stranded genomic RNA of flaviviruses encodes a single polyprotein that is cleaved into ten functionally distinct proteins, including three structural proteins incorporated into the virus particle. High resolution structures of portions of all three structural proteins have been reported [9]. The envelope protein (E) is a ~53kDa elongated protein that orchestrates the processes of viral entry and virion budding [10]. It is composed of three distinct domains and may be modified by the addition of one or two asparagine-linked (N-linked) carbohydrates, depending on the flavivirus strain (Figure 1A). E proteins are arranged on mature virions as 90 anti-parallel dimers [11]. E domain III (E-DIII) is an immunoglobulin-like domain that forms small protrusions on the surface of an otherwise smooth spherical mature virus particle (Figure 1B); this structure is thought to interact with cellular receptors on target cells [12-14]. Domain II (E-DII) is composed of two “finger-like” structures involved in E protein dimerization and contains a highly conserved 13 amino acid hydrophobic fusion loop at its distal end [15]. These two structures are linked through a third central domain I (E-DI) via short flexible loops. The complex structural changes in E that occur during the course of virion maturation and fusion involve rotation between these three domains [16-19]. The E protein is anchored to the viral membrane through the stem anchor helical domain and two anti-parallel transmembrane domains [20,21]. The pre-membrane protein (prM) is a seven β-stranded glycoprotein that facilitates E protein folding and regulates the oligomeric state of E proteins to prevent adventitious fusion during the egress of virus particles from infected cells, as detailed below [22,23].
Figure 1. Structure of the flavivirus envelope proteins and their organization on the virus particle.
Flaviviruses are small spherical virions that incorporate a dense array of prM and E proteins that function to promote virus assembly, budding, and entry. (A) The E protein is composed of three structuraly distinct domains and is present on mature virions as anti-parallel homodimers. The dimeric arrangement of DENV E proteins is shown from the top and side. Domain III (E-DIII, shown in blue) is thought to interact with receptors on target cells. The conserved 13 amino-acid fusion loop (shown in green) is located at the distal end of domain II (E-DII, shown in yellow). E-DIII and E-DII are connected by the central domain I (E-DI, shown in red). The carbohydrate modifications of E-DI and E-DII are shown in the side view using a ball and stick representation and vary in number among different flaviviruses. The stem anchor that anchors the E protein to the viral membrane is not shown. (B) The arrangment of E proteins on the mature DENV virion is depicted. Each virus particle is composed of 30 rafts of three antiparallel dimers in a herringbone pattern. (C) The structure of the “pr” portion of DENV prM is shown in complex with the E protein. prM is shown in purple, whereas the domain organization of the E protein is represented as described in panel A. (D) The arrangement of prM and E proteins on the fully immature DENV virion is displayed. Each immature virion is composed of sixty prM-E heterotrimeric spikes arranged with icosahedral symettry. The arrangment of the prM and E protein within each spike is shown on the right.
Flavivirus biogenesis and structure
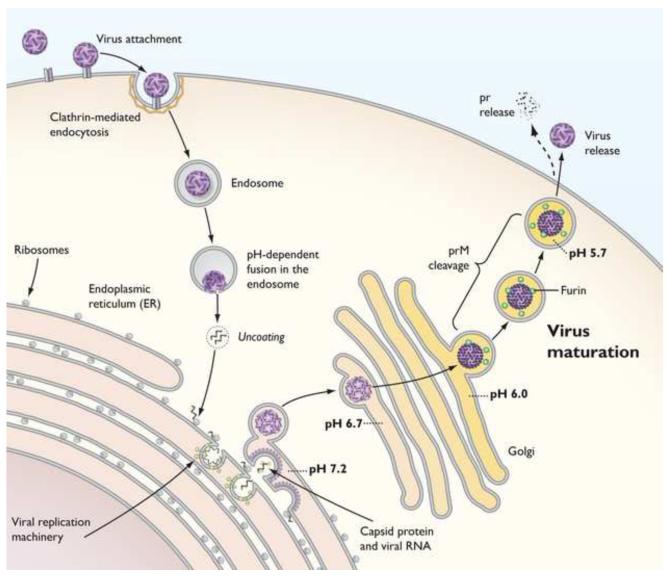
Flavivirus infection results in a marked proliferation and reorganization of membranes in the cytoplasm of cells [24]. While the cell biology of flavivirus assembly is incompletely understood, virions are thought to assemble on membranes derived from the endoplasmic reticulum (ER) at locations proximal to the site of viral RNA replication [25-27] (Figure 2). Nascently synthesized virions bud into the lumen of the ER as immature virus particles that incorporate 60 heterotrimeric spikes of prM and E arranged with icosahedral symmetry [19,28] (Figure 1D). On immature virions, prM is located at the distal end of these spikes adjacent to the E-DII fusion loop; a recent structure of the DENV prM-E heterodimer details the molecular basis for this interaction [18] (Figure 1C). Assembled virions exit the cell via the secretory pathway (Figure 2). Transit of virions through acidic compartments of the trans-Golgi network (TGN) results in a reorganization of the surface of the virus particle. The E proteins of immature virions at low pH lay flat against the surface of the virus in a herringbone pattern; prM remains attached on the surface of the immature virion positioned atop the fusion loop [29**]. This pH-dependent change in virion structure exposes on prM a cleavage site recognized by the cellular serine protease furin [30]. Mutagenesis studies with TBEV demonstrate that cleavage of prM is a required event in the infectious viral life cycle [31], presumably because the interactions between prM and E prevent the conformational changes in the E proteins required to drive pH-dependent membrane fusion after entry [23,32]. Secretion of the virion from cells into the neutral pH of the extracellular space triggers the release of the ~91 amino acid “pr” portion of prM [29**]. The resulting mature flavivirus has a relatively smooth spherical structure on which antiparallel dimers of E are arranged with T=3 pseudo-icosahedral symmetry [11,33] (Figure 1B). In the event cleavage of prM does not occur during egress through the TGN, immature virions decorated by trimeric prM-E heterotrimeric spikes are released from cells [19,28].
Figure 2. The maturation of flaviviruses.
Flaviviruses enter cells via a clathrin-dependent endocytic pathway and fuse with endosomal membranes of the target cell in a pH-dependent fashion. Flaviviruses assemble at and bud into membranes derived from the endoplasmic reticulum (ER) as immature virions on which prM and E proteins interact as heterotrimeric spikes. Assembling virions are shown on the ER membrane; the viral capsid protein is depicted as yellow spheres in complex with viral RNA. Flavivirus assembly sites are thought to be located proximal to the site of viral RNA replication (shown schematically as invaginations of membrane decorated with components of the viral replication complex (green spheres)). Transit of immature virions through the mildly acidic compartments of the secretory pathway results in a pH-dependent change in the arrangement of E proteins on the surface of the virus particles. E proteins on immature virions at an acidic pH are positioned flat against the surface of the virion in a herringbone pattern. The prM protein (represented as dark purple spheres) remains associated with the virus particle. In this conformation, prM may be cleaved by the cellular serine protease furin. Release of the virion from cells into the more neutral extracellular milieu results in the disassociation of the “pr” portion of prM and the formation of a mature virion.
Partially mature virions
Biochemical analysis of the protein composition of flaviviruses reveals the presence of uncleaved prM protein, suggesting the process of virion maturation may be inefficient under some circumstances (see [31] and references within). As a majority of virions released from DENV-infected cells can be immunoprecipitated using monoclonal antibodies (mAbs) specific for prM, virions containing significant amounts of prM may be quite prevalent [34**,35]. Factors that control the extent of virion maturation within cells remain poorly understood. The prM content of flaviviruses produced in mosquito cells appears greater than that of virus propagated in mammalian cells [36,37]. An ability to increase the efficiency of prM cleavage through the ectopic expression of furin suggests exposure to a furin-like protease in the TGN may be limiting in some cellular contexts [38,39*]. Mutations in both the prM [35] and E proteins (S. Nelson and T. Pierson, data not shown; [40]) also have been shown to impact the efficiency of prM cleavage.
Genetic studies with TBEV demonstrate that cleavage of prM is required for the production of infectious virions [31]. In support, the production of virus under conditions that reduce the efficiency of virion maturation (e.g. treatment of cells with ammonium chloride (NH4Cl) or in cell lines that do not express furin) markedly decreases the specific infectivity of virus preparations ([31,41,42] and references within). However, the residual (albeit significantly reduced) infectivity of virions produced under these conditions suggests that virions that retain uncleaved prM protein might be infectious. Three lines of experimental evidence indicate uncleaved prM are present on infectious virions: (i) the prM content of flaviviruses modulates the conditions required to trigger the conformational changes that drive membrane fusion and promote infection. Murray Valley encephalitis virus grown in the presence NH4Cl was more resistant to inactivation by acidic conditions [43]; (ii) uncleaved prM on virions may participate in the attachment and entry of infectious virus particles. Interactions between the C-type lectin DC-SIGNR and the carbohydrate present on uncleaved prM were sufficient to promote the infectious entry of WNV into cells [38]; and (iii) the prM content of flaviviruses markedly impacts the outcome of antibody-virion interactions, as discussed in detail below.
Partially mature virus particles share structural characteristics with both mature (complete prM cleavage) and immature (no prM cleavage) virus particles. Because partially mature virions are heterogeneous and lack icosahedral symmetry, structural information is limited. Examination of electron micrographs of WNV and DENV identify individual virions characterized by a mixture of smooth and spiky surfaces [28,34**,44*]. A recent cryo-electron tomographic analysis of the structure of partially mature DENV suggested that partially mature virions are mosaics of two regions corresponding to E proteins in mature and immature arrangements; the relative size of these two regions varied among the virus particles analyzed [45**]. Viral proteins at the junction of these two regions appear to be arranged in a unique fashion that was not resolved by the tomographic model.
Impact of virion heterogeneity on the interaction of flaviviruses with antibody
The presence of uncleaved prM on flaviviruses impacts the interaction of virions and antibodies in several functionally significant ways. Antibody-mediated neutralization of flavivirus infection can be modeled as a “multiple-hit” process requiring engagement of the virion with a stoichiometry that exceeds a required threshold number of antibody molecules [5,46]. From this perspective, the number of antibodies that simultaneously bind the virion is the critical parameter determining neutralizing activity. Conversely, the engagement of virions with a stoichiometry that does not exceed the neutralization threshold has the potential to promote more efficient infection of cells that express Fcγ-receptors. This antibody-dependent enhancement (ADE) of infection is thought to contribute mechanistically to the severe clinical manifestations associated with secondary DENV infections [5,47].
Epitope accessibility governs the activity of neutralizing antibodies [7]. Many of the epitopes recognized by mAbs are not predicted to be accessible on the surface of the mature virion, yet display considerable neutralizing activity [48-51]. The molecular basis for this neutralizing activity may be explained in part by the complex structure of partially mature virions. Differences in arrangement and oligomeric state of E proteins on mature and immature virions translate into changes in antibody reactivity [43,52]. Antibodies may differentially interact with the mature and immature portions of individual partially mature virions, or the envelope proteins located at the poorly resolved junction between these ordered regions. For example, the WNV E-DII fusion loop-reactive mAb E53 efficiently binds virions that contain uncleaved prM [44*]. In support of this, the neutralizing activity of many (perhaps most) WNV mAbs are strongly influenced by the maturation state of virion [39*]. WNV produced under conditions that promote more efficient cleavage of prM are markedly less sensitive to neutralization by several classes of mAbs (including E53), presumably due to a reduction in epitope accessibility.
While the majority of neutralizing mAbs are directed against the E protein, antibodies specific for prM have been reported in mice [53-57] and humans [58*,59*]. Two recent studies of the repertoire of antibodies present in DENV-immune individuals suggest anti-prM antibodies may be prevalent [58*,59*]. Anti-prM antibodies can contribute to protection from flavivirus infection through effector functions mediated by the Fc-portion of the antibody molecule [60], after interaction with uncleaved prM on virions. Studies with anti-prM mAbs reveal they nonetheless possess limited neutralizing activity. It is presently unclear whether these molecules inefficiently neutralize infection because they fail to bind virions with a required stoichiometry (too few prM molecules on infectious partially mature virions) or because they are unable to interfere with a required step in the virus entry process, such as virion attachment or fusion. In contrast, antibodies against prM readily promote ADE in vitro [42,56,58*,59*,61**] and in vivo [62]. These antibodies can markedly increase the specific infectivity of largely immature preparations of DENV [42,61**], and have been shown to enhance the infectivity of WNV in vivo [63]. Because of the significant cross-reactivity of many prM-reactive antibodies and their propensity to promote ADE, antibodies of this specificity have been proposed to be a primary contributor to the more severe clinical manifestations of secondary DENV infection [59*].
Maturation delayed: cleavage of prM during virus entry
A hallmark of virion maturation is the cleavage of prM by a cellular furin-like protease. While furin is enriched in the TGN, it also traffics to the cell surface and is recycled in endosomes [45**]. Flaviviruses enter cells via clathrin-dependent endocytosis and fuse in acidic compartments of the late endosome [64] (Figure 2). Thus, incoming flaviviruses may colocalize with furin during the virus entry process in the low pH-environment required for both exposure of the furin cleavage site and E protein-mediated viral membrane fusion [30,65]. Recently, Rodenhuis-Zybert and colleagues raised the possibility that furin may act on incoming immature virions to increase infectivity [61**] (Figure 3A). Treatment of DENV produced in furin-deficient Lovo cells with prM- or E-specific antibodies markedly enhanced the infection of Fcγ-receptor-expressing cells in a furin-dependent fashion [61**,66]. Cleavage of prM during virus entry provides a mechanistic explanation for the observation that prM antibodies increase the specific infectivity of prM-containing virions discussed above [42,61**,66]. Nonetheless, additional studies are required to determine the cellular contexts in which furin-mediated cleavage of prM during the entry of flaviviruses is important. For example, the infectivity of WNV was not significantly reduced by inhibitors of furin-like proteases, even when virions were produced in the presence of NH4Cl or infections were performed in the presence of enhancing concentrations of antibody [67]. In contrast, the replication and release of Lovo cell-derived WNV was enhanced under conditions that support ADE [66]. Because the stoichiometric threshold for prM cleavage for virus infectivity is unknown, and whether this differs in the presence or absence of enhancing antibody, it is possible that a requirement for furin-like proteases exists only for a subset of virions that retain exceptionally large numbers of uncleaved prM molecules. The size of the population of prM-containing virions released from cells for which infectivity can be enhanced through the activities of furin may be virus strain- and producer cell-type dependent.
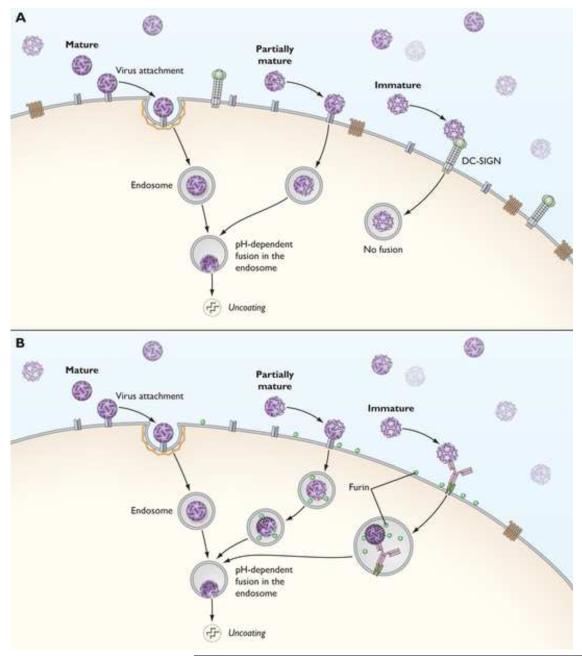
Figure 3. The heterogeneity of flaviviruses complicates an understanding of the interactions of virions with antibodies and the cell surface.
(A) Populations of flaviviruses released from cells are heterogeneous with respect to the extent of prM cleavage. Mature virions are defined as those virus particles on which prM has been completely cleaved and are infectious. Conversely, immature virions retain 180 copies of uncleaved prM. Genetic studies indicate these virus particles are non-infectious. Partially mature virions describe a heterogeneous population of virions that retain varying amounts of uncleaved prM. Recent structural studies indicate that the partially mature virions contain distinct regions of mature and immature character [45]. At least some partially mature virions are infectious, although the relationship between the specific infectivity of virions and prM content has not yet been investigated. The significant differences in the arrangement of the E proteins on mature, immature, and partially mature viruses raises the interesting (yet unexplored) possibility that they differentially interact with cellular factors on the surface of target cells. (B) A role for furin-like proteases during flavivirus entry? The presence of the furin protease in endocytic compartments raises the possibility that the prM protein on partially mature and immature virions may be processed during entry [61**]. This phenomenon may contribute to the marked increase in the specific infectivity of largely immature populations of DENV in the presence of enhancing concentrations of antibody [42,61**].
Conclusions and future directions
While recent progress has been considerable, the biology of partially mature virions and their contribution to pathogenesis remains incompletely understood. Partially mature virions are defined by the presence of uncleaved prM protein; an unknown proportion of these virions are infectious. The recent model of the structure of partially mature virions suggest their immature character is restricted to a single region on the virion surface, presumably the size of which will vary as a function of the prM content of the individual virus particle [45**]. However, the relationship between the number of prM molecules on the virion (the size of the immature region) and its specific infectivity has not yet been studied. Understanding the stoichiometric requirements for prM cleavage will provide insights into the number of E proteins molecules required for fusion, and how virions interact with target cells. Because prM controls the oligomeric state of E proteins and regulates fusion [23,32], presumably E protein molecules in immature regions cannot contribute to fusion. If E-DIII indeed binds cellular receptors on target cells [12-14], the orientation of the E protein could modulate access to this receptor binding structure; E-DIII at the bottom of a trimeric spike may render it less able to attach to its cognate receptor. Is it possible that the cellular factor used by a virion during entry into cells varies as a function of the prM content of the virus particle? Does the retention of uncleaved prM modulate the efficiency of interactions with other host factors, such as mannose binding lectin, that may be important for immune control or pathogenesis [68]? Studies with TBEV suggest cleavage of prM is significantly more efficient than that observed for mosquito-borne flaviviruses [30]. What viral and cellular factors control the extent of prM cleavage and the proportion of mature, immature, and partially mature virions released from cells? What is the efficiency of prM cleavage in vivo? Resolution of many of these issues will require new approaches that enable the analysis of the functional and biochemical properties of individual virus particles with controlled levels of uncleaved prM.
Highlights.
Partially mature flaviviruses with features of both mature and immature viruses are released from infected cells and may be infectious.
The maturation state of flaviviruses modulates the functional outcome of the interaction with antibody
Anti-prM antibodies are elicited by infection and increase the specific infectivity of flaviviruses via interactions with Fc-γ receptors
The maturation of flaviviruses may be enhanced during the virus entry process by furin proteases recycling through endosomal compartments.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the NIH, National Institutes of Allergy and Infectious Diseases (NIAID), R01-AI077955 and U01-AI061373. The authors thank Mr. Ethan Tyler and Phong Lee for preparation of the figures. We are grateful to Drs. Kimberly Dowd and Swati Mukherjee for their thoughtful comments on our manuscript. We thank members of our laboratory for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Monath TP. Yellow fever vaccine. Expert review of vaccines. 2005;4(4):553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 2.Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25(43):7559–7567. doi: 10.1016/j.vaccine.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Beasley DW, Lewthwaite P, Solomon T. Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther. 2008;8(1):95–106. doi: 10.1517/14712598.8.1.95. [DOI] [PubMed] [Google Scholar]
- 4.Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001;951:286–297. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- 5.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell host & microbe. 2008;4(3):229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monath TP, Cetron MS, Teuwen DE. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Saunders Elsevier; 2008. pp. 959–1055. [Google Scholar]
- 7.Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411(2):306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodenhuis-Zybert IA, Wilschut J, Smit JM. Partial maturation: an immune-evasion strategy of dengue virus? Trends Microbiol. 2011;19(5):248–254. doi: 10.1016/j.tim.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Perera R, Kuhn RJ. Structural proteomics of dengue virus. Curr Opin Microbiol. 2008;11(4):369–377. doi: 10.1016/j.mib.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nature reviews. 2005;3(1):13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375(6529):291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nature medicine. 1997;3(8):866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 14.Chiu MW, Yang YL. Blocking the dengue virus 2 infections on BHK-21 cells with purified recombinant dengue virus 2 E protein expressed in Escherichia coli. Biochemical and biophysical research communications. 2003;309(3):672–678. doi: 10.1016/j.bbrc.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 15.Allison SL, Schalich J, Stiasny K, Mandl CW, Heinz FX. Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. J Virol. 2001;75(9):4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, Lescar J, Heinz FX, Rey FA. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. Embo J. 2004;23(4):728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427(6972):313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science (New York, NY. 2008;319(5871):1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. Structures of immature flavivirus particles. Embo J. 2003;22(11):2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Chipman PR, Corver J, Johnson PR, Zhang Y, Mukhopadhyay S, Baker TS, Strauss JH, Rossmann MG, Kuhn RJ. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat Struct Biol. 2003;10(11):907–912. doi: 10.1038/nsb990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allison SL, Stiasny K, Stadler K, Mandl CW, Heinz FX. Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E. J Virol. 1999;73(7):5605–5612. doi: 10.1128/jvi.73.7.5605-5612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz IC, Allison SL, Heinz FX, Helenius A. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J Virol. 2002;76(11):5480–5491. doi: 10.1128/JVI.76.11.5480-5491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinz FX, Stiasny K, Puschner-Auer G, Holzmann H, Allison SL, Mandl CW, Kunz C. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology. 1994;198(1):109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 24.Westaway EG, Mackenzie JM, Kenney MT, Jones MK, Khromykh AA. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71(9):6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie JM, Westaway EG. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J Virol. 2001;75(22):10787–10799. doi: 10.1128/JVI.75.22.10787-10799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell host & microbe. 2009;5(4):365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. Journal of virology. 2010;84(20):10438–10447. doi: 10.1128/JVI.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. Structure of immature West Nile virus. J Virol. 2007;81(11):6141–6145. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science (New York, NY. 2008;319(5871):1834–1837. doi: 10.1126/science.1153264. ** The authors preasent the structure of the immature virion at acid pH. This structure provides an important intermediate between the immature and mature virus products of the biogenesis and maturation pathway of flaviviruses.
- 30.Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71(11):8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elshuber S, Allison SL, Heinz FX, Mandl CW. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J Gen Virol. 2003;84(Pt 1):183–191. doi: 10.1099/vir.0.18723-0. [DOI] [PubMed] [Google Scholar]
- 32.Guirakhoo F, Heinz FX, Mandl CW, Holzmann H, Kunz C. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J Gen Virol. 1991;72(Pt 6):1323–1329. doi: 10.1099/0022-1317-72-6-1323. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science (New York, NY. 2003;302(5643):248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 34**.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, Kasinrerk W, et al. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J Virol. 2010;84(16):8353–8358. doi: 10.1128/JVI.00696-10. ** This study provides an estimate of the proportion of mature, immature, and partially mature DENV released from cells using structural and immunoprecipitation approaches. The authors conclude that a majority of DENV virions may retain uncleaved prM.
- 35.Junjhon J, Lausumpao M, Supasa S, Noisakran S, Songjaeng A, Saraithong P, Chaichoun K, Utaipat U, Keelapang P, Kanjanahaluethai A, Puttikhunt C, et al. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. Journal of virology. 2008;82(21):10776–10791. doi: 10.1128/JVI.01180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray JM, Aaskov JG, Wright PJ. Processing of the dengue virus type 2 proteins prM and C-prM. The Journal of general virology. 1993;74(Pt 2):175–182. doi: 10.1099/0022-1317-74-2-175. [DOI] [PubMed] [Google Scholar]
- 37.Vogt MR, Dowd KA, Engle M, Tesh RB, Johnson S, Pierson TC, Diamond MS. Poorly Neutralizing Cross-Reactive Antibodies against the Fusion Loop of West Nile Virus Envelope Protein Protect In Vivo via Fc{gamma} Receptor and Complement-Dependent Effector Mechanisms. Journal of virology. 2011;85(22):11567–11580. doi: 10.1128/JVI.05859-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80(3):1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS, Pierson TC. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS pathogens. 2008;4(5):e1000060. doi: 10.1371/journal.ppat.1000060. * The authors demonstrate that changes in the maturation state of WNV modulates sensitivity to antibody-mediated neutralization by mAbs and some polyclonal antibody responses.
- 40.Nelson S, Poddar S, Lin TY, Pierson TC. Protonation of individual histidine residues is not required for the pH-dependent entry of west nile virus: evaluation of the “histidine switch”a hypothesis. J Virol. 2009;83(23):12631–12635. doi: 10.1128/JVI.01072-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zybert IA, van der Ende-Metselaar H, Wilschut J, Smit JM. Functional importance of dengue virus maturation: infectious properties of immature virions. J Gen Virol. 2008;89(Pt 12):3047–3051. doi: 10.1099/vir.0.2008/002535-0. [DOI] [PubMed] [Google Scholar]
- 42.Randolph VB, Winkler G, Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174(2):450–458. doi: 10.1016/0042-6822(90)90099-d. [DOI] [PubMed] [Google Scholar]
- 43.Guirakhoo F, Bolin RA, Roehrig JT. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191(2):921–931. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, et al. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J. 2009;28(20):3269–3276. doi: 10.1038/emboj.2009.245. * This study provides the structural basis for the maturation-state dependent binding of a neutralizing antibody to WNV.
- 45**.Plevka P, Battisti AJ, Junjhon J, Winkler DC, Holdaway HA, Keelapang P, Sittisombut N, Kuhn RJ, Steven AC, Rossmann MG. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO Rep. 2011;12(6):602–606. doi: 10.1038/embor.2011.75. ** This cryo-electon tomographic study provides the first model of the structure of partially mature DENV.
- 46.Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont DH, Diamond MS. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell host & microbe. 2007;1(2):135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. The American journal of tropical medicine and hygiene. 1989;40(4):444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 48.Stiasny K, Kiermayr S, Holzmann H, Heinz FX. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J Virol. 2006;80(19):9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80(24):12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437(7059):764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, Diamond MS, et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nature structural & molecular biology. 2008;15(3):312–317. doi: 10.1038/nsmb.1382. [DOI] [PubMed] [Google Scholar]
- 52.Holzmann H, Stiasny K, York H, Dorner F, Kunz C, Heinz FX. Tick-borne encephalitis virus envelope protein E-specific monoclonal antibodies for the study of low pH-induced conformational changes and immature virions. Archives of virology. 1995;140(2):213–221. doi: 10.1007/BF01309857. [DOI] [PubMed] [Google Scholar]
- 53.Calvert AE, Kalantarov GF, Chang GJ, Trakht I, Blair CD, Roehrig JT. Human monoclonal antibodies to West Nile virus identify epitopes on the prM protein. Virology. 2011;410(1):30–37. doi: 10.1016/j.virol.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 54.Falconar AK. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Archives of virology. 1999;144(12):2313–2330. doi: 10.1007/s007050050646. [DOI] [PubMed] [Google Scholar]
- 55.Vazquez S, Guzman MG, Guillen G, Chinea G, Perez AB, Pupo M, Rodriguez R, Reyes O, Garay HE, Delgado I, Garcia G, et al. Immune response to synthetic peptides of dengue prM protein. Vaccine. 2002;20(13-14):1823–1830. doi: 10.1016/s0264-410x(01)00515-1. [DOI] [PubMed] [Google Scholar]
- 56.Huang KJ, Yang YC, Lin YS, Huang JH, Liu HS, Yeh TM, Chen SH, Liu CC, Lei HY. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol. 2006;176(5):2825–2832. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
- 57.Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246(2):317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 58*.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell host & microbe. 2010;8(3):271–283. doi: 10.1016/j.chom.2010.08.007. * This study identifies prM-reactive antibodies as a substantial component of the human antibody repertoire against DENV.
- 59*.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science (New York, NY. 2010;328(5979):745–748. doi: 10.1126/science.1185181. * This study identifies prM-reactive antibodies as a substantial component of the human antibody repertoire against DENV.
- 60.Kaufman BM, Summers PL, Dubois DR, Cohen WH, Gentry MK, Timchak RL, Burke DS, Eckels KH. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. The American journal of tropical medicine and hygiene. 1989;41(5):576–580. doi: 10.4269/ajtmh.1989.41.576. [DOI] [PubMed] [Google Scholar]
- 61**.Rodenhuis-Zybert IA, van der Schaar HM, da Silva Voorham JM, van der Ende-Metselaar H, Lei HY, Wilschut J, Smit JM. Immature dengue virus: a veiled pathogen? PLoS pathogens. 2010;6(1):e1000718. doi: 10.1371/journal.ppat.1000718. ** This study implicates the activity of furin protease on incoming immature flaviviruses as a mechanism to increase the specific infectivity of prM-containing flaviviruses
- 62.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell host & microbe. 2010;7(2):128–139. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colpitts TM, Rodenhuis-Zybert I, Moesker B, Wang P, Fikrig E, Smit J. prM-antibody renders immature West Nile virus infectious in vivo. J Gen Virol. 2011 doi: 10.1099/vir.0.031427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Schaar HM, Rust MJ, Chen C, van der Ende-Metselaar H, Wilschut J, Zhuang X, Smit JM. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS pathogens. 2008;4(12):e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stiasny K, Heinz FX. Flavivirus membrane fusion. J Gen Virol. 2006;87(Pt 10):2755–2766. doi: 10.1099/vir.0.82210-0. [DOI] [PubMed] [Google Scholar]
- 66.Rodenhuis-Zybert IA, Moesker B, da Silva Voorham JM, van der Ende-Metselaar H, Diamond MS, Wilschut J, Smit JM. A fusion-loop antibody enhances the infectious properties of immature flavivirus particles. Journal of virology. 2011;85(22):11800–11808. doi: 10.1128/JVI.05237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukherjee S, Lin TY, Dowd KA, Manhart CJ, Pierson TC. The Infectivity of prM-Containing Partially Mature West Nile Virus Does Not Require the Activity of Cellular Furin-Like Proteases. Journal of virology. 2011;85(22):12067–12072. doi: 10.1128/JVI.05559-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuchs A, Lin TY, Beasley DW, Stover CM, Schwaeble WJ, Pierson TC, Diamond MS. Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin. Cell host & microbe. 2010;8(2):186–195. doi: 10.1016/j.chom.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]